Translate this page into:
Isolation of new compound and neuroprotective studies from Dodonaea viscosa
⁎Corresponding authors. nsiddiqui@ksu.edu.sa (Nasir A. Siddiqui), oalmarfadi@ksu.edu.sa (Omer M. Almarfadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Objective
Neurological disorders is an increasing problem in the society. Our study aims to explore the secondary metabolites and evaluate the neuroprotective effect of leave extract of Dodonaea viscosa.
Methods
The ethyl acetate extract was chromatographed and produced one new entity which was characterized by spectroscopy (IR, UV, NMR, MS) studies. This entity was named as (6S, 9R)-vomifoliol-9-β-D-glucopyranosyle (1 -3)- O-α-L-rhamnopyranoside (DVE-8) while the known entities were identified as viscosine (DVE-12), and catechin (DVE-18). The neuroprotective potential of D. viscosa leaves extract was evaluated using the transient middle cerebral artery occlusion (MCAO) method to induce focal cerebral ischemia–reperfusion injury in rats.
Results
The levels of proinflammatory cytokines such as TNF-α, IL-6, and NF-κB gene expression along with the level of anti-apoptotic Bcl–2, BAX, and caspase–3 gene expression was evaluated. Neurological findings showed a significant reduction in the levels of pro-inflammatory cytokines (TNF-α and, IL-6; both P < 0.05). Also, the NF-κB gene expression was significantly downregulated (P < 0.05). Furthermore, the level of anti-apoptotic Bcl–2 gene expression was significantly upregulated (P < 0.05), whereas the level of BAX and, caspase–3 gene expression was significantly downregulated (P < 0.05).
Conclusion
The neuroprotective effect of D. viscosa leaves extract on MCAO-induced ischemic stroke may be due to the anti-inflammation, and anti-apoptosis activities. The findings of the present study may suggest that D. viscosa leaves extract supplementation may serve as valuable adjuvant therapy for neuronal protection.
Keywords
Neuroprotection
MCAO
D. viscosa
Cytokines
Apoptosis
1 Introduction
Dodonaea viscosa (L.) Jacq (Sapindaceae) is a flowering evergreen woody perennial shrub widespread in tropical and subtropical regions. In Saudi Arabia, D. viscosa grows in the Southern, Eastern, and Hijaz regions (Vasudevan et al., 2019). It has been used in traditional medicine to treat diverse illnesses. D. viscosa has been reported to contain a wide range of phytochemicals such as flavonoids, terpenoids, saponins, sterols, phenolics, sugars, alkaloids, and tannins (Al-Snafi A, 2017). According to a literature survey, D. viscosa possesses several specific phytochemicals, such as C-alkylated flavonol derivatives (Muhammad et al., 2012). It has been established that hautriwaic acid is one of the active constituents responsible for anti-inflammatory activity in this species (Osvaldo et al., 2012). Isoprenylated flavonol derivatives named dodoviscins are also reported (Gao et al., 2013). Terpenoids were also obtained from D. viscosa such as β-pinene, myrcene, limonene, p-cymene, citronellal, linalool, linalyl acetate, γ-terpineol, geraniol (monoterpenoides) (Mata et al., 1991), and dodonic acid (diterpenoid) (Gao et al., 2013). Triterpenoid saponins were isolated from D. viscosa, such as dodonaeaside A and dodonaeaside B, 21,22-diangeloyl, barringtogenol C, 21-angeloyl-R1-barringenol, 21,22-diangeloyl-R1-barringenol, and cleomiscosin, R1-barrigenol, R1- barrigenol 21,22-diangelate, jegosapogenol, and jegosapogenol 21-(2,3-dihydroxy- 2- methyl butyryl) 22-angelate. Furthermore, previous reports have documented many sterols were isolated from D. viscosa, such as lupeol, stigmasterol and stigmast-7-en-3-ol, α-spinasterol and β-sitosterol, besides, myo-inositol derivatives such as 1-L-O-methyl-2-acetyl-3-p-cis-coumaryl-myo-inositol and 1-L- 1-O-methyl-2-acetyl-3-p-coumaryl-myo-inositol], were also isolated from this species (Al-Asmari et al., 2013). A literature review has shown that D. viscosa is least explored in Saudi Arabia; only one phytochemical report suggests the presence of flavonoids and new clerodane diterpenoids (Al-Asmari et al., 2013), which supports selecting this plant for further explorations.
Neurological disorders are a variety of diseases that affect the central and peripheral nervous systems. However, neurological disorders represent the major cause of disability and the second leading cause of mortality worldwide. The absolute number of deaths and individuals disabled by neurological disorders has increased dramatically in the last 30 years, especially in low- and middle-income countries (Feigin et al., 2020).
A variety of biological and biochemical mechanisms are implicated in several neurodegenerative diseases and these mechanisms include the release of inflammatory mediators, and evoking apoptotic factors. Among the various neurological disorders stroke is one of the most serious and life-threatening neurological disorders and the most frequent cause of death worldwide. Ischemic strokes represent 80–85 % of all stroke cases. It occurs due to hindrance of blood flow to the brain due to vascular obstruction in the cerebral arteries depriving brain cells of oxygen and glucose. Neurons are highly responsive to ischemia and reperfusion culminating in neuronal death and brain dysfunction (Prabhakaran et al., 2015).
Inflammation is induced during brain stroke and consequently leads to neuronal damage (Ahmad et al., 2019). Microglial cells are the primary immune effectors of the CNS. In stroke, they are activated, especially in the penumbra, change their shape to an amoeba-like form, proliferate, and become phagocytically active. They also produce a wide range of pro-inflammatory cytokines, oxygen-free radicals, and enzymes (Allan and Rothwell, 2001). In addition, leukocytes, neurons, and astrocyte cells also contribute to producing cytokines and chemokines (Dawes et al., 2018). During brain ischemia, the most predominant cytokines are TNFα, IL-1, and IL-6 which play a notable role in the inflammatory response (Wytrykowska et al., 2016).
A programmed cell death is called apoptosis which takes place during physiological cellular turnover and in pathological conditions such as ischemia (Ünal-Çevik et al., 2004). Apoptosis is induced by two pathways; intrinsic and extrinsic pathways (Broughton et al., 2009). At the onset intrinsic pathway, calcium ions accumulated intracellularly due to stimulation of NMDA receptors causing induction of calapine and cleveite of Bcl-2 interacting domain (BID) to truncated Bid (tBid) (Nikoletopoulou et al., 2013😉 Truncated Bid (tBid) interact with apoptotic proteins (such as Bad, Bak, etc.) leading to opening the mitochondrial transition pores (MTP) and releasing mitochondrial cytochrome c (Cyt c) or/and apoptosis-inducing factor (AIF). On the other side, Bcl-2 and BcL-XL proteins act as anti-apoptotic proteins throughout the prevention of (MTP) formation (Broughton et al., 2009). Apoptosome is created due to the interaction of Cyt c with apoptotic protein-activating factor-1(Apaf-1); it activates caspase-3 that cleaves nuclear DNA repair enzymes, causing damage to nDNA and cell death (apoptosis). In addition, AIF enters the nucleus and causes caspase-independent DNA fragmentation and cell death (Martin-Villalba et al., 1999). The caspase proteins are activated during the extrinsic pathway by the TNF ligands such as the FASL and TNF (Broughton et al., 2009), Which activate, sequentially, caspase-8, caspase-9 and caspase-3. This cascade of activation enhances mitochondrial permeability, condensation of chromatin, fragmentation of DNA, and finally, cell death (apoptosis) (Li et al., 2000). It has been reported that the down-regulation of BAX and the up-regulation of Bcl‐2 have anti-apoptotic activity (Yaidikar & Thakur, 2015). In addition, over-expression of Bcl-2 inhibits the release of Cyt c and blocks the activation of caspase-3 expression (Zhao et al., 2003). In general, inhibition of intrinsic and extrinsic pathways of apoptosis may protect against brain injury (Guan et al., 2006). Previous studies have shown the traditional use of D. viscosa for stomach pain, piles, and ulcers. Anti-inflammatory, antimicrobial, local anesthetic and smooth muscle relaxing effect of D. viscosa has been reported (Ramkumar and Periyasamy, 2019).
2 Experimental
2.1 Materials
2.1.1 Plant material
The leaves of D. viscosa (3.0 kg) were collected from the Botanical Garden, College of Pharmacy, King Saud University (KSU), Riyadh, Saudi Arabia. The identity of the sample was confirmed by Dr. Mohammed Yusuf, Field Taxonomist, Department of Pharmacognosy, College of Pharmacy, KSU. The voucher specimen (voucher #15787) has already been deposited in the Herbarium, Pharmacognosy Department, College of Pharmacy, KSU, Riyadh, Saudi Arabia.
2.1.2 Animals
Male Wistar rats (170–202 g) were acquired from the Central Animal House Facility of King Saud University, Riyadh, Saudi Arabia. They were kept in animal cages with 12-hour light and dark cycles at 25 °C ± 2 °C. The rats were fed on standard rat chow and provided water ad libitum. Ethical approval for the in vivo experimental protocol used in this study was obtained from the research ethics committee, King Saud University, Riyadh, Saudi Arabia (KSU-SE-19–62).
2.1.3 Solvents and reagents
Analytical and spectroscopic grade solvents like n-hexane, dichloromethane, chloroform, ethyl acetate, n-butanol, and methanol were used in the extraction, fractionation, chromatographic and spectroscopic analysis. But, deuterated solvents like methanol and dimethyl sulfoxide are used in NMR analysis. All solvents were purchased from (Sigma Aldrich, MO, USA). ELISA kits of TNF-α and IL-6 and standard biomarkers involving caspase-3 (sc-22171-R), Bax (sc-6236), Bcl-2 (sc-492), NF-κB (p65) (sc-398442), and β-actin (housekeeping antibodies) (sc-47778), all kits and standard biomarkers were purchased from (Santa Cruz Biotechnology, Inc., California, United States), were used for evaluation of neuroprotective activities.
2.1.4 Apparatus and equipment
A rotary vacuum evaporator was used to dry the extract and fractions. Spectroscopic instruments like an ultraviolet lamp, UV–VIS spectrophotometer (Shimadzu Corporation, Kyoto, Japan), Fourier-transform infrared spectrometer (FTIR) (Shimadzu Corporation, Kyoto, Japan), Triple quadrupole mass spectrometer (TQMS) (Waters Corp., Milford, MA, USA), and Bruker Avance DRX 700 MHz spectrometer (Massachusetts, United States) was used to obtain 1D and 2D NMR spectra at 700 and 175 MHz for 1H and 13C, respectively. Bruker Bioapex FT–MS (Massachusetts, United States) was used to obtain the EI mass spectra. In addition, Blade Scalpel (size 15), sutures (size 5–0), monofilament (size 0.4–0.45 mm) were used in a surgical procedure. LI-COR C-Di-Git blot scanners (LI-COR Biosciences, Lincoln, NE, USA) were used in the Western blot assay.
2.2 Experimental design
Twenty-four rats were randomly divided equally into four groups (n = 6) as follows:
Group I: Control (CN): Each rat was given normal saline for 30 days and placebo surgery without ischemia/reperfusion (MCAO).
Group II: MCAO: Each rat was treated with normal saline for 30 days before reperfusion. Reperfusion continued for 24 h following 45-min middle cerebral artery occlusion (MCAO), and the rats were sacrificed 24 h after ischemia.
Group III: Treated (DVME + MCAO): Each rat was given a dose of 150 mg/ kg body wt. by gastric gavage for 30 days as pre-treatment. Reperfusion proceeded for 24 h following 45 min MCAO, and rats were sacrificed 24 h after ischemia.
Group IV: Treated (DVME + MCAO): Each rat was given a dose of 300 mg/ kg body wt. and followed a similar procedure as for group III.
2.3 Surgical procedure
The animals were anesthetized according to the method described previously by Lee et al., 2014 and the surgical procedure technique was followed as mentioned by Lemmerman et al., 2022.
2.4 Phytochemical evaluation methods
2.4.1 Extraction
The size reduction of air-dried leaves (3 kg) was done in a grinder and extracted by cold maceration with (8 L) 90 % methanol. The extraction time was five days with occasional shakings to exhaust the material and produce of good yield of extract. The extract was concentrated using a rotary vacuum evaporator (Buchi Rotavap) under reduced pressure. After drying, a total of dry crude extract (DVME, 1.1 kg) was obtained.
2.4.2 Fractionation, isolation and purification of secondary metabolites
Leaves extract (750 g) was subjected to fractionation using organic solvents of successive increasing polarity (i.e. n-hexane, chloroform, ethyl acetate, and n-butanol). It was transferred to a separatory funnel, suspended in 1500 ml of distilled water, and partitioned with 3 × 1250 ml of each solvent. The filtrate was concentrated using a rotary vacuum evaporator (Buchi Rotavap) under reduced pressure (45 rpm) at 40 °C. The yield of the dried fractions was 3.3 g = 0.77 %, 130.6 g = 30.51 %, 36.84 g = 8.61 %, 92.6g = 21.62 %, and 164.7 g = 38.48 % for the n-hexane, chloroform, ethyl acetate, n-butanol and aqueous residues fractions, respectively. TLC of all fractions was run using CHCl3: MeOH: Formic acid (70: 30: 2.5) as a mobile phase. Then the dried fractions were transferred into separated glass containers and stored at − 20 °C until use.
2.4.3 Chromatographic methods
2.4.3.1 Compounds isolated from ethyl acetate fraction
Ethyl acetate fraction (15 g) was chromatographed on a silica gel column (72 g, 80 × 3 cm). Elution started with 100 % n-hexane, then with 50:50n-hexane: chloroform after that with 90: 10 chloroform: methanol, and polarity was increased with methanol to 100 %. The collected fractions (150 ml each) were pooled depending on their TLC behavior and homogeneity, and yielded eight major sub-fractions E1- E8, using EtOAc: formic acid: AcOH: H2O (30: 0.8: 1.2: 8) as a mobile phase.
2.4.3.2 The sub-fraction E-7
The sub-fraction E-7 (2 g) was re-chromatographed on a reversed-phase column that was eluted gradually starting from 90 % water with methanol. According to their RP-TLC similarities, the collected fractions were merged into six sub-fractions (1–6).
Sub-fraction 4 (70 mg) was eluted with water: methanol (70: 30) using a reversed-phase column and re-chromatographed again on Sephadex LH20 column that eluted with methanol: dichloromethane (1: 2) to obtain four sub-fractions (1–4) based on their TLC similarity. Sub-fraction 1 showed one major spot on TLC with (Rf = 0.32), CHCl3: MeOH (75: 25) was used as a mobile phase. It afforded 7.4 mg of compound (DVE-8).
2.4.3.3 The sub-fraction E-1
The sub-fraction E-1 (84 mg) was re-chromatographed on a reversed-phase column. The column was eluted gradually starting from water: methanol (90: 10). The collected fractions were merged into five sub-fractions according to their TLC similarities. Sub-fraction 2 showed one major spot on the TLC (Rf = 0.21) using n-hexane: EtOAc (65: 35) as mobile phase and afforded 21.9 mg compound DVE-12.
2.4.3.4 Sub-fraction E-5
The sub-fraction E-5 (1 g) was re-chromatographed using a reversed-phase column. The column was eluted gradually starting from water: methanol (70: 30). The collected sub-fractions were merged into nine sub-fractions according to their TLC similarities. Sub-fraction 8 exhibited four spots, but one was major. Sephadex column was used to purify the major spot using DCM: MeOH (2: 1) as eluent. Four sub-fractions (1–4) were obtained; sub-fraction 2 showed one spot with (Rf = 0.30) using RP-TLC, which developed with ACN: H2O (70: 30). It afforded 22 mg compound (DVE-18).
2.5 Estimation of biomarkers of inflammation and apoptosis
2.5.1 Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) tests
The injured areas of the brain tissue were removed 24 h after MCAO that induced ischemia/reperfusion injury. The brain tissues are washed in cold phosphate buffer slain (PBS) and placed into a homogenate tube. An appropriate volume of PBS was added into the tubes at 4 °C, to allow the tissues to be ground into 10 % homogenate. The supernatant was collected following centrifugation at 4,000 × g for 15 min. The protein levels of TNF-α and IL-6 were determined using ELISA kits according to the manufacturer's instructions, which included the addition of 100 µl of appropriately diluted samples to each well. Standards (triplicates) and blanks were run with each plate to ensure accuracy (Sinha et al., 2001).
2.5.2 Western blot analysis
‘Briefly, denatured protein (20 μg per well) was loaded onto a 10 % polyacrylamide gel containing 0.1% sodium dodecyl sulfate (SDS) buffer and electrophoresed at 120 V for 60 min. Immunoblotting was performed as previously described (Koob et al., 2012). 20 µg of protein was transferred to polyvinylidene difluoride (PVDF) membranes, blocked in 5 % skim milk in tris-buffered saline (TBS) buffer containing 1 % Tween 20, and incubated overnight with the caspase-3 (sc-22171-R), Bax (sc-6236), Bcl-2 (sc-492), NF-κB (p65) (sc-398442) and β-actin (housekeeping antibodies) (sc-47778), followed by incubation with HRP-conjugated anti-rat/rabbit/goat antibodies for 2 hrs at 25 °C. Bands were visualized with the Luminata™ Western Chemiluminescent HRP Substrates, and densitometric analysis of bands was performed using LI-COR C-Di-Git blot scanners (LI-COR Biosciences, Lincoln, NE, USA).
2.6 Statistical analysis
Data obtained from animal experiments were expressed as the mean and standard error of the mean (mean ± SEM). Statistical differences between the control and treated groups were evaluated by one-way ANOVA and post hoc Tukey’s tests helping GraphPad Prism-8 software. In all cases, P < 0.05 was considered to be significant.
3 Result and discussion
3.1 Characterization of isolated compounds from ethyl acetate fraction
3.1.1 Characterization of compound DVE-8
Compound DVE-8 (7.4 mg) was obtained as a yellowish-white powder. UV spectrum showed two λ max absorption bands at 232 and 204 nm, indicating olefinic and aliphatic groups, respectively. The IR spectrum showed strong absorption at 3437 cm1 indicating to a hydroxyl group, weak absorption at 2927 cm1 gave indication methyl group, and a strong absorption at 1640 cm−1 exhibited the olefinic group. LC-MS/MS scanning spectrum demonstrated a strong peak at m/z 531.32 [M−H]-, compatible with the molecular weight 532. Furthermore, the LC-MS/MS fragmentation spectrum clarified a molecular ion peak for the parent compound at m/z 531.29 [M−H]- and other significant fragments at 369.26 [C19H29O7•]- and 153.14 [C9H13O2•]-, suggested the molecular formula C25H40O12 which contains 6 degrees of unsaturation.
The 1H NMR spectrum showed resonances for three vinyl protons signals at δH 5.79 (brs, 1H, H-4), 5.72 (br. s, 1H, H-7), 5.73 (brs, 1H, H-8), isolated methylene δH 2.10 (d, J = 16.8 Hz, 1H, H-2a) and 2.35 (d, J = 16.8 Hz, 1H, H-2b), two tertiary methyl protons signals at δH 0.93 (s, 3H, H-11), 0.91 (s, 3H, H-12), a vinyl methyl protons signal at δH 1.81 (d, J = 1.3 Hz, 3H, H-13), and one secondary methyl at δH 1.16 (d, J = 6.3 Hz, 3H, H-10) were assigned as a megastigmane aglycone (2 1 5). Additionally, two anomeric protons at δH 4.68 (d, J = 1.3 Hz, 1H, H-1‘) and 4.34 (d, J = 7.8 Hz, 1H, H-1‘‘) suggest the presence of α and β sugar units, respectively, based on coupling constant, that assigned as aglycone moiety.
The 13C NMR spectrum displayed 25 carbon signals which were further differentiated helping DEPT and HSQC to 4 quaternary carbons (comprising one keto group at δC 197.3 (C-3), two allylic carbons at δC 19.0 (C-13), 77.7 (C-6) and an aliphatic carbon at δC 39.8 (C-1), 3 two olefinic methines at δC 125.7 (C-4), 130.1 (C-7), 132.7 (C-8), one oxygenated methine at δC 71.9 (C-9), one methylene at δC 49.3 (C-2), and four methyl carbons, at δC 23.1 (C-11), 24.0 (C-12), 20.4 (C-10), 19.0 (C-13), were assigned to megastigmane skeleton (Wang et al. 2011). Besides, two anomeric carbons at δC 98.2 (C-1‘), 104.6 (C-1‘‘), oxygenated aliphatic methine carbons at δC 69.8 (C-2‘), 81.9 (C-3‘),70.9 (C-4‘), 68.2 (C-5‘), 74.0 (C-2‘‘), 76.6 (C-3‘‘), 69.9 (C-4‘‘), 76.1 (C-5‘‘), one oxygenated methylene carbon at δC 61.4 (C-6‘‘), and one methyl carbon at δC 17.7 (C-6‘) were accounted for a glycone moiety (α- rhamnopyranosyl and β-glucopyranose units) (Fig. 1).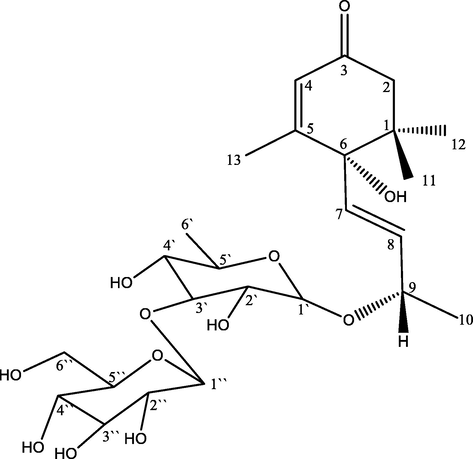
Chemical structure of compound DVE-8.
In the COSY spectrum δH 5.73 (brs, 1H, H-7) was coupled with 5.72 (bro. s, 1H, H-8) while, 4.23 (m, H-9, 1H) was coupled with both 5.72 (brs, H-8, 1H) and 1.16 (d, J = 5.5, H-10, 1H).
Also, HMBC correlations showed long bond correlation; δH 1.81 (d, J = 1.3 Hz, H-13, 3H) to C-4, C-5, C-6. Moreover, δH 5.79 (brs, H-4, 1H) to C-2, C-3, C-6. Also, vinylic protons at δH 5.73 (brs, H-7, 1H) correlated to C-6 and C-9, clarifying the keto group at C-3, Δ5 at C-4/C-5 and Δ7 at C-7/C-8, respectively, at megastigmane moiety. Besides, an anomeric proton at δH 4.68 (d, J = 1.3 Hz, H-1‘) correlated to C-9 and C-3‘ supporting the α- rhamnopyranosyl was attached to C-9 of megastigmane moiety via (1‘→9) linkage while secondary anomeric proton at δH 4.34 (d, J = 7.8 Hz, H-1‘‘, 1H) correlated to C-2‘, C-2‘‘, and C-3‘‘ confirming the connection of anomeric carbon C-1‘of α- rhamnopyranosyl unit to C-3‘, C-2‘‘ and C-3‘‘of β-glucopyranose moiety via (1‘‘→3‘) linkage (Fig. 2). The 1H NMR and 13C NMR data are shown below in Table 1.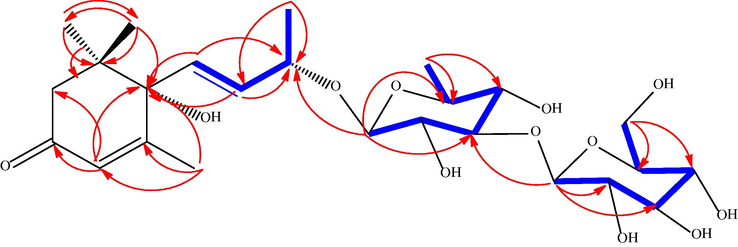
HMBC and COSY correlations of DVE-8.
Position
δC (ppm)
δH (ppm)
Multiplicity and J (Hz) value
1
39.8
–
–
2a
49.3
2.10
d, J = 16.8, 1H
2b
2.35
d, J = 16.8, 1H
3
197.3
–
–
4
125.7
5.79
brs, 1H
5
163.7
–
–
6
77.7
–
–
7
130.1
5.72
brs, 1H
8
132.7
5.73
brs, 1H
9
71.9
4.23
m, 1H
10
20.4
1.16
d, J = 6.3, 3H
11
23.1
0.93
s, 3H
12
24.0
0.91
s, 3H
13
19.0
1.81
d, J = 1.3, 3H
1‘
98.2
4.68
d, J = 1.3, 1H
2‘
69.8
3.81
brt, J = 4.6 1H
3‘
81.9
3.50
m, 1H
4‘
70.9
3.38
m, 1H
5‘
68.2
3.51
m, 1H
6‘
17.7
1.11
d, J = 6.2, 3H
1‘‘
104.6
4.34
d, J = 7.8, 1H
2‘‘
74.0
3.03
m, 1H
3‘‘
76.6
3.11
m, 1H
4‘‘
69.9
3.06
m, dd J = 9.1, 2.6,1H
5‘‘
76.1
3.14
m, 1H
6‘‘a
61.4
3.42
m, 1H
6‘‘b
3.63
dd, J = 11.6, 2.5,1H
6-OH
–
5.09
s, 1H
2‘–OH
–
4.54
d, J = 4.6, 1H
4‘–OH
–
4.89
d, J = 3.3, 1H
2‘‘–OH
–
5.24
brs, 1H
3‘‘- OH
–
4.99
brs, 1H
4‘‘ –OH
–
4.93
brs, 1H
6‘‘ –OH
–
4.43
t, J = 5.6, 1H
The spectroscopic data of DVE-8 are mostly similar to a previously isolated compound like corchoionoside C and vomifoliol from Capparis spinose and Zizyphi Fructus, respectively, exempted they contain only one glucose unit (Çalış İ et al., 2002) as well as megastigmane glucoside from Lonicera gracilipes var. Glandulosa, except for the presence of β - rhamnopyranose instead of α-L-arabinopyranosyl unit (Matsuda et al., 1997). Therefore, the structure of DVE-8 was determined as (6S, 9R) - vomifoliol −9-β-D-glucopyranosyl (1‘‘-3‘) -O- α-L-rhamnopyranoside. By referring to what was published in the literature, this constituent is a new compound and it is the first time to be isolated from a natural source. The chemical structures of isolated known compounds viscosine (DVE 12) and catechin (DVE 18) are depicted in Fig. 3 and the spectral details are attached as supplementary material.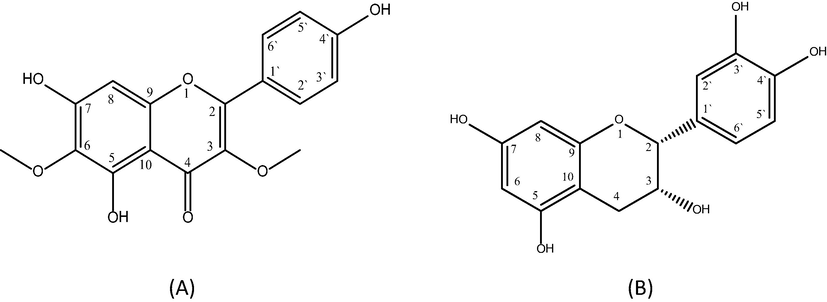
Chemical structure of viscosine DVE-12 (A) and catechin DVE-18 (B).
3.2 Neuroprotective findings
Cerebral ischemic stroke is one of the clinically severe brain diseases. However, there is a lack of effective neuroprotective therapy in its acute phase. Nowadays, it has become essential to solve this problem by exploring safe and effective neuroprotective drugs. Our study demonstrated that pre-treatment with DVME provides neuroprotective effects through anti-inflammatory, and anti-apoptotic activities after cerebral MCAO injury. According to previous studies, the MCAO model induced focal cerebral ischemia in experimental rats. Post-ischemia reperfusion restores blood flow to ischemic brain tissues associated with activation of the inflammatory response, upregulation of pro-apoptotic factors, and downregulation of anti-apoptotic factors expression (Gao et al., 2017). Toxicity study has been established in many research articles based on the toxic effects and anti-inflammatory effects of D. viscosa. The dose of D. viscosa was mentioned as 100–300 mg/ kg. There is no toxicity reported up to 2000 mg/ kg dose of D. viscosa (Ramkumar and Periyasamy, 2019).
3.2.1 Neuroinflammation
3.2.1.1 TNF-α
TNF-α was elevated significantly in the hippocampus region after induced transient focal cerebral ischemia in rats. It was significantly decreased (p < 0.05) in the MCAO group, as compared with the normal group. But in DVME pretreated groups TNF-α level in the hippocampus decreased significantly (p < 0.01) in a dose-dependent manner compared with the MCAO group. TNF-α abolished markedly in pretreated groups with different doses of DVME (150 and 300 mg/kg), by approximately (35.47and 49.32 %), respectively (Fig. 4).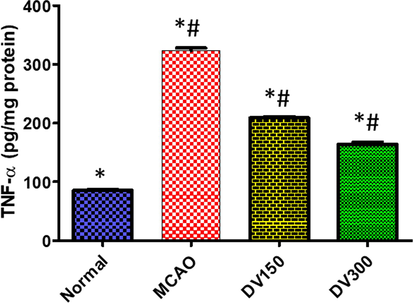
The effects of Dodonaea viscosa extract on TNF-α level in the brain of different groups. Statistical analysis was done using one-way ANOVA followed by post-hoc Tukey test. Values represent means ± (SEM) of each group, *P < 0.05 compared to normal group, #P < 0.05 compared to MCAO group.
3.2.1.2 Il-6
In the MCAO group, the IL-6 level was increased significantly (p < 0.05) in the brain as compared with the normal group. While pretreatment with DVME (150 and 300 mg/kg) showed a significant (p < 0.05) dose-dependent decline in IL-6 level as compared with the MCAO group. DVME pretreatment groups (150 and 300 mg/kg) exhibited a significant reduction in IL-6 level by approximately 83.49% and 122.58%, respectively, compared with the MCAO group (Fig. 5).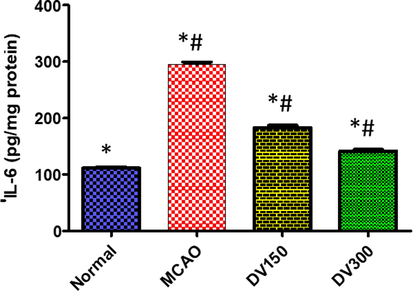
The effects of Dodonaea viscosa extract on IL-6 level in the brain of different groups. Statistical analysis was done using one-way ANOVA followed by post-hoc Tukey test. Values represent means ± (SEM) of each group, *P < 0.05 compared to normal group, #P < 0.05 compared to MCAO group.
3.2.1.3 Nf-κB
In comparison with the normal group, the MCAO group exhibited a significantly high NF-Κb expression in the brain (P < 0.05). While pretreated groups with DVME (150 and 300 mg/kg/d) showed a significant reduction in expression of NF-κB (P < 0.05), vs the MCAO group. Marked down-regulation in NF-kB expression (approximately 67.99% and 74.92%) was observed in pretreated groups with DVME (150 and 300 mg/kg/d, respectively) when compared with MCAO (Fig. 6).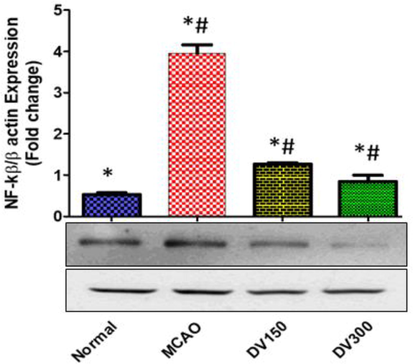
The effects of Dodonaea viscosa extract on NF-κB level in the brain of different groups. Statistical analysis was done using one-way ANOVA followed by post-hoc Tukey test. Values represent means ± (SEM) of each group, *P < 0.05 compared to normal group, #P < 0.05 compared to MCAO group.
Neuroinflammation is excited during cerebral I/R and participates in the deterioration of the neurological outcome and even brain cell death. In cerebral hypoxia, circulating neutrophil cells and leukocytes are stimulated to migrate toward the injured region, cross the BBB, and contribute to brain tissue damage (Franklin S, 2017). However, pro-inflammatory cytokines are released causing a secondary neuro-destructive cascade. Pro-inflammatory cytokines such as TNF‑α and IL-6 levels are markedly elevated during cerebral ischemia, this abnormal elevation contributes to brain damage as well as feedback stimulation of circular leukocytes adhesion, trans-endothelial migration, and expression of intracellular adhesion molecule‑1, exacerbating the post-ischemic injury (Feigin et al., 2020; Ahmad MS et al., 2021) Neuro-inflammatory response leads to losing the integrity of BBB, accumulation of fluid in the brain, loss of neuronal cell function, and finally neuronal cell death (Rasool et al., 2014). In our study, we evaluated the anti-inflammatory effect of DVME using experimental rats exposed to MCAO in order to induce focal cerebral ischemic after 30 days of oral administration of DVME (150 and 300 mg/kg). However, pro-inflammatory cytokines levels, TNF-α, and IL-6 were used as biomarkers to the evaluation of the neuroprotective activity of DVME.
NF-κB is the transcription factor that accounts for the key regulator of the inflammatory response. It regulates gene expression in a wide range of biological processes. In the central nervous system, NF-κB plays a dual role in neuronal survival after cerebral I/R through the regulation of neuro-inflammatory and apoptosis gene expression (Bähr, M., 2006). In a clinical study, the expression of NF-κB was detected in the cerebral infarct area in both ischemic and penumbra regions but not in the unaffected one in the postmortem human brain (Lo et al., 2003). Additionally, it has demonstrated that NF-kB expression increased in transient and permanent MCAO after cerebral ischemia in the animal model. Furthermore, a previous report established the contribution of NF-κB in worsening brain infarction during cerebral ischemia and suggested the neuroprotective effect may be attributable to the inhibition of NF-κB activation (Prabhakaran et al., 2015). In our study, the elevation of NF-κB expression was markedly detected in the brain of the MCAO group and was conjugated with an elevation of inflammatory, apoptotic markers as well infarct volume and neurological score. While the NF-κB expression was markedly downregulated in the pretreated DVME (150 and 300 mg/kg/d) groups in a dose-dependent manner ameliorating the inflammatory and apoptotic markers besides improvement of infarct volume and neurological behaviors. This result agrees with a previously published study that stated the anti-inflammatory effect of polyphenolics-rich plants like D. viscosa on cerebral endothelial cells (Testai and Aiyagari, 2008).
TNF-α is one of the pro-inflammatory cytokines which is rapidly upregulated in the brain after ischemic injury enhancing BBB permeability, oxidative stress, and apoptosis. However, the interventions targeting this biomarker showed a therapeutic value and protected against I/R injury in the brain (Khan et al., 2013). Our finding showed a significant elevation in TNF-α level in the MCAO group vs the baseline of the normal group, this elevation was suggested to deteriorate the structure and function of vascular and neuronal cells in the brain. But, DVME pretreatment (150 and 300 mg/kg) for 30 days reduced this elevation significantly and protected against cerebrovascular cell injury. These results agreed with a previous study which demonstrated a clear improvement in BBB integrity, ROS production, and apoptosis using TNF-α antibody in the cell culture model (Bryan and Waxman, 2006).
IL-6 is a pro-inflammatory cytokine its levels often correlate to the severity of a wide variety of pathological disorders involving ischemia-induced brain injury. It has been reported controversial role of IL-6, some studies suggested that IL-6 has anti-inflammatory activity (Sanderson et al., 2013) While others stated its role in saving the neural cell against viral-induced demyelination suggested using IL-6 in the treatment of neurodegenerative disorders (Uzdensky AB, 2019). Also, It has been reported that the infarct size was similar in IL-6 deficient mice in comparison with wild type suggesting that IL-6 does not participate in the pathogenesis of focal cerebral ischemia (Tiwari et al., 2019). On the other side, was closely correlated with the risk of incident stroke in a biracial population-based study, IL-6 mediated a racial difference in stroke through the inflammatory effects of risk factors (Wu & Cederbaum, 2003). In our study, the IL-6 level in the brain was significantly elevated in the MCAO group while DVME pretreatment groups (150 and 300 mg/kg/d) showed a marked reduction, dose-dependently, in IL-6 level and was associated with a significant reduction in infraction volume. Our observations agreed with previous studies that reported the destructive effect of induced IL-6 in cerebral I/R besides the neuroprotective effect of DVME through suppression of neuro-inflammatory response (Wu & Cederbaum, 2003).
Neuroinflammation is a major pathological process that is triggered by ischemic stroke (Chan et al., 1984). Inflammatory response induced by several signals such as NF-kB gen and cytokines like TNF-α and IL-6 proteins which are released from activated lymphocytes and microglia leads to further immune cell activation as well as damage of brain tissues (Javier Jiménez et al., 2016). Previously published studies reported that the inhibition of microglia activation and suppression of NF-kB, TNF-α, and IL-6 enhances neuroprotection by minimizing brain infarction and amelioration of neurological dysfunction (Kanamori et al., 2010). In our study, we observed those pro-inflammatory cytokines TNFα and IL-6 were evaluated after transient cerebral I/R. The mean TNF-α and IL-6 levels increased in the I/R injury group compared with the normal group. Treatment with DVME significantly attenuated the expression of NF-Kb and production of TNF-α and IL-6, which may attributable to the anti-inflammatory activity of DVME, these finding agreed with previous reports. D. viscosa contains a wide range of bioactive compounds mostly flavonoids, and phenolics with anti-inflammatory activity and owning suggested to produce neuroprotective activity against neurodegenerative disorders (Costa et al., 2015).
3.2.2 Apoptosis
3.2.2.1 Caspase-3
A caspase-3 expression was significantly evaluated in the hippocampus of the rat group with MCAO vs normal group, (p < 0.05). While pretreated groups with DVME (150 and 300 mg/kg) showed a significant Caspase-3 overexpression in a dose-dependent manner in comparison with the MCAO group, (p < 0.05). Both low dose (150 mg/kg/d) and high dose (300 mg/kg/d) of DVME exhibited a marked down-regulation of Caspase-3 expression by approximately 49.61% and 64.80 %, respectively, vis MCAO group (Fig. 7).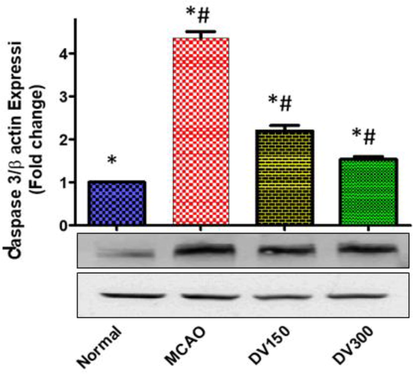
The effects of Dodonaea viscosa extract (DV) on Caspase-3 level in the brain of different groups. Statistical analysis was done using one-way ANOVA followed by post-hoc Tukey test. Values represent means ± (SEM) of each group,*P < 0.05 compared to normal group, #P < 0.05 compared to MCAO group.
3.2.2.2 BAX
In comparison with the normal group, the MCAO group revealed a significant upregulation of BAX protein expression in the brain (P < 0.05). While compared with the MCAO group, pretreated groups with DVME (150 and 300 mg/kg/d) showed significant downregulation of BAX expression (P < 0.05). In the DVME pretreated groups (150 and 300 mg/kg/d), BAX expression was downregulated by approximately 34.13% and 65.55%, respectively; vs the MCAO group (Fig. 8).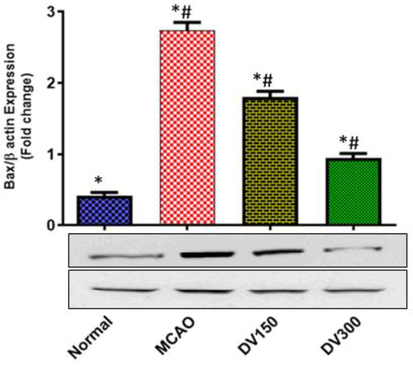
The effects of Dodonaea viscosa extract on BAX level in the brain of different groups. Statistical analysis was done using one-way ANOVA followed by post-hoc Tukey test. Values represent means ± (SEM) of each group, *P < 0.05 compared to normal group, #P < 0.05 compared to MCAO group.
3.2.2.3 Bcl-2
Expression of anti-apoptotic Bcl-2 protein was significantly downregulated in the brain of the MCAO group compared to the normal group of rats, (P < 0.05). On the other hand, the Bcl-2 expression was significantly upregulated in the brain of DVME pretreated (150 and 300 mg/kg/d) groups compared to the MCAO group of rats, (P < 0.05). DVME pretreatment groups showed a marked Bcl-2 overexpression, both low-dose and high doses (300 mg/kg/d) exhibited Bcl-2 overexpression, approximately 142.04% and 199.43% (Fig. 9).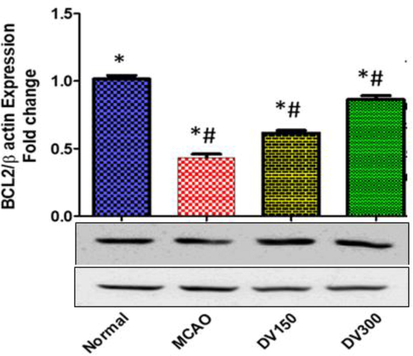
The effects of Dodonaea viscosa extract on Bcl-2 level in the brain of different groups. Statistical analysis was done using one-way ANOVA followed by post-hoc Tukey test. Values represent means ± (SEM) of each group, *P < 0.05 compared to normal group, #P < 0.05 compared to MCAO group.
It has been established that acute cerebral I/R injury may initiate pathological processes including glutamate release, calcium overload, and inflammatory response, all of which can contribute to neuronal apoptosis (Shao et al., 2020). Apoptosis (programmed cell death) stimulates the apoptotic-related protein cascade within apoptotic cells, which plays a key role in the etiology of cerebral ischemia–reperfusion injury (Ighodaro et al., 2018). Specific apoptosis proteins were measured and determined in cerebral I/R insult and suggested the occurrence of several apoptosis pathways involving extrinsic and intrinsic apoptotic pathways (Berghian et al., 2012). During the intrinsic pathway, the mitochondria under the effect of oxidative stress release Cyt c which interacts with procaspases leading finally to activation of caspase-3 which induces the occurrence of apoptosis. Extrinsic pathway depends on the activation of cell surfaces death receptors such as Fas receptor and TNF-receptor, resulting in the activation of caspase-3. While intrinsic apoptotic pathways are regulated by the Bcl-2 family including Bcl-2 and Bax, a group of mitochondrial proteins (Ahmad et al., 2019).
Caspase-3 is a member of the cysteine protease family playing a critical role in apoptosis. Several previous studies have shown that caspase-3 is a major effector in the apoptosis process triggered by cerebral ischemia (Allan and Rothwell, 2001). In the mammalian brain, caspase-3 serves as a cell death mediator that is upregulated during hypoxia suggesting the possible benefits of Caspase-3 signaling pathway suppression to cataract the cell death during cerebral I/R injury (Dawes et al., 2018). Our result showed that expression of Caspase-3 was upregulated in the MCAO group vs normal group, but in the DVME pretreated group (150 and 300 mg/kg/d), it was markedly downregulated in a dose-dependent manner in comparison with the MCAO group suggesting the inhibition of the apoptotic cell death. This finding was compatible with a previous report that stated the neuroprotective activity of the Sapindus laurifolia, (Sapindaceae) family, through attenuation of apoptotic markers such as caspase-3 in neurodegenerative diseases (Wytrykowska et al., 2016).
Bcl-2 is an anti-apoptotic protein that inhibits apoptosis and enhances cell survival, Bcl-2 overexpression inhibits neural cell death by stabilizing of mitochondrial membrane preventing the release of Cyt C that activates executive caspase-3 protein. A previous study demonstrated the relationship between Bcl-2 overexpression and the neuroprotective effect against hypoxia-induced cerebral injury (Han et al., 2002). Our findings clearly depict that the MCAO-induced Bcl-2 downregulation was significantly contracted by DVME Pretreatment (150 and 300 mg/kg/d) and suggested that Bcl-2 protects the brain tissues against apoptotic cell death. This result agreed with the previously published (Nogawa et al., 1997).
BAX is a potent pro-apoptotic protein. It plays role in the regulation of programmed cell death. It has been established that during hypoxia-induced stress, BAX expression is upregulated and translocated from the cytosol to the mitochondria enhancing the release of Cyt C and activation of terminal caspases. While interrupted Bax-mediated apoptosis pathway may protect against ischemic neuronal injury (Fonnum, F., 1984). Our study showed a significant elevation of BAX expression in the MCAO group vs the normal group. However, BAX expression was significantly downregulated in dose-dependent in DVME pretreated groups (150 and 300 mg/kg/d) enhancing brain cell survival against I/R stress. These findings were compatible with a previous study that evaluated the neuroprotective effect of pinocembrin, a flavonoid isolated from a variety of plants like D. viscosa, against ROS-induced neurotoxicity through downregulation of BAX expression preventing apoptosis and enhancing cell survival (Pellerin and Magistretti, 1994). It Has been demonstrated that cerebral I/R induces the expression of BAX and caspase-3 while downregulating BcL- expression aggravating neural cell apoptosis and infarct volume in brain tissues, However, herbal drugs that counteract the endogenous apoptotic proteins and showing marked improvement in infarction volume may protect the brain cells against cerebral ischemia (Nogawa, et al., 1997).). In our study, DVME showed ant-apoptotic potential activity through upregulating Bcl-2 expression and down regulation Bax expression and caspase-3 activation suggesting the increased livability of the viable of brain cells at risk after focal cerebral I/R injury, reducing the infarct volume and prevent further ischemic injury (Nogawa, et al., 1997).
4 Conclusion
The structure of the new compound was elucidated as (6S, 9R)-vomifoliol-9-β-D-glucopyranosyle (1‘‘-3‘)-O-α-L-rhamnopyranoside (DVE-8) while the structures of the known compounds were elucidated as viscosine (DVE-12), and catechin (DVE-18). All compounds were characterized on the basis of spectroscopic analysis and by comparing literature data. Generally, this study may suggest that the isolated compounds could be responsible for the neuroprotective effect of D. viscosa leaves extract mediated through anti-inflammatory and anti-apoptotic mechanisms. The levels of proinflammatory cytokines were significantly reduced (TNF-α and IL-6; both P < 0.05), and NF-κB gene expression was significantly downregulated (P < 0.05). Furthermore, the level of anti-apoptotic Bcl–2 gene expression was significantly upregulated (P < 0.05), whereas the level of BAX and caspase–3 gene expression were significantly downregulated (P < 0.05). Therefore, the neuroprotective effect of D. viscosa leaves extract on MCAO-induced ischemic stroke may be due to the anti-inflammation, and anti-apoptosis activities. The findings of the present study may suggest that D. viscosa leaves extract supplementation may serve as valuable adjuvant therapy in the treatment of ischemic stroke due to its neuroprotective effects. However, further studies may be required to explore some new leads and the isolated new compounds from D. viscosa leaves extract and their involvement in neuroprotection through different possible mechanisms and pathways.
Funding Information
This research work was supported by the Agency for Research and Innovation in the Ministry of Education, Saudi Arabia, Project # IFKSURG-2-1347.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project no IFKSURG-2-1347.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmad, M.S., Batool, S., Islam, A., Jabeen, A., Noureen, A., Shamshad, S., Zainab, T., Shahid, M., Ahmad, W., 2021. Neurological Disorders: Biochemistry of Drug Resistance and Future Challenges. Biochemistry of Drug Resistance. 255-77. Springer, Cham. https://doi.org/10.1007/978-3-030-76320-6_9.
- Acetyl-11-keto-β-boswellic acid (AKBA) Attenuates Oxidative Stress, Inflammation, Complement Activation and Cell Death in Brain Endothelial Cells Following OGD/Reperfusion. Neuromolecular Med.. 2019;21(4):505-516.
- [CrossRef] [Google Scholar]
- An Updated Phyto-pharMCAOlogical Review on Medicinal Plant of Saudi Arabia- Dodonaea viscoa Linn. American Journal of Research Communication.. 2013;1(12):519-531.
- [Google Scholar]
- Cytokines and acute neurodegeneration. Nat Rev Neurosci.. 2001;2(10):734-744.
- [CrossRef] [Google Scholar]
- A review on Dodonaea viscosa: A potential medicinal plant. International Organization of Scientific Research Journal of Pharmacy.. 2017;7(2):10-21.
- [Google Scholar]
- Bähr, M. (Ed.), 2006. Neuroprotection: models, mechanisms and therapies. John Wiley & Sons. ISBN: 978-3-527-60386-2 .
- Berghian, A.C., Moldovan, R., Decea, N., Tache, S., 2012. Oxidant/Antioxidant Balance in Carnitine Supplemented Rats Exposed to Chronic Hypothermic Stress. Veterinary Medicine. 69(1–2):42–8. http://journals.usamvcj.ro/veterinary.
- Activated Microglia Contribute to the Maintenance of Chronic Pain after Spinal Cord Injury. J. Neurosci.. 2006;26(16):4308-4317.
- [CrossRef] [Google Scholar]
- (6S)-Hydroxy-3-oxo-α-ionol glucosides from Capparis spinosa fruits. Phytochemistry. 2002;59(4):451-547.
- [CrossRef] [Google Scholar]
- Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology. 1984;34(3):315.
- [CrossRef] [Google Scholar]
- The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology. 2015;48:68-76.
- [CrossRef] [Google Scholar]
- Human neural stem cell-derived neuron/astrocyte co-cultures respond to La Crosse virus infection with proinflammatory cytokines and chemokines. J. Neuroinflammation. 2018;15(1):1-15.
- [CrossRef] [Google Scholar]
- The global burden of neurological disorders: translating evidence into policy. The Lancet Neurology.. 2020;19(3):255-265.
- [CrossRef] [Google Scholar]
- Franklin, S., 2017. The peripheral and central nervous system. In: Conn’s Translational Neuroscience. ACADEMIC Press, 113–29. https://doi.org/10.1016/B978-0-12-802381-5.00007-5.
- Isoprenylated flavonoids and clerodane diterpenoids from Dodonaea viscosa. Natural Products and Bioprospecting. 2013;3:250-325.
- [CrossRef] [Google Scholar]
- Sesamol attenuates oxidative stress, apoptosis and inflammation in focal cerebral ischemia/reperfusion injury. Exp. Ther. Med.. 2017;14(1):841-887.
- [Google Scholar]
- Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res.. 2006;1092(1):36-46.
- [CrossRef] [Google Scholar]
- Influence of Mild Hypothermia on Inducible Nitric Oxide Synthase Expression and Reactive Nitrogen Production in Experimental Stroke and Inflammation. J. Neuroscience.. 2002;22(10):3921-4398.
- [CrossRef] [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine.. 2018;54(4):287-293.
- [CrossRef] [Google Scholar]
- An update on the role of nitric oxide in the neurodegenerative processes of parkinson’s disease. Curr. Med. Chem.. 2016;23(24):2666-2679.
- [CrossRef] [Google Scholar]
- Neuroprotection against superoxide anion radical by metallocorroles in cellular and murine models of optic neuropathy. J. Neurochemistry.. 2010;114(2):488-498.
- [CrossRef] [Google Scholar]
- Molecular docking of viscosine as a new lipoxygenase inhibitor isolated from Dodonaea viscosa. Bangladesh Journal of Pharmacology. 2013;8(1):36-39.
- [CrossRef] [Google Scholar]
- Protein analysis through Western blot of cells excised individually from human brain and muscle tissue. Anal. Biochem.. 2012;425(2):120-214.
- [CrossRef] [Google Scholar]
- Middle cerebral artery occlusion methods in rat versus mouse models of transient focal cerebral ischemic stroke. Neural Regen. Res.. 2014;9(7):757-778.
- [CrossRef] [Google Scholar]
- Transient Middle Cerebral Artery Occlusion with an Intraluminal Suture Enables Reproducible Induction of Ischemic Stroke in Mice. Bio-protocol. 2022;12(3):e4305.
- [Google Scholar]
- Caspase inhibitors reduce neuronal injury after focal but not global cerebral ischemia in rats. Stroke. 2000;31(1):176-180.
- [CrossRef] [Google Scholar]
- Lo, E.H., Dalkara, T., Moskowitz, M.A., 2003. Mechanisms, challenges and opportunities in stroke. Nat. Rev.Neurosci. 2003; 4(5):399–414. doi: 10.1038/nrn1106.
- Martin-Villalba, A., Herr, I., Jeremias, I., Hahne, M., Brandt, R., Vogel, J., Schenkel, J., Herdegen, J., Debatin, K.M., 1999. CD95 Ligand (Fas-L/APO-1L) and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Mediate Ischemia-Induced Apoptosis in Neurons. J. Neurosci. 1999, 15(9), 3809-17. doi: 10.1523/JNEUROSCI.19-10-03809.1999.
- Mata, R., Contreras, J. L., Crisanto, D., Pereda- Miranda, R., Castaneda, P., Rio, F. D., 1991. Chemical studies on mexican plants used in traditional medicine, XVIII. New secondary metabolites from Dodonaea viscosa. J. Nat. Prod. 1991; 54(3):913–17. https://doi.org/10.1021/np50075a033.
- Matsuda, N., Isawa, K., Kikuchi, I., 1997. Megastigman glycosides from Lonicera gracilipes var. glandulosa. Phytochemistry. 1997; 45(4):777–79. https://doi.org/10.1016/S0031-9422(97)00045-9.
- Biologically Active C-Alkylated Flavonoids from Dodonaea viscosa. Arch. Pharm. Res.. 2012;35(3):431-446.
- [CrossRef] [Google Scholar]
- Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta.. 2013;1833(12):3448-3459. Epub 2013 Jun 13
- [CrossRef] [Google Scholar]
- Cyclo-Oxygenase-2 Gene Expression in Neurons Contributes to Ischemic Brain Damage. J. Neurosci.. 1997;17(8):2746-2755.
- [CrossRef] [Google Scholar]
- Anti-inflammatory Activity of Hautriwaic Acid Isolated from Dodonaea viscosa Leaves. Molecules. 2012;17(4):4292-4429.
- [CrossRef] [Google Scholar]
- Pellerin, L., Magistretti, L.P., 1994. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci., USA. 1; 91(22):10625–9. doi: 10.1073/pnas.91.22.10625.
- Acute stroke intervention: a systematic review. JAMA. 2015;313(14):1451-1462. PMID: 25871671 Review
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of Dodonaea viscosa leaves. International Journal of Research and Analytical Reviews.. 2019;6(2):223-225. www.ijrar.org
- [Google Scholar]
- Rasool, M., Malik, A., Qureshi, M. S., Manan, A., Pushparaj, P.N., Asif, M., Qazi, M. H., Qazi, A. M., Kamal, M. A., Gan, S. H., Sheikh, I. A., 2014. Recent updates in the treatment of neurodegenerative disorders using natural compounds. Evid.Based Complement. Alternat. Med., Article ID 979730, 7 pages, DOI: 10.1155/2014/979730.
- Molecular mechanisms of ischemia–reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol.. 2013;47(1),9–23.PMID:23011809.
- [CrossRef] [Google Scholar]
- Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis.. 2020;11(6):1537.
- [CrossRef] [Google Scholar]
- Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol.. 2001;428(2):185-192.
- [CrossRef] [Google Scholar]
- Acute hemorrhagic stroke pathophysiology and medical interventions: blood pressure control, management of anticoagulant-associated brain hemorrhage and general management principles. Neurol. Clin.. 2008;26(4):963-985.
- [CrossRef] [Google Scholar]
- Inflammation, Oxidative Stress, and Cerebral Stroke: Basic Principles. Advancement in the Pathophysiology of Cerebral Stroke, World J. of. Stem Cells 2019:11-21.
- [CrossRef] [Google Scholar]
- Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke. 2004;35(9):2189-2194.
- [CrossRef] [Google Scholar]
- Apoptosis regulation in the penumbra after ischemic stroke: expression of pro-and antiapoptotic proteins. Apoptosis. 2019;24(9–10):687-702.
- [CrossRef] [Google Scholar]
- CNS activity of ethyl acetate extract of stem bark of Dodonaea viscosa Linn. Indian J. Tradit. Knowl.. 2019;18(1):184-189.
- [Google Scholar]
- New megastigmane diglycoside from Litsea glutinosa (Lour.) CB Rob. J. Braz. Chem. Soc.. 2011;22(11):2234-3228.
- [CrossRef] [Google Scholar]
- Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health.. 2003;27(4):277-284. PMID: 15540798; PMCID: PMC6668865
- [Google Scholar]
- IL-1β, TNF-α, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J. Oral Sci.. 2016;58(4):509-513.
- [CrossRef] [Google Scholar]
- Punicalagin attenuated cerebral ischemia–reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol. Cell. Biochem.. 2015;402(1–2):141-218.
- [CrossRef] [Google Scholar]
- Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J. Neurochem.. 2003;85(4):1026-1036.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102704.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







