Translate this page into:
Kinetic investigation of 1,9-dimethyl-methylene blue zinc chloride double salt removal from wastewater using ferrate (VI) and ultraviolet radiation
⁎Corresponding author. atalaie@jami.ac.ir (Amirreza Talaiekhozani),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ferrate (VI) oxidation process and UV radiation can be used for the removal of dye from wastewater. The aims of this study are 1) investigation of optimal conditions for removal of 1,9-dimethyl-methylene blue zinc chloride double salt from wastewater using ferrate (VI) oxidation process and UV radiation and 2) Study on kinetics coefficient of removal of this compound from wastewater by both ferrate (VI) oxidation and UV radiation. For determination of the optimum conditions for removal of the 1,9-dimethyl-methylene blue zinc chloride double salt, response surface methodology was used. The parameters were pH, temperature, hydraulic retention time (HRT) and initial dye concentration. The results showed that the optimum conditions for the removal of dye by ferrate (VI) oxidation process were at pH of 1.4, temperature of 50 °C, HRT in 50 min and initial dye concentration of 70 mg/L. Also, the optimum conditions for dye removal by UV radiation were at pH 13.5, temperature at 45 °C, HRT in 43 min and initial dye concentration of 66 mg/L. In addition, it is confirmed that the removal of dye from wastewater by both ferrate (VI) oxidation process and UV radiation were first order kinetics.
Keywords
Ferrate (VI)
Wastewater treatment
Chemical oxidation
UV radiation
1 Introduction
Dyes are toxic and harmful for human’ body and the removal of dye from wastewater is essential (Soltani et al., 2013). Meanwhile, releasing of wastewater contaminated with dye into the revers or lakes can decrease light penetration (Vinayagam et al., 2017). Then, lower amount of light penetration means lower self-purification capacity of rivers and lakes (Khataee et al., 2015). The majority of dyes are categorized as recalcitrant compounds which are difficult to remove by biological processes (Bahadori et al., 2017; Soltani et al., 2016). 1,9-Dimethyl-methylene blue zinc chloride double salt is an organic dye with empirical formula of C18H22ClN3S·0.5ZnCl2 and molecular weight of 416.05 (Zheng and Levenston, 2015). This dye is widely used in textile industries and easily can be found in textile wastewater.
Nowadays, oxidation processes are considered as a reliable method for treatment of recalcitrant compounds (Tichonovas et al., 2017). Ozone (O3), chlorine (Cl), chlorine dioxide (ClO2) and hydrogen peroxide (H2O2) are well-known oxidants which are usually used for toxic wastewater treatment (Glaze et al., 1987; Lee and Von Gunten, 2010). Chlorine is an inexpensive oxidant which formed as trihalomethanes by-products during wastewater treatment (Postigo et al., 2017). In addition, trihalomethanes have carcinogenic effects and can be considering as a serious environmental pollutants (Lodhi et al., 2017). Therefore, using chlorine as an oxidant can remove a wide range of pollutants from wastewater and generate other dangerous pollutants such as trihalomethanes (Kumari and Gupta, 2017). In the other hand, ozone and chlorine dioxide are very expensive and don’t have any by-products (López-Gálvez et al., 2017; Papageorgiou et al., 2017; Spiliotopoulou et al., 2017).
Hydrogen peroxide is an cheap and unstable compound and cannot be easily produced in situ (Martin et al., 2017). Recent studies introduced new oxidants that can be used in wastewater treatment (Talaiekhozani et al., 2016a; Talaiekhozani et al., 2017a; Zhou et al., 2017). Ferrate (VI) is a multipurpose chemical that can be used as a safe, cheap and effective oxidant which gradually can be converted to Fe (III) that is a well-known coagulant. As ferrate (VI) is able to destruct microorganisms, it can be considered as a disinfectors. In addition, three processes occurred once ferrate (VI) is applied as oxidant for wastewater treatment including oxidation, coagulation and disinfection. Several reports showed that ferrate (VI) is an effective compound for degradation of organic and inorganic compounds (Aslani et al., 2017; Talaiekhozani et al., 2016b). Although, ferrate (VI) produces less sludge with high pollutants removal during wastewater treatment that making the process more economically (Malik et al., 2017).
Photo-degradation is defined as alternative chemical method that using visible and invisible light radiation (Sun et al., 2017). Ultraviolet (UV) is a powerful radiation which can be applied for wastewater treatment (Paredes et al., 2018). Some studies showed that UV radiation can enhance the removal rate of pollutants by oxidants (García and Hodaifa, 2017; Talaiekhozani et al., 2016b; Talaiekhozani et al., 2017b; Talaiekhozani et al., 2016c). Besides, simultaneous usage of hydrogen peroxide and UV radiation have a higher removal rate of pollutants in comparison with UV or hydrogen peroxide solely (Im et al., 2015). High removal rate is due to generation of hydroxide ion (OH−) under UV radiation (Chakma and Moholkar, 2015). UV is able to increase oxidation power of ferrate (VI) (Talaiekhozani et al., 2016a). Iovino et al. (2016) studied the removal of Ibuprofen from synthetic wastewater by changing hydraulic retention time (HRT), pH and UV light intensities on Ibuprofen degradation by UV radiation. They found that increasing of HRT had a positive effect on Ibuprofen removal by UV radiation while lower wastewater heights during UV radiation is more suitable to remove Ibuprofen. (Iovino et al., 2016) revealed that as theincreasing of light intensity from 100 to 400 mJ/m2, decreased Ibuprofen concentration. Capocelli et al. (2014a) studied the removal rate of p-nitrophenol by cavitation as an advanced oxidation process. The first-order kinetic constant values showed the existence of an optimal configuration: k = 1.13 × 10−2 min−1 at 0.45 MPa with a value for the electrical energy per order EEO = 66.7 kWh m−3. In another study, (Capocelli et al., 2014b) evaluated the removal of p-nitrophenol in a lab scale Venturi reactor. They used a numerical simulations model to estimate the optimum configuration. Using the empirical experiments numerical simulations model could be validated and considered as a theoretical tool to identify the best configuration of hydrodynamic cavitation operating parameters (Capocelli et al., 2014a).
Although, the removal of different pollutants from wastewater using ferrate (VI) and UV radiation has been investigated, kinetics of their reactions has not been studied yet. Therefore, the aim of this study are (1) to investigate kinetic of 1,9-dimethyl-methylene blue zinc chloride double salt removal from synthetic contaminated wastewater by UV radiation and ferrate (VI) oxidation and (2) to evaluate the optimum condition of removal of this compound from wastewater using UV radiation and ferrate (VI) oxidation.
2 Materials and methods
2.1 Optimization of pollutants removal
In this study, several symbols have been used which are introduced in Table 1.
Symbol
Definition
k0
Frequency factor of reaction
Ea
Activation energy
T
Temperature
R
Universal gas constant
k2
The reaction rate constant of second order kinetic
k1
Reaction rate constant
CA0
Initial concentration of pollutants
CA
Pollutant concentration
rA
Reaction rate
t
Time
HRT
Hydraulic retention time
pH
Initial pH of the environment
C0
Initial concentration of dye
C
Concentration of dye after treatment
R2
Correlation coefficient square
Fe
Concentration of Ferrate (VI)
Dye0
Initial concentration of dye
The optimum condition was defined as the best dye removal efficiency which can be obtained under specific conditions. In this study, the optimum value of some parameters specifically pH, temperature, HRT and initial dye concentration were investigated. The response surface methodology (RSM) was used to optimize the removal of dye using ferrate (VI) and UV and Mini Tab software was used to design experiments. The input data for the experimental design are shown in Table 2.
Min
Max
Ferrate (VI)
C0 (mg/l)
10.0
70.0
Ferrate (mg/l)
1.0
30.0
pH
1.4
13.5
Temperature (°C)
23.0
50.0
Time (min)
1.0
60.0
UV radiation
C0 (mg/l)
10.0
70.0
pH
1.4
13.5
Temperature (°C)
23.0
50.0
Time (min)
1.0
60.0
2.2 Kinetics investigation
Kinetics investigation of a reaction leads to a better understanding of the reaction and provides the necessary information for the industrial applications. In this study, a first-order kinetic equation (Eq. (1)) and a second-order kinetic equation (Eq. (4)) was used to investigate the kinetics. Kinetic experiments were carried out under the optimized conditions. The reaction rate constant (k) is the most important parameter that should be determined in this study.
where, rA is reaction rate (mg/L.min), CA is concentration of pollutants (mg/L), t is time (min) and k1 is the reaction rate constant (1/min). Eq. (1) can be rewritten as Eq. (2). Eq. (3) is obtained by integrating Eq. (2).
Eq. (3) is similar of y = ax equation. Therefore, k1 coefficient can be calculated by plotting ln(CA/CA0) versus t. Eq. (4) was used to investigate second order kinetic.
where, rA is reaction rate (mg/L.min), CA is the pollutant concentration (mg/L), t is time (min) and k2 is the reaction rate constant of second order kinetic (L/mg.min). Eq. (5) can be obtained after integration of Eq. (4). Then Eq. (5) can be rearranged in the form of Eq. (6).
where, CA0 is initial concentration of pollutants in mg/L, CA is pollutant concentration in time of t in mg/L. k2 can be calculated by plotting of 1/CA versus t.
2.3 Determination of activation energy
The correlation between temperature and reaction rate is different in various temperatures. Arrhenius was a chemist who has suggested an equation to show relation between temperature and reaction rate (Eq. (7)) (Cui et al., 2017).
where, k0 is frequency factor of reaction, Ea is activation energy in kJ/mol, T is temperature in kelvin and R is universal gas constant in kJ/K.mol. Ea are almost independent from temperature (Dyre et al., 2006). Eq. (7) can be rewritten as Eq. (8).
With regression of lnk versus 1/T a liner equation is obtained that –Ea/R is the slope of the equation and lnk0 is its intercept.
2.4 Analytical methods
All the experiments in this section of study were carried out in the laboratory scale. All the chemicals were purchased from Merck Company. The pH was measured using AZ digital pH meter model AZ-86502 made in Taiwan. Dye concentration was measured by Unico spectrophotometer model S 2100 SUV wavelength of 631 nm (made in US). A lamp emitting monochromatic UV light at a fixed wavelength (254 nm) was used to UV radiation in the experiments. UV power was measured by a Digital Light Meter (made in Taiwan). The temperature was measured using digital ZH-08 thermometer (made in China).
2.5 Production of ferrate (VI)
Two rectangular iron with dimension of 60 × 24 mm and thickness of 0.63 mm were employed as anode and cathode electrodes, respectively. The DC voltage used within the range of 1–24 V. An electrolysis system was assembled to produce ferrate (VI) as demonstrated in Fig. 1. In this study, 56 g of sodium hydroxide was dissolved in 100 ml of distilled water to prepare a 14 M solution. Next, the electrolysis container was filled with 100 ml of 14 M sodium hydroxide (Fig. 1). Subsequently, electrodes were charged using DC current with voltage of 9 V and amperage of 1 A for 30 min. Since, ferrate (VI) is converted to ferric (III) as time goes by, the prepared ferrate (VI) solution must be used in the shortest time.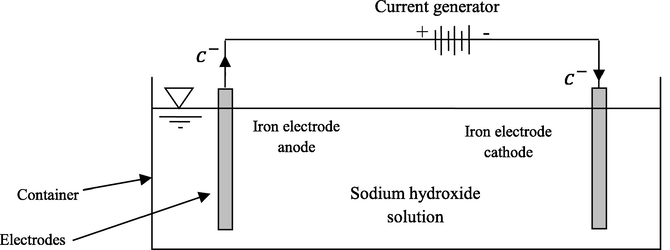
Schematic of the electrochemical cell for ferrate (VI) synthesis.
3 Results and discussion
3.1 Optimization of UV radiation
Initially, the optimum conditions for dye removal using UV radiation were determined (Table 3). The results showed that the dye removal was highest in initial dye concentration of 66 mg/L using UV radiation. Hence, it was very close to the optimal initial concentration of 70 mg/L for dye removal using ferrate (VI) oxidation process.
Removal Method
Pollutants
pH
Time (min)
Temperature (°C)
UV intensity (mW/cm2)
Initial dye concentration
Dye removal in optimum conditions (%)
Ferrate (VI)
Dye
1.4
50
50
–
70.0
95
UV radiation
Dye
13.5
43
45
17
66.5
95
The optimum pH was ranged from 9.8 to 13.5 for the dye removal using UV radiation. The results showed that the rate of pollutants removal increased by increasing in pH using UV radiation which was in contrast with oxidation by ferrate (VI). Then, dyes may disappear due to reaction with hydroxide ion (Du et al., 2013). Therefore, increasing dye removal under alkaline conditions was not only related to UV radiation.
HRT is another important factor that should be considered for designing of wastewater treatment reactors (Talaiekhozani et al., 2016a). The results showed that the optimum HRT was 43 min for dye removal using UV radiation. Malik and Sanyal (2004) reported that dye molecules are attacked by UV photons; therefore longer UV radiation leads to higher rate of wastewater decolonization. As reported by Takahashi (1990), for complete removal of organic compounds from wastewater under optimal conditions using UV, 2 h HRT is required. The increasing UV radiation intensity has a positive effect on the pollutants removal from wastewater (Tan et al., 2013). Therefore, increasing UV radiation intensity may be used instead of increasing HRT in wastewater treatment by UV radiation alone.
Temperature is another effective parameter on the removal of organic compounds by both ferrate (VI) and UV radiation (Talaiekhozani et al., 2016a). The optimum temperature was as high as 45 °C for dye removal. Eq. (9) is a multidimensional regression equation which is obtained by RSM. Minitab software which is a famous and standard software for statistical tests was used to design and analysis of the data. In statistics, RSM explores the relationships between several explanatory variables and one or more response variables. The main idea of RSM is to use a sequence of designed experiments to obtain an optimal response. Asadi and Zilouei (2017) suggest using a second-degree polynomial model to do this. They acknowledge that this model is only an approximation, but they use it because such a model is easy to estimate and apply, even when little is known about the process. RSM can be employed to maximize the production or removal of a special substance by optimization of operational factors. In contrast to conventional methods, the interaction among process variables can be determined by statistical techniques (Asadi and Zilouei, 2017).
where, CA0 is initial concentration of dye (mg/L), CA is concentration of dye after treatment by UV radiation (mg/L), HRT is hydraulic retention time (min), T is temperature (°C) and pH is initial pH of the environment.
Fig. 2(a) shows the relation between HRT and Temperature. It showed that in higher temperature and HRT, high amount of dye was removed using UV radiation. Meanwhile, higher pH and HRT had a better condition for dye removal using UV radiation Fig. 2(b). In addition, in higher HRT and lower dye concentration, the dye removal efficiency increased using UV radiation. In addition, in pHs from 2 to 5, temperature was not an effective parameters for dye removal using UV radiation while in it was effective in pH more than 5 (Fig. 2d). Iovino et al. (2016) found high removal of Ibuprofen in alkaline conditions using UV radiation. As pH rises from 2.25 to 6.6 and 8.25, the Ibuprofen concentration, after an hour of treatment, decreases to 45, 34 and 27% respectively. As reported by Gupta et al. (2011), the pollutants removal was increased in pH 11 in a photocatalytic process under UV radiation. As a result, these studies showed that the pollutants removal from water using UV radiation could be enhanced under alkaline condition.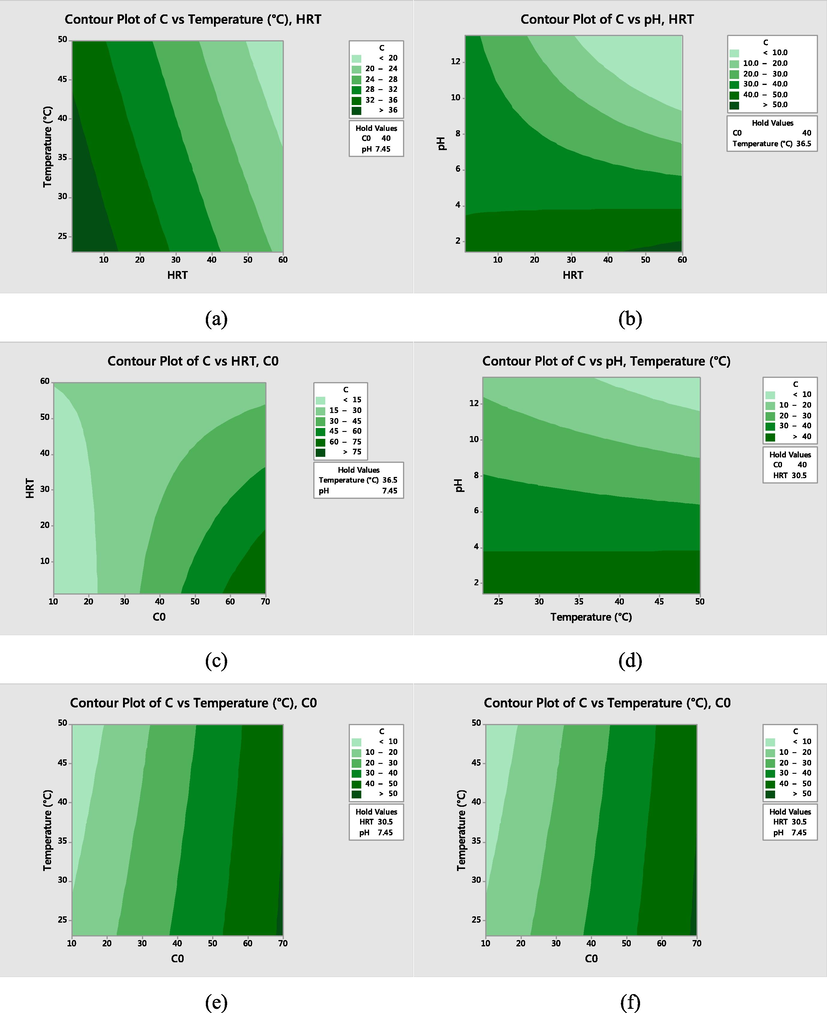
The contour plot of dye removal by using UV radiation.
As shown in Fig. 2(c), in concentration of dye less than 20 mg/L, HRT was not an effective factor using UV radiation. In this condition, dye can be completely removed from wastewater in 10 min. HRT was an effective factor once the initial concentration of dye increased to more than 50 mg/L. The results showed that the most important factor for dye removal using UV radiation was the initial concentration of dye while temperature does not have a significant effect (Fig. 2(e)). Almost all the dye can be removed using UV radiation in pH more than 12 and initial dye concentration up to 20 mg/l (Fig. 2(f)). In this study, the removal of dye from water using UV radiation in optimum condition was 95%.
Several by-products such as organic aldehydes halides, inorganic acids, metals and organic acids can be formed during the removal of dyes by photocatalytic reactions (Yang et al., 1998). The amount of by-products depends on the amount of impurities in water. Some advantages were reported for using UV to remove dye from water such as no sludge is produced and foul odors are greatly reduced (Robinson et al., 2001).
Table 4 illustrates the ANOVA analysis for dye removal using UV radiation. The model P value was lower than 0.0001, which indicates that the model terms were significant. It should be noted that a P value lower than 0.05 shows the significant factors of the model. Initial dye concentration, HRT and pH parameters were the most significant parameters of the proposed correlation based on Eq. (1). The results showed the P value was 0.709 which showed 2-way interaction between CA0 and pH and there was no significant effect on the dye removal using UV radiation. Also, the P value for 2-way interaction between HRT and T, HRT and pH and T and pH were calculated that they do not had significant effects on the dye removal.
Source
DF
Adj SS
Adj MS
F-Value
P-Value
Model
11
5932.15
539.29
10.71
0.000
Blocks
1
69.45
69.45
1.38
0.256
Linear
4
5337.62
1334.40
26.50
0.000
CA0
1
2747.97
2747.97
54.57
0.000
HRT
1
451.79
451.79
8.97
0.008
T
1
46.62
46.62
0.93
0.349
pH
1
2091.23
2091.23
41.53
0.000
2-Way Interaction
6
525.09
87.51
1.74
0.169
CA0 × T
1
280.14
280.14
5.56
0.030
CA0 × T
1
2.29
2.29
0.05
0.834
CA0 × pH
1
7.22
7.22
0.14
0.709
HRT × T
1
0.17
0.17
0.00
0.954
HRT × pH
1
213.53
213.53
4.24
0.054
T × pH
1
21.74
21.74
0.43
0.519
Error
18
906.46
50.36
Lack-of-Fit
14
876.44
62.60
8.34
0.027
Pure Error
4
30.02
7.51
Total
29
6838.60
3.2 Optimization of ferrate (VI) oxidation process
The ferrate (VI) oxidation process was done in laboratory scale and into the Erlenmeyer flasks. The optimum conditions for dye removal from wastewater using ferrate (VI) are shown in Table 3. The results showed that initial concentration of 66 mg/L was the best value for dye removal using ferrate (VI) oxidation. It was close to best value of initial concentration for dye removal using UV radiation. In addition, the optimum pH was 1.4 for dye removal using ferrate (VI). Talaiekhozani et al. (2017a) reported that decreasing of pH has a positive effect on removal of organic compounds by ferrate (VI). Li et al. (2009) reported that ferrate (VI) solution is an unstable compound and very strong oxidant especially in pH below 6. They also found that ferrate (VI) is not an effective oxidant when pH is higher than 9. In another study, Aslani et al. (2017) reported that the pH 3 is the optimum pH for the removal of trichloroacetic using ferrate (VI). It is reported that pH 7 is the best pH for degradation of Benzene and Toluene while pH 9 is a suitable pH for the removal of Ethyl benzene and Xylenes from wastewater using ferrate (VI) (Minetti et al., 2017).
In this study, the optimum concentration of ferrate (VI) for dye removal was 17 mg/L. Aslani et al. (2017) found that the optimum concentration for removal of trichloroacetic was 4.26 mg/L of ferrate (VI). Malik et al. (2017) showed that ferrate (VI) alone is not able to remove considerable amount of COD, dye and toxicity from textile wastewater. Meanwhile, in combination with ferrous sulfate it can remove 40 mg/L of the pollutants in the optimal ferrate (VI). Minetti et al. (2017) also reported that methyl tert-butyl ether could not be easily removed by ferrate (VI); therefore, MTBE was categorized as a recalcitrant compound which is difficult for degradation by ferrate (VI).
The results showed that the optimum HRT was at 50 min for dye removal using ferrate (VI) oxidation. As reported by Aslani et al. (2017), HRT did not have any significant effect on pollutants removal using ferrate (VI). This is due to the quick reaction between ferrate (VI) and dye; therefore HRT does not contribute for removal of dye as the mixing speed is the limiting factor. The results of this study showed that ferrate (VI) oxidation process needs a very low HRT compared by other oxidants. Low HRT means that smaller wastewater treatment plants can be designed with lower construction cost.
Based on the results, the optimum temperature was at 50 °C for dye removal using ferrate (VI) oxidation. Eskandari (2016) and Talaiekhozani et al. (2016a) reported that by increasing temperature to more than 50 °C, the conversion rate of ferrate (VI) to Fe (III) increased while the pollutants removal is decreased. Eq. (10) was developed based on the RSM experiments to predict concentration of dye after ferrate (VI) oxidation process.
where, CA0 is initial concentration of dye (mg/L), CA is concentration of dye after treatment by UV radiation (mg/L), HRT (min), T is temperature (°C), Fe is concentration of Ferrate (VI) (mg/L) and initial pH.
Fig. 3(a) shows interaction between dye concentration and ferrate concentration. As can be seen, initial dye concentration had a great effect on dye removal using ferrate (VI) oxidation process. In higher concentrations of dye, higher amount of ferrate (VI) is needed, therefore ferrate (VI) concentration was considered as a limiting factor. In contrast, in dye removal by UV radiation temperature was an important factor in ferrate (VI) oxidation process (Fig. 3b). The results revealed that in higher temperatures, lower HRT was required for dye removal from wastewater using ferrate (VI) oxidation process. Average of annual temperature can be used to design a ferrate (VI) oxidation process.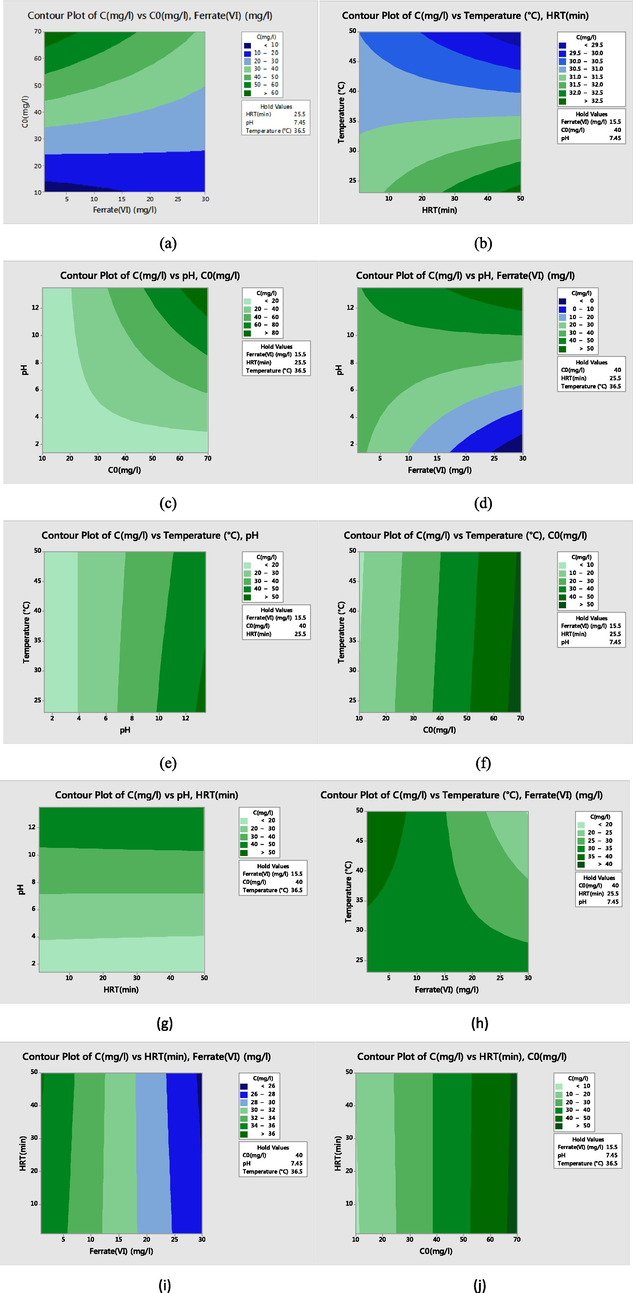
The contour plot of dye removal by using ferrate (VI) oxidation process.
It was reported that for removal of organic pollutants by ferrate (VI) oxidation process no by-products are formed (Talaiekhozani et al., 2016b). Therefore, using ferrate (VI) oxidation process is better than using other common oxidant such as chlorine as they can produce several dangerous by-products.
The ANOVA analysis for dye removal by ferrate (VI) oxidation process is shown in Table 5. The model P value was lower than 0.0001, which indicates that the model terms are important. According to the ANOVA analysis, the proposed correlation is valid (Eq. (10)). CA0, pH, Fe × CA0, Fe × pH and CA0×pH parameters were the most significant terms of the proposed correlation.
Source
DF
Adj SS
Adj MS
F-Value
P-Value
Model
16
7381.90
461.37
33.77
0.000
Blocks
1
90.69
90.69
6.64
0.014
Linear
5
6339.86
1267.97
92.81
0.000
Ferrate (VI)
1
188.08
188.08
13.77
0.001
C0
1
3473.21
3473.21
254.23
0.000
HRT
1
0.00
0.00
0.00
0.992
pH
1
2669.59
2669.59
195.40
0.000
Temperature
1
8.98
8.98
0.66
0.423
2-Way Interaction
10
951.35
95.13
6.96
0.000
Fe × CA0
1
79.44
79.44
5.81
0.021
Fe × HRT
1
0.12
0.12
0.01
0.926
Fe × pH
1
228.23
228.23
16.71
0.000
Fe × T
1
28.20
28.20
2.06
0.159
CA0 × HRT
1
0.36
0.36
0.03
0.872
CA0 × pH
1
610.23
610.23
44.67
0.000
CA0 × T
1
0.02
0.02
0.00
0.971
HRT × pH
1
0.66
0.66
0.05
0.827
HRT × T
1
0.47
0.47
0.03
0.855
pH × T
1
3.62
3.62
0.26
0.610
Error
37
505.49
13.66
Lack-of-Fit
27
497.79
18.44
23.96
0.000
Pure Error
10
7.70
0.77
Total
53 7887.38
3.3 Kinetics
3.3.1 Kinetics of pollutants removal by ferrate (VI)
Fig. 4 shows the variations of ln(CA/CA0) versus t and also, variation of 1/CA versus t to calculate kinetic coefficients (k1 and k2) when ferrate (VI) oxidation process was used. In Fig. 4, CA0 means initial concentration of dye in each experiment which was calculated at different temperatures. Also, first and second order kinetic coefficients of removal of dye by ferrate (VI) are shown in Table 6. The results showed that kinetic coefficients for both first and second order reactions increased with increasing in temperature (Fig. 4a and b). It shows that dye removal depends on the temperature as the higher amount of dye removed in the higher temperatures.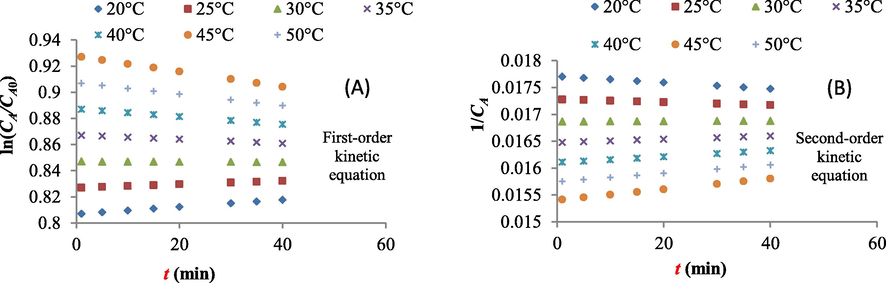
(A) variations of ln(CA/CA0) versus t and (B) variation of 1/CA versus t when ferrate (VI) oxidation process was used (t was varied from 1 to 40 min).
No.
Temperature (°C)
Second order kinetics
First order kinetics
K2 (mg/L.min)
R2
K1 (1/min)
R2
1
20
0.000006
1
0.0004
1
2
25
0.000003
1
0.0002
1
3
30
0.0000003
1
0.00002
1
4
35
0.000003
1
0.0002
1
5
40
0.000005
1
0.0004
1
7
45
0.000008
1
0.0007
1
7
50
0.00001
0.99
0.0005
1
3.3.2 Kinetics of pollutants removal by UV radiation
Kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Kinetics includes investigations of how different effective parameters such as temperature can influence the speed of a chemical reaction. In this section of study, kinetics of pollutants removal by UV radiation were investigated. The variations of 1/CA versus of t for different temperatures using UV radiation are shown in Fig. 5. The slope of regression equation was the kinetic coefficient (k2). Also, kinetics reaction coefficients of dye removal by UV radiation are shown in Table 7. Results showed that k2 is equal of 0.00005 L.mg/min for all tested temperatures. Also, k1 is between 0.0033 and 0.0035 L/min.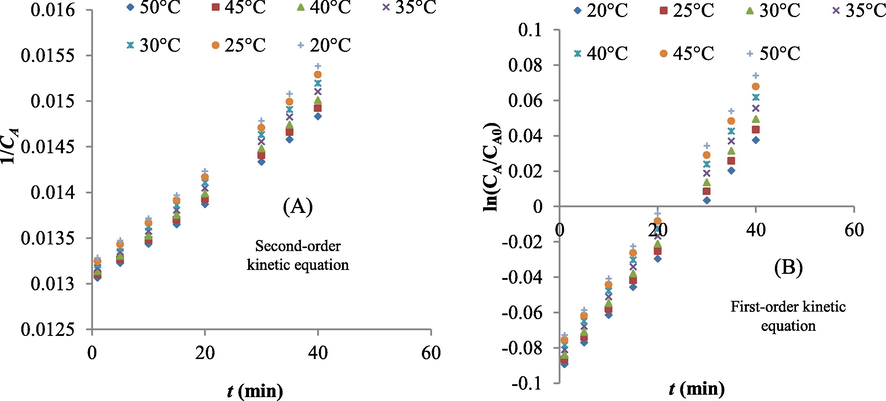
(A) Variations of 1/CA versus of t and (B) variations of ln(CA/CA0) versus t when UV radiation was used (t was vitiated from 1 to 40 min).
No.
Temperature (°C)
Second order kinetics
First order kinetics
k2 (L/mg.min)
R2
k1 (L/min)
R2
1
20
0.00005
0.99
0.0033
0.99
2
25
0.00005
0.99
0.0033
0.99
3
30
0.00005
0.99
0.0034
0.99
4
35
0.00005
0.99
0.0035
0.99
5
40
0.00005
0.99
0.0036
0.99
7
45
0.00005
0.99
0.0037
0.99
7
50
0.00005
0.99
0.0038
0.99
3.4 Activation energy
Activation energy is defined as the energy that must be accessible in a chemical process to result in a chemical reaction. Activation energy can also be defined as the minimum energy required to start a chemical reaction. The rate controlling step can be partly characterized by the observed activation energy for a process, and studying the effect of temperature on rate yields information relating to optimum operations conditions and also permits evaluation of the activation energy, thus leading to a means for determining the nature of the rate limiting reactions (Fell et al., 2017).
Lin and Leu (1999) reported that the activation energy for removal of anionic alkylbenzene sulfonate and linear alkylbenzene sulfonate by Fenton process is 10.620 and 8.081 kJ/mol, respectively. Mckay et al. (1980) found activation energy of 13.2 + 0.6 kJ/kg for removal of dye from wastewater. Calculated activation energy for both ferrate (VI) oxidation process and UV radiation are shown in Table 8. Also, (Gleston et al., 1941) reported that the activation energy of the dye removal by adsorbents should be within the range of values of 8–22 kJ/kg.
Removal Method
Reaction order
Activation energy (kJ/mol)
k0
R2
Ferrate (VI) Oxidation process
First order
33.400
0.008
1.00
Second order
34.089
2.13
1.00
UV radiation
First order
3.974
60.2
0.97
Second order
0.082
1
0.99
4 Conclusion
Both UV radiation and ferrate (VI) oxidation process were able to remove 1,9-dimethyl-methylene blue zinc chloride double salt. The optimum conditions for the removal of dye using ferrate (VI) oxidation process were pH of 1.4, temperature of 50 °C, HRT at 50 min and initial dye concentration of 70 mg/L. Also, the optimum conditions for the dye removal using UV radiation were pH of 13.5, temperature of 45 °C, HRT at 43 min and initial dye concentration of 66 mg/L. It was found that removal of dye from wastewater by both ferrate (VI) oxidation process and UV radiation followed first order kinetics. In addition, the activation energy for the dye removal by ferrate (VI) oxidation process in first order kinetics was 33.4 kJ/mol. As the mechanism of dye removal by ferrate (VI) is not clear; therefore, further studies are needed to understand exact mechanisms of dye removal using UV radiation. Since several studies reported that ferrate (VI) is a cheap and powerful oxidant, it can be a good option for the removal of recalcitrant compounds from wastewater.
References
- Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol.. 2017;227:335-344.
- [Google Scholar]
- Haloacetic acids degradation by an efficient Ferrate/UV process: byproduct analysis, kinetic study, and application of response surface methodology for modeling and optimization. J. Environ. Manage.. 2017;203:218-228.
- [Google Scholar]
- Photo-activated degradation of tartrazine by H2 O2 as catalyzed by both bare and Fe-doped methyl-imogolite nanotubes. Catal. Today. 2017;34:199-207.
- [Google Scholar]
- Hydrodynamic cavitation of p-nitrophenol: a theoretical and experimental insight. Chem. Eng. J.. 2014;254:1-8.
- [Google Scholar]
- Chemical effect of hydrodynamic cavitation: Simulation and experimental comparison. AIChE. J.. 2014;60(7):2566-2572.
- [Google Scholar]
- Investigation in mechanistic issues of sonocatalysis and sonophotocatalysis using pure and doped photocatalysts. Ultrason. Sonochem.. 2015;22:287-299.
- [Google Scholar]
- Kinetic analysis of the temperature effect on polyhydroxyalkanoate production by haloferax mediterranei in synthetic molasses wastewater. J. Polyme. Environ.. 2017;25(2):277-285.
- [Google Scholar]
- Kinetics of the reaction of crystal violet with hydroxide ion in the critical solution of 2-butoxyethanol+ water. J. Physical. Chem. A. 2013;117(2):283-290.
- [Google Scholar]
- Elastic models for the non-Arrhenius viscosity of glass-forming liquids. J. Non-Cryst. Solid. 2006;352(42):4635-4642.
- [Google Scholar]
- Control of Hydrogen Sulfide and Organic Compounds in Municipal Wastewater by Using Ferrate (VI) Produced by Electrochemical Method. (M.Sc). Isfahan, Iran: Jami Institute of Technology; 2016.
- Origins of the unfavorable activation and reaction energies of 1-azadiene heterocycles compared to 2-azadiene heterocycles in Diels-Alder reactions. J. Organic. Chem.. 2017;82(4):1912-1919.
- [Google Scholar]
- Real olive oil mill wastewater treatment by photo-Fenton system using artificial ultraviolet light lamps. J. Clean. Prod.. 2017;162:743-753.
- [Google Scholar]
- The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone. Sci. Eng. J.. 1987;9(4):335-352.
- [Google Scholar]
- The Theory of Absolute Rate Processes. New York: McGraw-Hill Book Company; 1941.
- Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng: C.. 2011;31(5):1062-1067.
- [Google Scholar]
- Sonocatalytic-TiO2 nanotube, Fenton, and CCl 4 reactions for enhanced oxidation, and their applications to acetaminophen and naproxen degradation. Separat. Purif. Technol.. 2015;141:1-9.
- [Google Scholar]
- Ibuprofen photodegradation in aqueous solutions. Environ. Sci. Pollu. Res.. 2016;23(22):22993-23004.
- [Google Scholar]
- Sonocatalytic removal of an organic dye using TiO 2/montmorillonite nanocomposite. Ultrason. Sonochem.. 2015;22:404-411.
- [Google Scholar]
- Multi-pathway risk assessment of trihalomethanes exposure in drinking water supplies. In: Trends in Asian Water Environmental Science and Technology. Springer; 2017. p. :223-235.
- [Google Scholar]
- Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical) Water. Res.. 2010;44(2):555-566.
- [Google Scholar]
- Decolorization of azo dye Orange II by ferrate (VI)–hypochlorite liquid mixture, potassium ferrate (VI) and potassium permanganate. Desalination. 2009;249:936-941.
- [Google Scholar]
- Operating characteristics and kinetic studies of surfactant wastewater treatment by Fenton oxidation. Water. Res.. 1999;33(7):1735-1741.
- [Google Scholar]
- Effect of trihalomethanes (chloroform and bromoform) on human haematological count. J. Water. Health.. 2017;15(3):367-373.
- [Google Scholar]
- Modelling of E. coli inactivation by chlorine dioxide in irrigation water. Agricult. Water. Manage.. 2017;192:98-102.
- [Google Scholar]
- Kinetics of decolourisation of azo dyes in wastewater by UV/H2 O2 process. Sep. Purif. Technol.. 2004;36(3):167-175.
- [Google Scholar]
- Comparison of coagulation, ozone and ferrate treatment processes for color, COD and toxicity removal from complex textile wastewater. Water. Sci. Technol.. 2017;76(5–6):1001-1010.
- [Google Scholar]
- Toolbox study for application of hydrogen peroxide as a versatile, safe and industrially-relevant green oxidant in continuous flow mode. Green. Chem.. 2017;19(6):1439-1448.
- [Google Scholar]
- The removal of colour from effluent using various adsorbents—III. Silica: rate processes. Water. Res.. 1980;14(1):15-20.
- [Google Scholar]
- In situ chemical oxidation of BTEX and MTBE by ferrate: pH dependence and stability. J. Hazard. Mater.. 2017;324:448-456.
- [Google Scholar]
- Effects of ozonation pretreatment on natural organic matter and wastewater derived organic matter–Possible implications on the formation of ozonation by-products. Chemosph.. 2017;170:33-40.
- [Google Scholar]
- Formation of iodo-trihalomethanes, iodo-haloacetic acids, and haloacetaldehydes during chlorination and chloramination of iodine containing waters in laboratory controlled reactions. J. Environ. Sci.. 2017;58:127-134.
- [Google Scholar]
- Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresourc. Technol.. 2001;77(3):247-255.
- [Google Scholar]
- Ultrasonically induced ZnO–biosilica nanocomposite for degradation of a textile dye in aqueous phase. Ultrason. Sonochem.. 2016;28:69-78.
- [Google Scholar]
- Preparation of bio-silica/chitosan nanocomposite for adsorption of a textile dye in aqueous solutions. Int. Biodeter. Biodegrad.. 2013;85:383-391.
- [Google Scholar]
- Use of fluorescence spectroscopy to control ozone dosage in recirculating aquaculture systems. Water. Res.. 2017;111:357-365.
- [Google Scholar]
- High photodegradation ability of dyes by Fe (III)-tartrate/TiO2 nanotubular photocatalyst supported via photo-Fenton reaction. J. Photochem. Photobiol. A: Chem.. 2017;334:20-25.
- [Google Scholar]
- An overview on production and applications of ferrate (VI) Jundishapur J. Health. Sci.. 2016;8(3)
- [Google Scholar]
- Removal of H2S and COD Using UV, Ferrate and UV/Ferrate from Municipal Wastewater. J. Hum. Environ. Health Promot. 2016;2(1):1-8.
- [Google Scholar]
- Formaldehyde removal from wastewater and air by using UV, ferrate (VI) and UV/ferrate (VI) J. Environ. Manage.. 2016;184:204-209.
- [Google Scholar]
- An Overview on Production and Application of Ferrate (VI) for Chemical Oxidation, Coagulation and Disinfection of Water and Wastewater. J. Environ. Chem. Eng.. 2017;5(2):1828-1842.
- [Google Scholar]
- Hydrogen sulfide and organic compounds removal in municipal wastewater using ferrate (VI) and ultraviolet radiation. Environ. Health. Eng. Manage. J.. 2017;4(1):7-14.
- [Google Scholar]
- Degradation of antipyrine by UV, UV/H2 O2 and UV/PS. J. Hazard. Materi.. 2013;260:1008-1016.
- [Google Scholar]
- Ozone-UV-catalysis based advanced oxidation process for wastewater treatment. Environ. Sci. Pollut. Res.. 2017;24(21):17584-17597.
- [Google Scholar]
- Photocatalytic degradation of orange G dye using ZnO/biomass activated carbon nanocomposite. J. Environ. Chem. Eng. 2017
- [CrossRef] [Google Scholar]
- What happens with organic micropollutants during UV disinfection in WWTPs? A global perspective from laboratory to full-scale. J. Hazard. Materi.. 2018;342:670-678.
- [Google Scholar]
- Decolorization of Dyes Using UV/H2O2 Photochemical Oxidation. Textile. Chemist. Colorist.. 1998;30(4):27-35.
- [Google Scholar]
- Fact versus artifact: avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs. European. Cells. Mater.. 2015;29:224.
- [Google Scholar]
- Trials of Treating Decentralized Domestic Sewage from a Residential Area by Potassium Ferrate (VI) Water. Air. Soil. Pollut.. 2017;228(8):316.
- [Google Scholar]







