Translate this page into:
Isolation, screening, and molecular characterization of indigenous Trichoderma isolates from West Java, Indonesia and their plant growth-promoting capability on rice plants (Oryza sativa L.)
⁎Corresponding author. febri@unpad.ac.id (Febri Doni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Trichoderma species are widely acknowledged as growth-promoting fungi that have been utilized to enhance the growth and yield of numerous crops. This research examined the capacity of 30 Trichoderma strains, isolated from two organic rice fields in West Java, Indonesia, to enhance the germination, growth, and physiological characteristics of rice plants (Oryza sativa L.). In general, Trichoderma strain TM10 demonstrated the greatest ability to increase seed germination (97.25 %), vigor index (3122.83), and germination speed (59.91 seeds/day). This strain also increased seedling root length and seedling height by 101.62 % and 112.20 %, respectively. Plants treated with TM10 exhibited a notable improvement in root length, plant height, fresh weight, and dry weight compared to control plants, demonstrating increases of 188.68 %, 69.90 %, 157.41 %, and 159.38 %, respectively. Furthermore, the total chlorophyll content and stomatal number in TM10-treated plants exhibited increments of 75.23 % and 75.53 %, respectively. Five selected isolates (TM7, TM10, SB2, SB8, and SB14) were evaluated for their potential to produce plant growth-promoting compounds, including phosphatase enzyme (ranging from 0.54 to 11.14 µg pNP g−1h−1), indole-3-acetic acid (IAA) (ranging from 28.96 to 63.91 µg/mL), ammonia (ranging from 1.96 to 5.79 µg/mL), and hydrogen cyanide (HCN) (ranging from 221.76 to 274.82 ppm). The best strain, namely TM10, was then molecularly identified as Trichoderma yunnanense. This investigation demonstrates that Trichoderma spp. isolated from organic rice fields could be used as a bioinoculant in sustainable rice production.
Keywords
Trichoderma
Plant growth promoting fungi
Rice
Symbiosis
Bioinoculant
Plant-microbe interactions
- PGPM
-
plant growth-promoting microorganisms
- PGP
-
plant growth promoting
- IAA
-
indole-3-acetic acid
- HCN
-
hydrogen cyanide
- PDA
-
potato dextrose agar
- pNPP
-
p-nitrophenyl phosphate
- PDB
-
potato dextrose broth
- ITS
-
transcribed spacer region
- BLAST
-
basic local alignment search tool
- NCBI
-
National Center for Biotechnology Information
- MEGA
-
molecular evolutionary genetics analysis
- DNA
-
deoxyribonucleic acid
- ACC
-
1-aminocyclopropane-1-carboxylate
Abbreviations
1 Introduction
Rice serves as a crucial staple food for the people of Indonesia, playing a central role in their daily diet and cultural traditions (Panuju et al., 2013). The demand for rice has surged in parallel with the escalating rates of economic and population growth in the country (Rozaki, 2021). From 2008 to 2020, there was a notable increase in rice demand, totaling 4.66 million tons, reflecting an average annual growth rate of 1.16 % (FAO, 2021). Rice production in Indonesia experienced fluctuations from 2018 to 2022, with corresponding figures of 59.20 million tons, 54.60 million tons, 54.64 million tons, 54.42 million tons, and 55.7 million tons (Statistics Indonesia, 2022). The challenge arises from domestic consumption outpacing production, leading the government to resort to rice imports to ensure ample supplies and prevent a spike in rice prices (Rifin, 2022). Accordingly, there is an immediate need to increase rice production.
Previously, the Indonesian government endeavored to uphold and enhance local rice output by employing methods such as reducing crop losses, improving irrigation systems, intensifying farming practices, maximizing land usage, and expanding cultivation areas (Rachman et al., 2022). However, efforts to increase rice production cause environmental pollution and harm due to the overutilization of chemical fertilizers and synthetic biocides (Huang et al., 2022). The excessive use of agrochemical products has a notable impact on diminishing soil fertility, elevating rice yield losses attributable to pests and diseases, and diminishing beneficial soil microbial communities essential for soil biochemical processes (Meena et al., 2020). Consequently, the application of sustainable agricultural techniques is required to improve soil biological processes, reduce reliance on external inputs, and improve the structure and fertility of the soil (Abd-Alhamid et al., 2015).
Microorganisms perform an important function in environmental management and sustainable agriculture, and utilizing them in agroecosystems has achieved excellent results (Kaviyarasu et al., 2016; Magdalane et al., 2017; Valsalam et al., 2019). Utilizing microorganisms has several beneficial effects, including enhancing plant growth and productivity, enhancing plant resilience, and effectively managing diseases and pests (van Jaarsveld et al., 2021). Plant growth-promoting microorganisms (PGPM), including fungi, bacteria, cyanobacteria, and mycorrhiza, serve as environmentally friendly alternatives (Primavesi et al., 2023).
One of the PGPM members that can be used to promote the growth of rice plants is Trichoderma (Pang et al., 2017). Trichoderma species produce a variety of bioactive chemical compounds which enhance plant growth (phytohormones), control pathogens (e.g., cellulases, peptaibols, and hydrogen cyanide), and improve soil health (e.g., phosphate-solubilizing enzymes and ammonia) (Sharma et al., 2019). Trichoderma species are beneficial soil microorganisms and are widely studied as accessible and sustainable solutions for improving plant growth and development (Rawal et al., 2022). Trichoderma enhances plant growth through various mechanisms, encompassing both biochemical and molecular modulation of plants (Gajera et al., 2016). Studies have shown that the utilization of Trichoderma has the potential to enhance the growth and physiological characteristics of rice plants (Khadka and Uphoff, 2019).
The isolation of indigenous Trichoderma strains holds significant importance in agricultural practices, particularly in relation to the production of rice in an environmentally friendly way (Tegene et al., 2021). Trichoderma can enhance crop productivity and quality, reduce dependence on synthetic pesticides, and improve soil health (Abdullah et al., 2021; Akbari et al., 2024). It functions as a biofertilizer, biopesticide, and bioremediation agent, inhibiting harmful microorganisms and promoting beneficial ones (Sharma et al., 2019). In recent years, there has been a growing and intensive search for Trichoderma species with the potential to serve as bioinoculants in rice production (Abdullah et al., 2021). Therefore, this research aims to isolate the Trichoderma species from organic rice fields in West Java, Indonesia, and to elucidate the potential of Trichoderma strains in improving the growth and physiological characteristics of rice plants.
2 Materials and methods
2.1 Trichoderma spp. isolation
Trichoderma spp. were isolated from soil samples obtained from two organic rice fields in West Java, Indonesia: Nusantara Organic SRI Center in Sukabumi and SRI Mukti Sadaya Organic Rice Farm in Tasikmalaya. Purified isolates were transferred to fresh potato dextrose agar (PDA) medium. Morphological identification of the isolates was conducted through macroscopic and microscopic observations. Detailed procedures are provided in Supplementary Table S1.
2.2 Evaluating the effect of Trichoderma on rice seedling growth
The growth-promoting effect of 30 isolated Trichoderma strains was evaluated on IR64 rice variety seedlings in gnotobiotic conditions, tracking growth progress over 14 days, using a completely randomized design with 31 treatments (30 Trichoderma isolates and a control), and 10 replications were performed in a greenhouse at the Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran. Seeds were surface-sterilized and inoculated with Trichoderma spore suspension (107 spores/mL) for 24 h. Seeds were then assigned to each experimental condition—treatment groups (Tasikmalaya: TM and Sukabumi: SB, inoculated with Trichoderma) and the control group (C, not inoculated). Seedlings were grown in sterilized growth medium in a greenhouse with controlled conditions for 14 days. Root and shoot lengths, as well as fresh and dry weights, were measured. Furthermore, germination percentage, vigor index, and germination speed were then calculated. Detailed procedures are provided in Supplementary Table S1.
2.3 Evaluating the effect of Trichoderma on the growth and physiological characteristics of rice plants
After the initial 14-day germination period, seedlings were inoculated with Trichoderma spore suspension and transplanted into plastic pots under controlled greenhouse conditions for 60 days. This experiment utilized a completely randomized design with 31 treatments, consisting of 30 different isolates of Trichoderma and a control group without Trichoderma inoculation. The experiment was conducted with four replications. The planting medium composition was the same as in the previous experiment. The 2 kg of soil was put into a plastic pot with a diameter of 20 cm. Plant maintenance involved ensuring a water level 2 cm above the soil and aerating the soil surface physically every 10 days. Growth parameters such as plant height, root length, number of leaves, fresh weight, and dry weight were measured. Physiological characteristics included measuring chlorophyll content and stomatal density. Procedures are detailed in Supplementary Table S1.
2.4 Plant growth promoting traits and molecular identification of Trichoderma
The five best-performing Trichoderma isolates in enhancing rice germination, rice growth, and rice physiological traits will be tested for their plant growth promoting (PGP) abilities. The PGP observed is the activity of phosphatase and the production of indole-3-acetic acid (IAA), ammonia, and hydrogen cyanide (HCN). The molecular identification of two highest-performing isolates involved genomic DNA extraction, ITS region amplification, sequencing, and phylogenetic analysis using MEGA software. Detailed procedures are provided in Supplementary Table S1.
2.5 Statistical analysis
A statistical analysis of all the data was conducted using one-way analysis of variance (ANOVA). For significantly different parameters, mean separation was performed using Duncan’s Multiple Range Test (DMRT) at a significance level of p ≤ 0.05.
3 Results
3.1 Isolation and morphological identification of Trichoderma
Trichoderma spp. were successfully isolated from the soil of organic rice fields in Tasikmalaya (TM) and Sukabumi (SB) with 15 isolates, respectively. The isolated Trichoderma strains exhibited similar macroscopic and microscopic characteristics (Supplementary Table S2). Fig. 1 shows the morphological appearance of TM10 and SB8 isolates.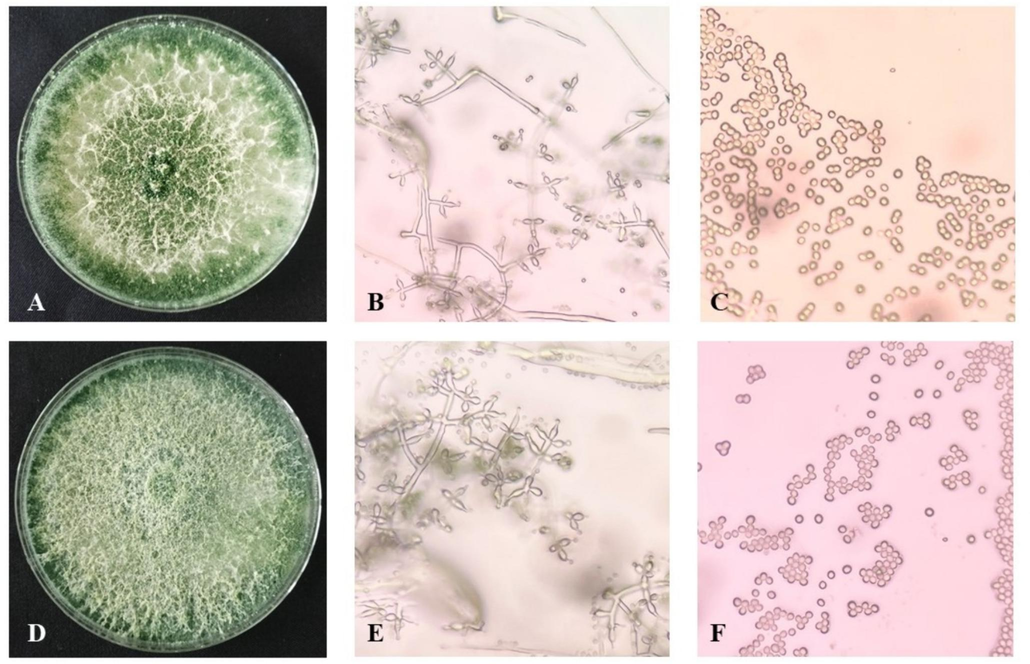
Colony of TM10 on PDA after 7 days (A), phialides of TM10 (B), conidia of TM10 (C), colony of SB8 on PDA after 7 days (D), phialides of SB8 (E), conidia of SB8 (F).
3.2 Rice seed germination and vigor
The germination capacity of rice seeds varied, with the lowest percentage shown by the TM7 treatment (80.25 %) and the highest percentage shown by the TM10 (97.25 %). TM10 treatment increased seed vigor significantly, as indicated by a high seed vigor index (3122.83). Likewise, in the germination speed parameter, the highest average number of seeds capable of germinating in a day was shown by the TM10 treatment (59.91 seeds/day). The findings summarized in Table 1 demonstrate that Trichoderma inoculation improved seed germination and seedling early growth. Note: Means with the same letter in the same column are not significantly different according to DMRT (p ≤ 0.05).
Treatments
Germination (%)
Vigor index
Speed of germination (seeds/day)
Root length (cm)
Shoot length (cm)
Seedling fresh weight (g)
Seedling dry weight (g)
C
87.25 hij
1342.64 k
50.58 bcdefgh
6.16 g
9.26 h
0.09 d
0.01 c
TM1
92.25 cdef
2274.76 efghi
56.36 abc
8.02 bcdefg
16.66 cdef
0.13 abc
0.01 c
TM2
89.25 fghi
2410.22 defgh
47.81 cdefgh
8.81 bcdefg
18.21 abcde
0.13 abc
0.02 ab
TM3
89.25 fghi
2185.43 fghi
47.29 defgh
7.95 bcdefg
16.53 defg
0.12 abc
0.01 abc
TM4
90.25 efgh
2469.65 cdefg
48.47 cdefgh
9.02 bcdefg
18.34 abcde
0.14 abc
0.02 a
TM5
87.25 hij
2256.57 efghi
49.42 bcdefgh
8.47bcdefg
17.40 abcde
0.13 abc
0.02 abc
TM6
86.25 ijk
2085.11 hi
46.22 efgh
7.68 bcdefg
16.49 defg
0.12 abc
0.01 c
TM7
80.25 jk
2278.30 efghi
45.09 fgh
9.79 bc
18.63 abcd
0.15 ab
0.02 a
TM8
87.25 hij
2137.77 ghi
47.56 defgh
7.98 bcdefg
16.54 defg
0.12 abc
0.01 bc
TM9
89.25 fghi
2264.50 efghi
50.65 bcdefgh
8.20 bcdefg
17.19 bcde
0.13 abc
0.01 abc
TM10
97.25 a
3122.83 a
59.91 a
12.42 a
19.65 a
0.15 ab
0.02 a
TM11
85.25 jk
2294.11 defghi
49.61 bcdefgh
8.74 bcdefg
18.19 abcde
0.13 abc
0.02 ab
TM12
87.25 hij
2399.04 defgh
51.28 bcdefgh
9.16 bcdef
18.37 abcde
0.14 abc
0.02 ab
TM13
85.25 jk
2136.16 ghi
44.20 gh
8.11 bcdefg
16.92 bcde
0.13 abc
0.01 bc
TM14
87.25 hij
2331.16 defgh
49.86 bcdefgh
8.62 bcdefg
18.12 abcde
0.13 abc
0.02 ab
TM15
83.25 jk
1739.78 j
43.93 gh
6.52 fg
14.42 g
0.11 cd
0.01 c
SB1
88.25 ghij
2479.72 cdef
48.64 cdefgh
9.60 bcd
18.50 abcd
0.15 abc
0.02 a
SB2
94.25 abcd
2754.72 bc
53.72 abcde
10.28 abc
18.95 abc
0.15 a
0.02 a
SB3
89.25 fghi
2361.68 defgh
48.65 cdefgh
8.52 bcdefg
17.92 abcde
0.13 abc
0.02 ab
SB4
83.25 kl
2076.54 hi
43.17 h
6.73 defg
18.21 abcde
0.12 bcd
0.01 bc
SB5
94.25 abcd
1992.84 ij
54.72 abcde
6.67 efg
14.50 fg
0.12 cd
0.01 bc
SB6
96.25 ab
2532.35 cde
60.30 a
8.50 bcdefg
17.81 abcde
0.13 abc
0.02 ab
SB7
91.25 defg
2473.09 cdefg
52.88 abcdf
8.89 bcdefg
18.24 abcde
0.13 abc
0.02 abc
SB8
96.25 ab
2856.82 ab
57.39 ab
10.60 ab
19.09 ab
0.15 a
0.02 a
SB9
89.25 fghi
2498.89 cdef
51.16 bcdefgh
9.58 bcde
18.39 abcd
0.14 abc
0.02 a
SB10
88.25 ghij
2264.13 efghi
46.24 efgh
8.28 bcdefg
17.36 abcde
0.13 abc
0.02 abc
SB11
93.25 bcde
2565.58 bcde
50.42 bcdefgh
9.12 bcdef
18.35 abcde
0.14 abc
0.02 ab
SB12
93.25 bcde
2189.73 fghi
52.87 abcdf
7.53 cdefg
15.98 efg
0.12 abc
0.01 bc
SB13
94.25 abcd
2615.68 bcd
52.48 abcdfg
9.41 bcdef
18.38 abcd
0.14 abc
0.02 a
SB14
95.25 abc
2752.40 bc
55.26 abcd
10.19 abc
18.70 abcd
0.15 a
0.02 a
SB15
90.25 efgh
2277.26 efghi
50.97 bcdefgh
8.19 bcdefg
17.03 bcde
0.13 abc
0.01 bc
3.3 Rice seedling growth
The Trichoderma treatment resulted in significant improvements in root length, seedling height, and the fresh and dry weight of seedlings, as shown in Table 1. The TM10 treatment yielded the greatest results in root length, measuring 12.42 cm, which represents a 101.62 % increase. Seedling height increased significantly, up to 112.20 %, with the TM10 treatment. In addition, TM7, TM10, SB2, SB8, and SB14 treatments increased fresh weight and dry weight significantly compared to the control.
3.4 Rice plant growth
The findings presented in Table 2 indicate that the use of Trichoderma led to improved root length, plant height, fresh weight, and dry weight in comparison to the uninoculated plants. Meanwhile, the Trichoderma treatment had no noticeable effect on the observed leaf number. Note: Means with the same letter in the same column are not significantly different according to DMRT (p ≤ 0.05).
Treatments
Root length (cm)
Plant height (cm)
Total fresh weight (g)
Total dry weight (g)
Leaf number (per plants)
C
4.24 o
35.05 n
2.16 q
0.32 q
5
TM1
7.41 ijkl
40.95 ij
3.26 jkl
0.49 jkl
5
TM2
8.74 defghi
45.55 efg
3.66 ghijk
0.55 ghijk
5
TM3
6.65 klm
40.05 jk
3.06 lmn
0.46 lmn
4.33
TM4
8.94 defgh
46.05 ef
3.86 fghi
0.58 fghi
5
TM5
8.19 fghij
42.05 i
3.38 ijkl
0.51 jkl
4.33
TM6
6.65 klm
39.15 kl
2.76 mno
0.41 mno
5
TM7
9.74 cde
49.05 cd
4.56 bcd
0.68 bcd
5
TM8
6.9 1jklm
40.55 ijk
3.16 klm
0.47 klm
5
TM9
7.74 ghijk
41.25 ij
3.36 jkl
0.50 jkl
5
TM10
12.24 a
59.55 a
5.56 a
0.83 a
5
TM11
8.61 efghi
45.05 fg
3.59 ghijk
0.54 ghijk
5
TM12
9.04defgh
46.55 ef
4.06 efg
0.61 efg
5
TM13
7.41 ijkl
41.175 ij
3.33 jkl
0.5 jkl
4.33
TM14
8.41 efghi
44.15 gh
3.56 hijk
0.53 hijkl
5
TM15
4.24 o
35.55 n
2.26 pq
0.34 pq
5
SB1
9.65 cdef
48.55 d
4.46 cde
0.67 cde
5
SB2
10.65 bc
52.05 b
4.76 bc
0.71 bc
5
SB3
8.26 efghij
43.55 h
3.46 ijkl
0.52 ijkl
5
SB4
5.61 mn
37.55 m
2.56 opq
0.38 opq
5
SB5
5.15 no
36.05 n
2.46 opq
0.37 opq
4.33
SB6
8.25 efghij
42.05 i
3.43 ijkl
0.51 ijkl
7
SB7
8.81 defghi
45.55 efg
3.76 fghij
0.56 fghij
4.33
SB8
11.24 ab
52.55 b
4.96 b
0.74 b
5
SB9
9.24 defg
47.05 e
4.16 def
0.62 def
5
SB10
8.11 ghij
41.55 ij
3.36 jkl
0.50 jkl
5
SB11
8.94 defgh
46.05 ef
3.96 fgh
0.59 fgh
5
SB12
6.24 lmn
38.55 lm
2.66 nop
0.40 nop
5
SB13
9.15 defgh
47.00 e
4.06 efg
0.61 efg
5
SB14
10.15 bcd
50.05 c
4.66 bc
0.70 bc
4.33
SB15
7.65 hijkl
41.25 ij
3.35 jkl
0.50 jkl
5
3.5 Chlorophyll content
The chlorophyll content exhibited a notable increase in the Trichoderma-treated plants, as shown in Table 3. The highest chlorophyll a content was shown in the TM10 treatment (2.22 mg/g), with an increase of 77.60 % when compared to the control. The highest chlorophyll b content was shown in the TM10 treatment (1.67 mg/g), followed by SB8 (1.49 mg/g), with an increase of 42.73 % and 27.35 %. Note: Means with the same letter in the same column are not significantly different according to DMRT (p ≤ 0.05).
Treatments
Chlorophyll a
(mg/g)
Chlorophyll b (mg/g)
Total chlorophyll
(mg/g)
Stomata/mm2
C
1.25 e
1.17 g
2.41 h
213.84 r
TM1
1.71 abcde
1.30 bcdefg
3.01 bcdefg
251.57 klmno
TM2
1.86 abcd
1.34 bcdefg
3.20 bcdef
276.73 efghi
TM3
1.59 bcde
1.28 cdefg
2.87 defgh
246.86 mnop
TM4
1.92 abc
1.35 bcdefg
3.27 bcde
289.31 defg
TM5
1.81 abcd
1.31 bcdefg
3.12 bcdef
264.15 hijklm
TM6
1.54 cde
1.27 defg
2.80 efgh
245.28 mnop
TM7
2.03 abc
1.44 bcd
3.46 abc
336.48 b
TM8
1.60 bcde
1.29 bcdefg
2.90 cdefgh
248.43 lmnop
TM9
1.80 abcd
1.31 bcdefg
3.11 bcdef
257.86 ijklmn
TM10
2.22 a
1.67 a
3.89 a
371.07 a
TM11
1.85 abcd
1.33 bcdefg
3.18 bcdef
276.73 efghi
TM12
1.94 abc
1.37 bcdef
3.31 bcde
295.60 cde
TM13
1.74 abcde
1.31 bcdefg
3.04 bcdefg
254.72 jklmn
TM14
1.83 abcd
1.33 bcdefg
3.16 bcdef
273.58 fghij
TM15
1.29 e
1.19 fg
2.48 gh
224.84 qr
SB1
2.03 abc
1.41 bcde
3.43 abcd
312.89 c
SB2
2.08 ab
1.47 bc
3.55 ab
342.77 b
SB3
1.82 abcd
1.32 bcdefg
3.15 bcdef
272.01 fghijk
SB4
1.53 cde
1.26 defg
2.78 efgh
232.70 opqr
SB5
1.40 de
1.22 efg
2.62 fgh
227.99 pqr
SB6
1.82 abcd
1.32 bcdefg
3.14 bcdef
268.87 ghijkl
SB7
1.91 abc
1.35 bcdefg
3.25 bcde
279.87 efgh
SB8
2.09 ab
1.49 b
3.58 ab
352.20 b
SB9
2.01 abc
1.40 bcdef
3.40 abcd
308.18 cd
SB10
1.81 abcd
1.31 bcdefg
3.12 bcdef
261.01 hijklm
SB11
1.92 abc
1.36 bcdefg
3.28 bcde
292.45 def
SB12
1.54 cde
1.26 defg
2.80 efgh
238.99 nopq
SB13
1.95 abc
1.39 bcdef
3.34 abcde
303.46 cd
SB14
2.06 ab
1.45 bcd
3.51 ab
339.62 b
SB15
1.79 abcd
1.31 bcdefg
3.10 bcdef
256.29 ijklmn
3.6 Stomatal density
The highest stomatal density was shown in the TM10 treatment (371.07 stomata/mm2), followed by SB8 (352.20 stomata/mm2) with an increase of 73.53 % and 64.70 %, respectively. The results summarized in Table 3 show that Trichoderma treatment increases the stomatal density of rice plants.
3.7 Plant growth-promoting traits of Trichoderma
Our study recorded the phosphatase activity, IAA production, ammonia production, and HCN production abilities of five Trichoderma isolates, revealing significant differences among treatments (Fig. 2).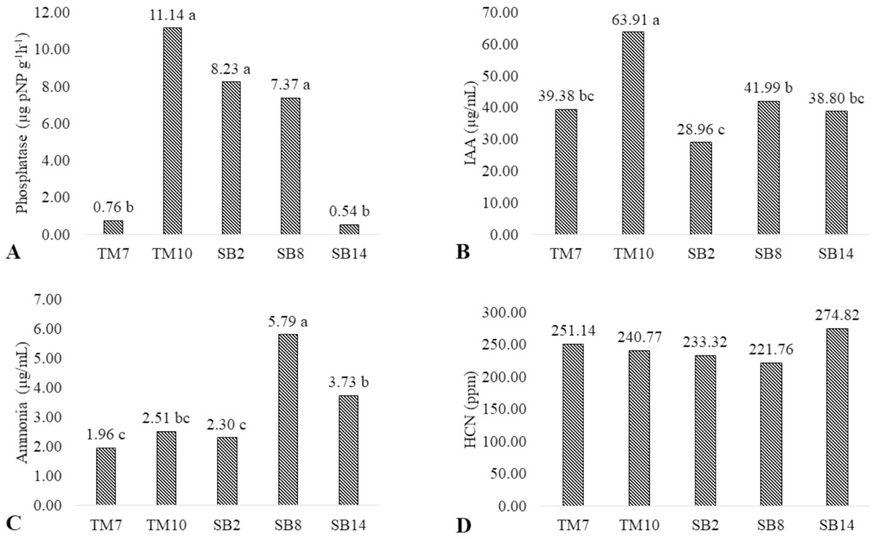
Plant growth promoting traits of five Trichoderma strains isolated from West Java, Indonesia. Phosphatase activity (A), IAA production (B), ammonia production (C), HCN production (D).
3.8 Molecular identification and phylogenetic analysis
According to the results of BLAST analysis, both TM10 and SB8 were identified as belonging to the T. yunnanense species (Fig. 3). The sequences of TM10 and SB8 were then deposited in GenBank with accession numbers OR915448 and OR915449, respectively.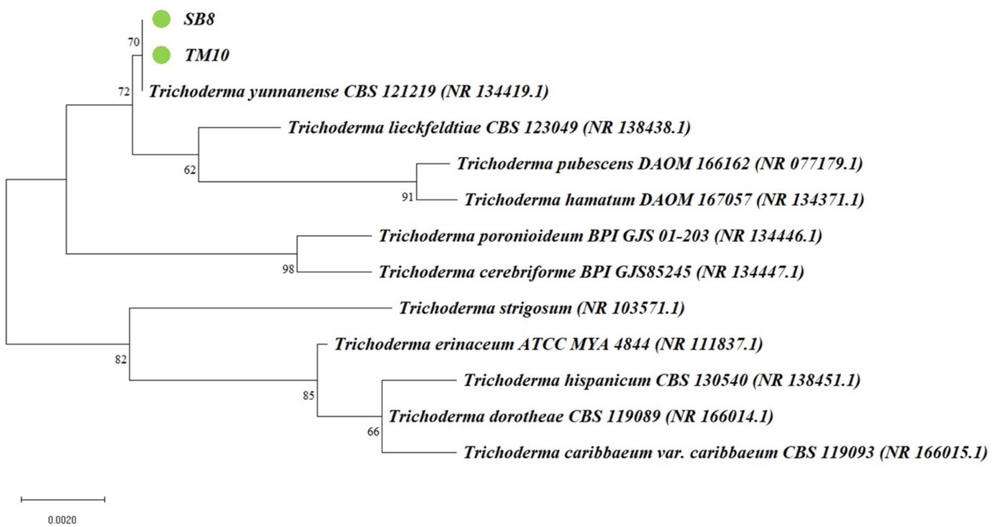
The phylogenetic tree uses DNA sequences from the isolated Trichoderma spp. (SB8 and TM10, green circle) used for the recent study which are constructed based on the neighbor-joining method and the p-distance genetic distance model with the MEGA11 application. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4 Discussion
Trichoderma-inoculated rice seeds in this study exhibited enhanced germination and vigor index, consistent with prior research (Doni et al., 2014). Phenolics, secondary metabolites, and phytohormones released by Trichoderma contribute to improved seed vigor (Swain et al., 2018), influenced by the strain and environmental factors (Nieto-Jacobo et al., 2017). However, some Trichoderma isolates in this study did not enhance germination percentage, aligning with observations of inhibitory effects reported by Santos et al. (2020).
Trichoderma has been discovered to synthesize secondary metabolites, such as harzianolide, that affect initial phases of seedling growth by increasing root and root tip length, thereby regulating general root development (Cai et al., 2013). These combined effects support the notable increase observed in root and shoot growth, as well as fresh and dry weight, in Trichoderma-inoculated seedlings. Such findings underscore the potential of Trichoderma-based bioinoculants as an environmentally friendly strategy for promoting rice seedling growth.
The enhanced growth in rice inoculated with Trichoderma during the late vegetative phase aligns with previous findings (Doni et al., 2017). Trichoderma strain TM10 exhibited high IAA production, correlating with increased root and shoot growth. The capability of Trichoderma to stimulate plant root growth is linked to IAA synthesis (Nieto-Jacobo et al., 2017). In seed treatments, Trichoderma can enhance the plants’ endogenous IAA production (Backer et al., 2018). IAA released by Trichoderma can directly enhance root growth through cell elongation or division and may have an indirect effect on ethylene (Contreras-Cornejo et al., 2016).
Efficient phosphorus absorption facilitated by phosphate-solubilizing microorganisms is crucial for chlorophyll biosynthesis, contributing to the increased chlorophyll observed in this study, consistent with a previous study by Swain et al. (2018). The five tested isolates exhibited acid phosphatase activity, essential for converting organic P into soluble inorganic forms, similar to that reported by Gaind (2016). The phosphatase production process by Trichoderma is influenced by various factors, with inorganic phosphate availability playing a pivotal role (Bononi et al., 2020).
Our findings align with earlier studies that revealed ammonia production by Trichoderma increased fresh weight, dry weight, plant height, and root length (Wang et al., 2021). In addition, the ample supply of nitrogen and phosphorus in the media can be utilized by plants for efficient photosynthesis, ultimately generating energy for growth (Singh et al., 2017). The five isolates tested for ammonia production align with several studies that reported ammonia production by Trichoderma strains (Gateta et al., 2023; Thakkar and Saraf, 2015).
An increase in stomatal density was observed in rice plants subjected to Trichoderma inoculation. These results are in agreement with the findings of Doni et al. (2017), who reported an increase in stomatal density (69 %) in rice plants following Trichoderma inoculation as opposed to those that were not inoculated. The improved stomatal density observed in this study holds potential implications for regulating water loss and augmenting CO2 absorption, which are essential for the growth of inoculated plants (Paradiso et al., 2017).
In addition, Trichoderma, as a biocontrol agent, demonstrates another plant growth-promoting property through the production of HCN, a gaseous volatile substance that hampers electron transport, leading to energy supply disruption and eventual cell death in targeted phytopathogens (Kolandasamy et al., 2023). Our findings showed the high production of HCN, which ranged from 221.76 ppm to 274.82 ppm, surpassing those reported by Vijayan et al. (2015), indicating that HCN production from various Trichoderma spp. isolates from India ranged from 0.8 ppm to 180.6 ppm. This holds significant implications for agricultural practices, offering a promising avenue for improving crop productivity and sustainability.
5 Conclusions
Trichoderma isolates significantly enhance rice growth and germination through mechanisms like IAA production, phosphate solubilization, ammonia production, and HCN regulation. The TM10 strain, in particular, showed superior results in improving rice growth and physiological traits of rice plants. For future studies, it is essential to investigate and compare different Trichoderma isolates, with a specific focus on discovering and characterizing secondary metabolites and proteins that may act as stimuli for plant defense systems. This comparative investigation of Trichoderma isolates will provide a deeper understanding of their potential use.
Funding
This work was funded by Universitas Padjadjaran through Riset Percepatan Lektor Kepala (RPLK) grant number 1616/UN6.3.1/PT.00/2024, awarded to Febri Doni.
CRediT authorship contribution statement
Sulistya Ika Akbari: Formal analysis, Methodology, Writing – original draft. Dedat Prismantoro: Data curation, Writing – review & editing. Joko Kusmoro: Supervision, Writing – review & editing. Rusdi Hasan: Supervision, Writing – review & editing. Mohamad Nurzaman: Supervision, Writing – review & editing. Nia Rossiana: Supervision, Validation, Writing – review & editing. Febri Doni: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Acknowledgement
The authors express their gratitude to Universitas Padjadjaran, Indonesia for the generous financial support for this research project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of mineral and bio-fertilization on vegetative growth, leaf mineral contents and flowering of manzanillo olive trees. Int. J. Chem. Technol. Res.. 2015;8:51-61.
- [Google Scholar]
- Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy. 2021;11:1-17.
- [CrossRef] [Google Scholar]
- Bioprospecting the roles of Trichoderma in alleviating plants’ drought tolerance: Principles, mechanisms of action, and prospects. Microbiol. Res.. 2024;283:127665
- [CrossRef] [Google Scholar]
- Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci.. 2018;871:1-17.
- [CrossRef] [Google Scholar]
- Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep.. 2020;10:1-14.
- [CrossRef] [Google Scholar]
- Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem.. 2013;73:1-8.
- [CrossRef] [Google Scholar]
- Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol.. 2016;92:1-17.
- [CrossRef] [Google Scholar]
- Formulation of Trichoderma sp. SL2 inoculants using different carriers for soil treatment in rice seedling growth. Springerplus. 2014;3:1-5.
- [CrossRef] [Google Scholar]
- Relationships observed between Trichoderma inoculation and characteristics of rice grown under System of Rice Intensification (SRI) vs. conventional methods of cultivation. Symbiosis. 2017;72:45-59.
- [CrossRef] [Google Scholar]
- Phosphate dissolving fungi: Mechanism and application in alleviation of salt stress in wheat. Microbiol. Res.. 2016;193:94-102.
- [CrossRef] [Google Scholar]
- Molecular evolution and phylogenetic analysis of biocontrol genes acquired from SCoT polymorphism of mycoparasitic Trichoderma koningii inhibiting phytopathogen Rhizoctonia solani Kuhn. Infect. Genet. Evol.. 2016;45:383-392.
- [CrossRef] [Google Scholar]
- The potential of endophytic fungi for enhancing the growth and accumulation of phenolic compounds and anthocyanin in maled phai rice (Oryza sativa L.) J. Fungi. 2023;9:1-19.
- [CrossRef] [Google Scholar]
- Research on the eco-efficiency of rice production and its improvement path: A case study from China. Int. J. Environ. Res. Public Health. 2022;19:1-20.
- [CrossRef] [Google Scholar]
- Rice husks as a sustainable source of high quality nanostructured silica for high performance Li-ion battery requital by sol-gel method - a review. Adv. Mater. Lett.. 2016;7:684-696.
- [CrossRef] [Google Scholar]
- Effects of Trichoderma seedling treatment with system of rice intensification management and with conventional management of transplanted rice. PeerJ. 2019;2019:1-22.
- [CrossRef] [Google Scholar]
- Multifaceted plant growth-promoting traits of indigenous rhizospheric microbes against Phomopsis theae, a causal agent of stem canker in tea plants. World J. Microbiol. Biotechnol.. 2023;39:1-20.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation effect of malachite green and catalytic hydrogenation by UV–illuminated CeO2/CdO multilayered nanoplatelet arrays: Investigation of antifungal and antimicrobial activities. J. Photochem. Photobiol. B Biol.. 2017;169:110-123.
- [CrossRef] [Google Scholar]
- Impact of agrochemicals on soil microbiota and management: A review. Land. 2020;9:1-21.
- [CrossRef] [Google Scholar]
- Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci.. 2017;8:1-18.
- [CrossRef] [Google Scholar]
- Trichoderma-enriched organic fertilizer can mitigate microbiome degeneration of monocropped soil to maintain better plant growth. Plant Soil. 2017;416:181-192.
- [CrossRef] [Google Scholar]
- The dynamics of rice production in Indonesia 1961–2009. J. Saudi Soc. Agric. Sci.. 2013;12:27-37.
- [CrossRef] [Google Scholar]
- Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci.. 2017;8:1-13.
- [CrossRef] [Google Scholar]
- The plant–soil microbiome: an overview. In: Uphoff N., Thies J., eds. Biological Approaches to Regenerative Soil Systems. Boca Raton, Florida, USA: CRC Press; 2023. p. :99-108.
- [Google Scholar]
- Sustainability status, sensitive and key factors for increasing rice production: A case study in West Java, Indonesia. PLoS One. 2022;17:1-19.
- [CrossRef] [Google Scholar]
- Novel Trichoderma isolates alleviate water deficit stress in susceptible tomato genotypes. Front. Plant Sci.. 2022;13:1-16.
- [CrossRef] [Google Scholar]
- Marketed surplus of Indonesian rice production. Cogent Econ. Financ.. 2022;10:1-14.
- [CrossRef] [Google Scholar]
- Food security challenges and opportunities in indonesia post COVID-19. In: Cohen M.J., ed. Advances in Food Security and Sustainability. Washington DC: Elsevier Inc.; 2021. pp. 119–168
- [CrossRef] [Google Scholar]
- Trichoderma spp. on treatment of Handroanthus serratifolius seeds: Effect on seedling germination and development. Heliyon. 2020;6:1–8
- [CrossRef] [Google Scholar]
- Sharma, S., Kour, D., Rana, K.L., Dhiman, A., Thakur, Shiwani, Thakur, P., Thakur, Sapna, Thakur, N., Sudheer, S., Yadav, N., Yadav, A.N., Rastegari, A.A., Singh, K., 2019. Trichoderma: Biodiversity, Ecological Significances, and Industrial Applications. In: Recent Advancement in White Biotechnology Through Fungi. Switzerland: Springer Nature, pp. 85–120. doi: 10.1007/978-3-030-10480-1.
- Effect of npk on plant growth, yield and quality of capsicum (Capsicum annum L.) c.v. Swarna under shade net condition. Int. J. Curr. Microbiol. Appl. Sci.. 2017;6:1007-1013.
- [CrossRef] [Google Scholar]
- Harvest Area and Rice Production in Indonesia, 2022. Statistics Indonesia, Jakarta: Official Statistics News; 2022.
- Novel Trichoderma strains. isolated from tree barks as potential biocontrol agents and biofertilizers for direct seeded rice. Microbiol. Res.. 2018;214:83-90.
- [CrossRef] [Google Scholar]
- Evaluation of native Trichoderma isolates for the management of sugarcane smut (Ustilago scitaminea) in sugar plantations of Ethiopia. Cogent Food Agric.. 2021;7:1-21.
- [CrossRef] [Google Scholar]
- Development of microbial consortia as a biocontrol agent for effective management of fungal diseases in Glycine max L. Arch. Phytopathol. Plant Prot.. 2015;48:459-474.
- [CrossRef] [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol.. 2019;191:65-74.
- [CrossRef] [Google Scholar]
- Investigation of Trichoderma species colonization of nursery grapevines for improved management of black foot disease. Pest Manag. Sci.. 2021;77:397-405.
- [CrossRef] [Google Scholar]
- Evaluation of native Trichoderma spp. against pathogens infecting small cardamom. J. Plant. Crop.. 2015;43:35-39.
- [Google Scholar]
- The endophytic strain Trichoderma asperellum 6s–2: An efficient biocontrol agent against apple replant disease in china and a potential plant-growth-promoting fungus. J. Fungi. 2021;7:1-27.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103378.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







