Translate this page into:
Local extinctions in the small mammal’s assemblages between late Holocene and historical times in Talagapa mountains (Patagonia, Argentina): The role of land use changes
⁎Corresponding author at: Centro Nacional Patagónico, Boulevard Almirante Brown 2915 (9120), Puerto Madryn, Chubut, Argentina. andrade@cenpat-conicet.gob.ar (Analia Andrade),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aim of this work was to evaluate possible changes in the small mammal composition in the Patagonian arid Extra-Andean steppes between the late Holocene and historical times. A bone sequence accumulated by the disintegration of owl pellets deposited inside a cave in the Talagapa mountains (Chubut province, Argentina) was analyzed. The paleontological sequence encompasses a time span since 2.857 ± 50 years BP and 2.285 ± 40 years BP and was covered by a thick and compact layer of sheep feces that set the boundaries of historical times. Rodent prey were identified and quantified on the basis of skulls and mandible pairs and Holocene and historical assemblages were compared with those recovered from modern owl pellets collected in the area. The sharpest change appears in recent times, after the modification in the land use in the area, with local extinction of Loxodontomys micropus, a species associated with mesic habitats, and the apparent modification in the frequencies of certain species. Hypotheses about climatic, anthropic, and environmental factors that may have influenced those variations are discussed.
Keywords
Local extinctions
Small mammal assemblages
Extra-Andean Patagonia
Holocene
1 Introduction
Patagonia is an extensive geographic region, that comprises around 800,000 km2 and is located at the southernmost end of South America. This region is characterized by a strong west-east environmental heterogeneity, determined mainly by a decreasing gradient of precipitation toward the east and a northwest-southeast gradient of temperature. In addition, the region is affected by air masses from the Atlantic Ocean resulting in a more even temporal distribution of precipitation (Paruelo et al., 1998). For the northern arid Extra-Andean region, gradients of many environmental variables are also associated with a marked altitudinal component. The biological productivity in the region is rather uniform with maximum gain reported in November (Austral spring) when the temperature and water availability peak (Paruelo loc cit). Distribution and abundance of small mammals are the expressed consequence of past and present climatic and anthropogenic related environmental changes. Temperature is a determining factor in such distributions and abundances in arid Patagonia, both in time and space (Andrade and Monjeau, 2015). Volcanic eruptions are among factors that influence the abundance of some rodent species. Despite possible immediate effects, after the explosion of the Hudson volcano in Santa Cruz province, with the subsequent coverage of the land with 5–15 cm of ash that had killed thousands of sheep, the large herbivore guanaco and other medium sized mammals, the rodents Eligmodontia morgani, Phyllotis xanthopygus and Reithrodon auritus were reported in greater-than-expected numbers and reproducing vigorously (Pearson, 1994; Saba and de Lamo, 1994). Intense periods of rain or extremely cold winters can also affect population numbers. Not only natural causes could produce alterations in species abundances changes in natural ecosystems by human activities often become unpredictable in several aspects, social, economic and human health. In western Patagonia, small mammal assemblages from the Andean forests include Oligoryzomys longicaudatus, rodent host of the Hantavirus, and potential cause of fatal diseases in humans by the Hantavirus Pulmonary Syndrome (HPS). Besides natural explosive population increases of this granivorous species associated with the availability of seeds of the flowering cane (Chusquea spp.), livestock and forest clearing dispersing the introduced and invasive sweet briar shrub lands (Rosa rubiginosa) make these anthropogenic environments a suitable habitat where this species reaches its highest densities, constituting a habitat with high epidemiological risk (Andreo et al., 2012).
Past climatic changes have also contributed to configure actual species distributions and abundances. Although paleoecology and paleoenvironmental reconstructions in Patagonia are still incomplete, a general faunal stability during the Holocene was inferred (Fernández et al., 2012). Specifically for the late Holocene (between 2.7 and 2 ky), a humid pulse with lower mean temperatures allowed minor changes in species abundances and the expansion in the distributional ranges of some particular forest species into the steppe (Teta et al., 2005). Archeological and paleontological stratigraphic sequences of small mammals become a useful tool to discriminate past natural and cultural causes of changes in species compositions and abundances. This work presents the preliminary results of the analysis of a stratigraphic sequence of small mammals recovered inside a cave located in the arid Extra-Andean steppes from northern Patagonia. The aim was to evaluate possible changes in the composition of the small mammal assemblages between the late Holocene and historical times and to propose hypotheses about climatic, anthropic, and environmental factors that may have influenced those variations.
2 Methods and materials
Abundance data come from the analysis of a bone sequence accumulated by the disintegration of pellets deposited inside a cave by owls that used the site as a nest and/or roost since late Holocene. The cave (Cerro Mesa) is located on the grassy steppes of the Patagonian phytogeographical province, on the top of a hill in the mountains called Sierra de Talagapa (42° 14′ S, 68° 14′ W, Chubut province, Argentina). The climate in the area is arid and cold, with a Mean Annual Precipitation of 187 mm and a wide thermal range (from −25 °C in winter to above 35 °C in summer, data given by DPA, Departamento Provincial de Aguas, Maquinchao weather station, Río Negro province).
Two radiocarbon dates on small mammal’s bones were obtained by AMS at the CEDAD laboratory (University of Lecce, Italy). The paleontological sequence encompasses a time span since 2.857 ± 50 years BP (layer 7, LTL2500A, raw data) and 2.285 ± 40 years BP (layer 1, LTL2501A, raw data), and was excavated by 7 artificial layers of 3 cm thick. Unfortunately, part of the sequence was missing; at the top of the late Holocene samples there was a thick and compact layer of sheep feces that set the boundaries of historical times.
The excavation grid (1 m2) was placed below the main roost used currently by an individual of the owl Tyto alba. The samples were recovered using a brush and a shovel and sieved with a 2 mm mesh. In the laboratory, small bone remains were picked out from the remaining sediments. Rodent prey were identified at the finest taxonomic level using reference collections housed at the Instituto Patagonico de Ciencias Sociales y Humanas (IPCSH, Cenpat-Conicet, Chubut, Argentina). Abundance data for each species were quantified by the MNI index (Minimum Number of Individuals) on the basis of skulls and mandible pair counts. Holocene and historical small mammal samples were compared with modern assemblages from two consecutive years (2001 and 2002) produced by the owl living inside the cave and with a pellet sample and a bone assemblage result of the disintegration of pellets (time averaged recent sample) collected at 3 km from Cerro Mesa in the Talagapa mountains (Teta and Andrade, 2002). Multivariate analyses were used to classify samples based on the frequency of small mammal species. Faunal databases were made and the Euclidean Distance Matrix employed in a cluster analysis built with the Unpaired Group Mean Average Algorithm (UPGMA). A Cophenetic Correlation Coefficient (CCC) higher than 0.8 was used as a threshold of cluster reliability. Cluster analysis was performed with the Past software (Hammer et al., 2001).
3 Results and discussion
A total of 7292 cranial and mandibular bone remains were identified in the sequence, which correspond to 12 species, 11 of which were rodents. They included 9 Sigmodontinae: Abrothrix hirta, A. olivaceus, Chelemys macronyx, Eligmodontia sp., Euneomys petersoni, Loxodontomys micropus, Notiomys edwardsii, Phyllotis xanthopygus and Reithrodon auritus, one Caviidae: Microcavia australis, one Ctenomyidae: Ctenomys sp. and one Didelphidae: Lestodelphys halli (Table 1). All of these are typical species from the Patagonian steppes but A. hirta, A. olivaceus, C. macronyx and L. micropus are also typical of forest assemblages or inhabitants of dense patches of vegetation. Current pellet samples registered the same species that were observed in late Holocene sequence but lacked L. micropus. This species was part of the small mammal assemblages from the Talagapa area until recent times, because it was observed not only in the paleontological sequence but also in the upper sheep feces layer. Eligmodontia sp. and E. petersoni were clearly dominant in Late Holocene (between 20% and 27%) as in the present. Abrothrix olivaceus, Ctenomys sp., N. edwardsii and R. auritus showed intermediate frequencies, while A. hirta, C. macronyx, L. halli, L. micropus, M. australis and P. xanthopygus showed frequencies lower than 5%. However, the sharpest change appears when the species assemblages of the sequence and the current pellet samples (Cerro Mesa and Talagapa samples) are compared. Besides the disappearance of L. micropus as mentioned, specific changes occur in the proportions of some of the species. Particularly, toward recent times E. petersoni has increased (between 22% and 38%, even higher if considering the time averaged recent bone assemblage), while N. edwardsii decreased (less than 1% and even absent in some of the pellet samples). Although environmental situation is different around the localities where actual pellet samples were collected (more rocky habitats suitable for the species characterize Talagapa compared with Cerro Mesa surroundings, Teta and Andrade, 2002), the increase in E. petersoni was also detected when comparing samples from two consecutive years collected inside the cave (Cerro Mesa I and II, Table 1). We do not discard the probability that differential preservation by trampling could alter frequencies of large species (like E. petersoni and other similar sized species like P. xanthopygus or R. auritus) in the sheep faces layer, but this situation must also alter the frequencies of larger species like the caviomorphs Ctenomys sp. and M. australis, but this was not the case.
Stratigraphical layer
Actual pellet samples
7
6
5
4
3
2
1
Sheep feces
Cerro Mesa I
Cerro Mesa II
Talagapa I
Talagapa II
E. petersoni
15 (25)
52 (21.2)
91 (24.1)
131 (27.3)
175 (27.4)
211 (26.4)
31 (27.2)
57 (34.3)
75 (22.6)
47 (32.6)
129 (38.5)
94 (45.9)
R. auritus
10 (16.7)
23 (9.4)
52 (13.8)
56 (11.7)
57 (8.9)
82 (10.2)
9 (7.9)
13 (7.8)
51 (15.4)
9 (6.2)
28 (8.4)
32 (15.7)
P. xanthopygus
1 (1.7)
7 (2.9)
9 (2.4)
10 (2.1)
14 (2.2)
20 (2.5)
3 (2.6)
7 (4.2)
11 (3.3)
4 (2.8)
15 (4.5)
10 (4.9)
L. micropus
2 (3.3)
8 (3.3)
7 (1.9)
8 (1.7)
25 (3.9)
25 (3.1)
3 (2.6)
7 (4.2)
0
0
0
0
C. macronyx
3 (5)
2 (0.8)
7 (1.9)
10 (2.1)
16 (2.5)
17 (2.1)
3 (2.6)
6 (3.6)
6 (1.8)
2 (1.4)
9 (2.7)
5 (2.4)
A. olivaceus
5 (8.3)
27 (11)
42 (11.1)
47 (9.8)
78 (12.2)
85 (10.6)
11 (9.6)
18 (10.8)
59 (17.1)
19 (13.2)
61 (18.2)
10 (4.9)
A. hirta
1 (1.7)
11 (4.5)
14 (3.7)
17 (3.5)
24 (3.8)
34 (4.2)
5 (4.4)
6 (3.6)
7 (2.1)
5 (3.5)
9 (2.7)
5 (2.4)
Eligmodontia sp.
15 (25)
60 (24.5)
80 (21.2)
105 (21.9)
142 (22.2)
193 (24.1)
26 (22.8)
26 (15.6)
79 (23.8)
40 (27.8)
48 (14.3)
11 (5.4)
N. edwardsii
2 (3.3)
13 (5.3)
32 (8.5)
35 (7.3)
47 (7.4)
55 (6.9)
9 (7.9)
7 (4.2)
3 (0.9)
1 (0.7)
0
1 (0.5)
Ctenomys sp.
5 (8.3)
37 (15.1)
35 (9.3)
55 (11.5)
53 (8.3)
64 (8)
8 (7)
15 (9)
33 (9.9)
15 (10.4)
32 (9.5)
31 (15.2)
M. australis
0
2 (0.8)
4 (1.1)
3 (0.6)
3 (0.5)
7 (0.8)
2 (1.7)
1 (0.6)
4 (1.2)
0
3 (0.9)
1 (0.5)
L. halli
1 (1.7)
3 (1.2)
5 (1.3)
3 (0.6)
5 (0.8)
7 (0.8)
4 (3.5)
3 (1.8)
4 (1.2)
2 (1.4)
1 (0.3)
3 (1.5)
Total MNI
60
245
378
480
639
800
114
166
332
144
335
204
Richness
11
12
12
12
12
12
12
12
11
10
10
11
This change toward recent time was also reflected in the cluster analysis performed on the small mammal frequency matrix (Fig. 1, CCC = 0.8711). Two main groups can be proposed, one with the samples from the late Holocene and a second grouping current pellet samples and historical samples from the stratigraphic sequence (small mammals from the sheep feces layer). The arrow in Fig. 1 depicts the separation between paleontological and historical times, before and after the changes in the use of land due to the introduction of cattle.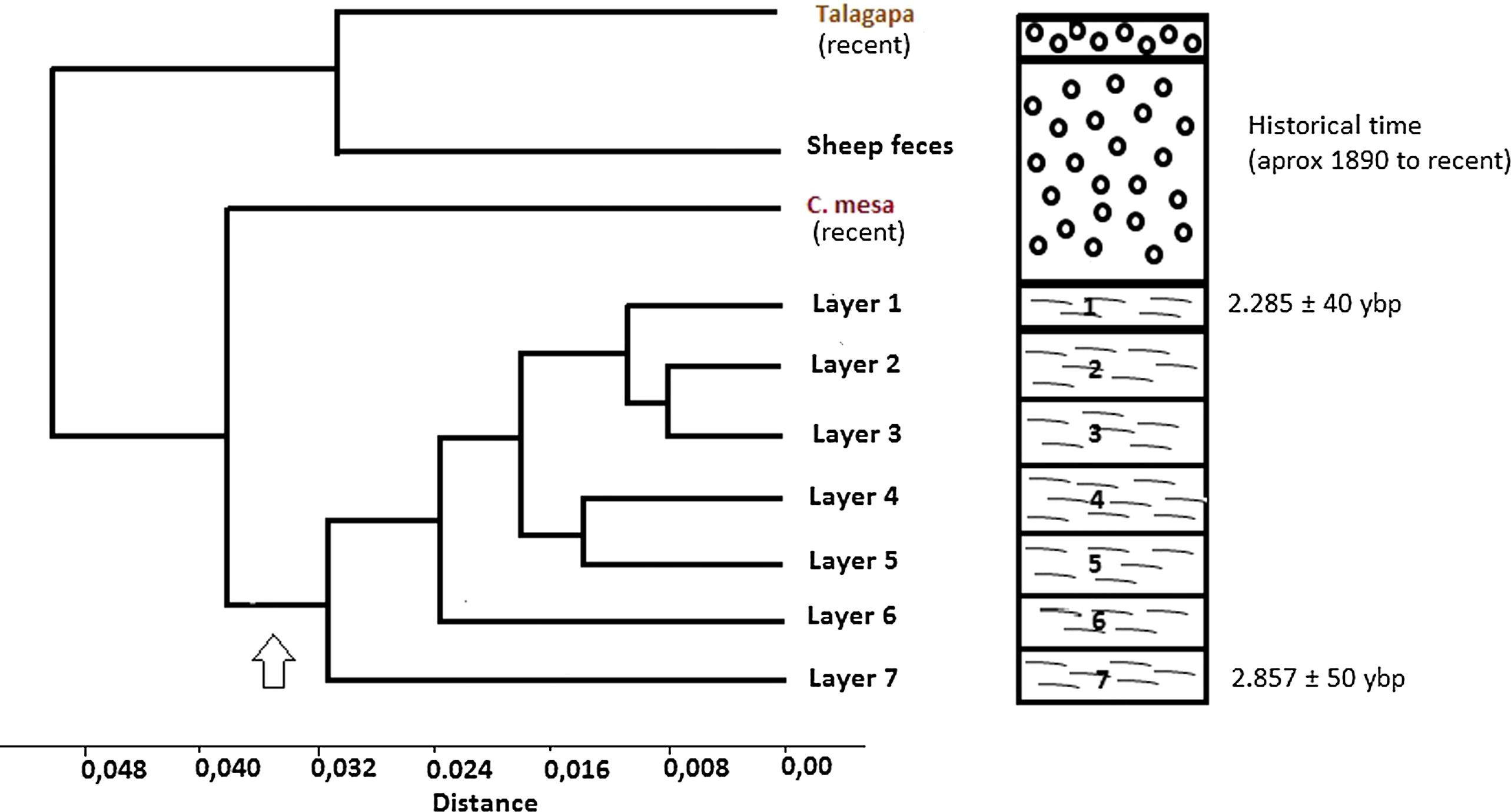
Cerro Mesa stratigraphy (right) and cluster analyses (left) based on the small mammals frequency matrix. The arrow shows the separation between paleontological (layers 1–7) and historical samples (sheep feces layer, Talagapa and Cerro Mesa pellet assemblages).
In Argentinean Patagonia, L. micropus is mostly restricted to forests and mesic brushy habitats in the Andean foothills, but some isolated populations have been documented in the central basaltic plateaus (Teta et al., 2002). These desert populations are restricted to mesic habitats into the steppe and may correspond to more extensive paleo-distributions. Although in low frequencies, L. micropus was found in several archeological deposits of the late Holocene from Extra-Andean Patagonia (Teta et al., 2005), showing that the expansion in its distributional range toward the east was associated with the cold and humid phases of the late Holocene. The same is true for C. macronyx; this forest and ecotonal species is restricted to mesic habitats in the steppe at higher elevations in the mountains ranges (Teta et al., 2002). It can be proposed that those mesic cores allow the persistence of these species at very low frequencies, although their populations are in clear decline and even some of them show signs of recent extinction. N. edwardsii is a small fossorial rodent species endemic from Argentinian and Chilean Patagonian steppes (Pearson, 1984; D́Elia et al., 2016). Its distribution was largely unknown but localities in the Somuncurá plateau allow to infer that this species lives in association with the Patagonian Phytogeographical Province, in habitats with bunchgrass steppe of Poa, Stipa and Festuca grasses with basaltic substrate at elevations above 1000 m a.s.l. (Andrade, 2008). Although N. edwardsii is still common in some steppe localities, mainly contained in Athene cunicularia pellets, it has been proposed that non-sustainable practices such as overgrazing of herbaceous steppes by cattle and wind erosion could lead to future local extinction of this species (Andrade, 2008). This hypothesis is supported by the study of Talagapa assemblages, because this species apparently declines in frequency after the introduction of sheep in the area, since percentage values were higher in late Holocene assemblages compared to those from the sheep feces layer and the extant pellet samples. The inverse situation is observed for E. petersoni. This species is common in bare, windswept and rocky habitats (Pearson, 1987) and is the dominant species in traps and pellet samples in the bunchgrass steppe from the upper levels of the central basaltic plateaus. Although preliminary, the decrease in the frequency of N. edwardsii and the increase in E. petersoni would indicate a further development of open and bare environments with decreasing areas occupied by grasses after the introduction of sheep in the area.
The Patagonian chinchilla mouse E. petersoni was the most abundant species in the Traful valley (western ecotone between the semiarid steppe and the temperate forests) during the last 10,000 years and disappeared in recent years (Pearson, 1987: named as E. chinchilloides). Despite climatic changes during glacial advances and retreats in the Patagonian Andes Mountains, the proportions of small mammal species did not change substantially except for this species. Pearson (loc cit) proposed that the retreating glaciers would have provided bare areas suitable for this species. Fires set purposefully by hunter-gatherers retarded the invasion of trees and shrubs slowing the return to the actual vegetation and E. petersoni retreated to isolated patches of bare ground. However, the effect of fires in the re-structuration of the small mammal’s composition in the arid Patagonia was not yet evaluated. The study of Pearson (loc cit) remains one of the first in demonstrating that changes in natural ecosystems by human activities could influence small mammal abundances in Patagonia. These and other small mammal sequences from the Holocene were re-evaluated (Pardiñas and Teta, 2012) and these authors arrived to the same conclusion proposed by Pearson (1987), but 25 years later: although minor expansion of desert adapted taxa to the west since the middle Holocene, small mammal assemblages remained stable over the Holocene and dramatic changes were only observed during the last 100–150 years. Changes included the disappearance of some species and remarkable increases of opportunistic taxa associated with sheep overgrazing and the introduction of exotic shrubs. For the Chubut valley in central Patagonia, a loss of small mammal diversity over the last 400 years was documented (Pardiñas et al., 2012), with the extirpation of five rodent species (between them L. micropus and N. edwardsii). The authors concluded that climatic changes but mainly human impact-sheep farming and development of crop fields-may be the main cause of this community re-structuration. We do not discard the possibility that climatic changes could have affected the small mammal communities in Talagapa. Moreover, it is likely that natural and cultural causes could have had synergistic effect. After a cold and humid pulse in late Holocene, a gradual desertification with the expansion of open areas was proposed for northern extra-Andean Patagonia (Teta et al., 2005). Probably, human activities acting in an environment that was already being modified by climatic causes, have accelerated the pace of landscape transformation in Talagapa. Beyond the differences in the scale of analysis, Teta et al. (2014) concluded that there is no evidence that current small mammal communities have been affected significantly by recent climate changes in Patagonia, but their structure was deeply affected after the arrival of the Europeans. Unfortunately the entire late Holocene is not registered in the sequence to evaluate this tendency; but undoubtedly human activities affected those communities because the main change in the sequence is observed after the introduction of cattle in the area.
European cattle was introduced by Jesuit Missions in western Patagonia in the eighteenth century and adopted by the indigenous people for subsistence in family type economies, although it was not until the nineteenth century when ovine livestock exploitation intensified (Bandieri, 2005). After the so-called Conquest of the Desert in 1879, when military campaigns were intended to defeat indigenous people, conquered lands were made available to private capital, many of them foreigners. Specifically for Talagapa, the chronicles mentioned that those mountains constituted a summer route, annually re-used by the indigenous people from northern Patagonia who were heading south toward the Chubut river; cattle (cows, horses and sheep) was yet exploited by indigenous families living in the area at least since 1879, and certainly before, as was observed by naturalists that went through their territories (Moreno, 1979).
The anthropogenic impact on the small mammal’s communities of Patagonia could have existed since prehistoric times. Based on historical, ethnographic and archeological evidence, it was demonstrated that some species of Caviomorpha rodents were intensively exploited by hunter-gatherer societies-bones, meat and skins of the animals were used-since at least late Holocene; this practice continued until last century (Andrade and Boschin, 2015). Hunting pressure carried out by human populations might be another possible cause of alteration in the abundance of the small mammals in Patagonia, not quantified yet.
Acknowledgements
Authors deeply thank Rizwan Irshad, editor of JKSUS, for his support and advice and the anonymous reviewers for their suggestions. Departamento Provincial de Aguas (DPA, Río Negro) offered the meteorological data. This research was funded by ANPCyT (Agencia Nacional de Promoción Científica y Técnica, Argentina, PICT-ANPCyT 3399).
References
- Mammalia, Rodentia, Cricetidae, Notiomys edwardsii (Thomas, 1890): distribution extension and geographic distribution map. CheckList. 2008;4(1):33-36.
- [Google Scholar]
- Explotación de roedores por las sociedades cazadoras-recolectoras de Patagonia durante el Holoceno tardío: de la evidencia arqueológica al registro histórico. Zephyrus. 2015;75:107-124.
- [Google Scholar]
- Patterns in community assemblage and species richness of small mammals across an altitudinal gradient in semi-arid Patagonia, Argentina. J. Arid Environ.. 2015;106:18-26.
- [Google Scholar]
- Summer-autumn distribution and abundance of the hantavirus host, Oligoryzomys longicaudatus, in northwestern Chubut, Argentina. J. Mammal.. 2012;93:1559-1568.
- [Google Scholar]
- Historia de la Patagonia. Buenos Aires: Sudamericana; 2005. p. :445.
- First record of the genus Notiomys Thomas 1890 (Rodentia, Cricetidae) for Chile. Theria. 2016;7(2)
- [Google Scholar]
- Small mammal remains from Cueva Huenul 1 (Late Pleistocene-Holocene), Patagonia, Argentina. Taphonomy and paleoenvironmental significance. Quat. Int.. 2012;278:22-31.
- [Google Scholar]
- PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electronica.
- Reminiscencias de Francisco P. Moreno. Buenos Aires: Eudeba; 1979.
- Holocene stability and recent dramatic changes in micromammalian communities of northwestern Patagonia. Quatern. Int.. 2012;278:22-31.
- [Google Scholar]
- Micromammal diversity loss in central-eastern Patagonia over the last 400 years. J. Arid Environ.. 2012;85:71-75.
- [Google Scholar]
- The climate of Patagonia: general patterns and controls on biotic processes. Ecologia Austral. 1998;8:85-101.
- [Google Scholar]
- Taxonomy and natural history of some fossorial rodents of Patagonia, Southern Argentina. J. Zool.. 1984;202:225-237.
- [Google Scholar]
- Mice and the postglacial history of the Traful Valley of Argentina. J. Mammal.. 1987;68:469-478.
- [Google Scholar]
- The impact of an eruption of Volcan Hudson on small mammals in Argentine Patagonia. Mastozoología Neotropical. 1994;1(2):103-112.
- [Google Scholar]
- Dynamic responses of mammals to the eruption of Volcan Hudson. Mastozoología Neotropical. 1994;1(2):113-122.
- [Google Scholar]
- Micromamíferos depredados por Tyto alba (Aves: Tytonidae) en las Sierras de Talagapa (provincia del Chubut, Argentina) Neotrópica. 2002;48:88-90.
- [Google Scholar]
- Novedosos registros de roedores sigmodontinos (rodentia: muridae) en la Patagonia central Argentina. Mastozoología Neotropical. 2002;9(1):79-84.
- [Google Scholar]
- Micromamíferos (Didelphimorphia y Rodentia) y paleoambientes del Holoceno tardío en la Patagonia noroccidental extra-andina (Argentina) Archaeofauna. 2005;14:183-197.
- [Google Scholar]
- Micromamíferos, cambio climático e impacto antrópico: ¿Cuánto han cambiado las comunidades del sur de América del Sur en los últimos 500 años? Therya. 2014;5:7-38.
- [Google Scholar]







