Translate this page into:
Isolation and characterisation of Streptomyces sp. Strain GLD25 with antimicrobial and antioxidant effects from Gueldaman cave (GLD1), Akbou-Algeria
⁎Corresponding authors. fatimazohra.djebbah@univ-tlemcen.dz (Fatima Zohra Djebbah), naldhabi@ksu.edu.sa (Naif Abdullah Al-Dhabi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The present study aimed to isolate potential antimicrobial and antioxidant Streptomyces sp from cave areas of Algeria.
Methods
Streptomyces sp.GLD25 was identified by biochemical, physiological and molecular analysis. The antimicrobial activity has been carried out by well diffusion method, microplates method has been used to perform the MIC values of the organic extracts. The antioxidant capacity of the extract was tested by DPPH and FRAP methods. GC–MS analysis was used to study the chemical profile of the crude active extract.
Results
The novel strain Streptomyces sp. GLD25 was isolated from Gueldaman cave in Algeria showed potential antioxidant and antimicrobial properties. According to the results of morphological, biochemical characteristics and complete 16S rRNA sequence, the strain revealed 99.71% of similarity with Streptomyces cyaneofuscatus strain WKFF71. The significant inhibition zone of the crude extract and MIC value were 30 mm and 0,015 mg/mL respectively against Staphylococcus aureus and Bacillus subtilis. In addition, the extract showed good antioxidant activity with IC50 = 20,12 mg/mL ± 2,05 for DPPH and IC50 = 8,80 mg/mL ± 0,2 for ferric reducing activity. The fundamental metabolites of the active extract were identified using GC–MS were Diisooctyl-phthalate, 6-hydroxy-heptanoic acid, Hexadecanoic acid, Benzeneacetic acid and 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid.
Conclusion
Overall, the Streptomyces sp.GLD25 isolated from Gueldamen cave have the potential to produce antimicrobials and antioxidants compounds with important biotechnological and medical applications.
Keywords
Streptomyces
Cave
Antimicrobial
Antioxidant
GC–MS
1 Introduction
The increase of resistance of pathogenic microorganisms against known drugs highlights the necessity for novel compounds with antimicrobial activity (Rashad et al., 2015). In addition, oxidative damages to lipids, proteins and DNA that may eventually lead to several complications including cardiovascular disease, aging, inflammatory disease mutations, carcinogenesis, and neurodegeneration are caused by reactive oxygen species and other radicals potentially oxidative (Tan et al., 2017). Actinomycetes are groupe of Gram positive, aerobic and filamentous bacteria. They constitute prolific sources of vital bioactive compounds which have a significant role in resolving public health (Hui et al., 2021). Especially, the genus Streptomyces produces >70% of all described natural products from actinomycets. Recently, studies have concentrated on exploring actinomycetes that thrive in extreme conditions to unearth their valuable bioactive metabolites for natural product drug discovery (Hui et al., 2021). However, over the past decade, caves have attracted the interest of microbiologists because of the diversity of bioactive metabolites produced by their mirobiom (Rangseekaew and Pathom-Aree, 2019). The inadequacy of energy in caves causes a complex interaction between different microorganisms, the result of these interactions is the production of metabolites, such as antibiotics, pigments, and siderophores (Gabriel and Northup, 2013). Furthermore, different groups of microorganisms are naturally present in caves sediments, and among heterotrophic bacteria actinomycetes are governing. Actinomycetes are Gram-positive bacteria despite they have the similar characteristics with bacteria and fungi. However, their reproduction is generally by producing conidia or spores, or even with binary fission. Moreover, Their DNA contains a cytosine and guanine with high percentage (Rai et al., 2018). Furthermore, the genus Streptomyces produce various secondary metabolites with varying biological activity, and GC–MS analysis is used to facilitate chemical profiling and to identify these active compounds (Law et al., 2017). The objective of this work was to isolate and characterize actinomycetes from Guldamen (GLD1) cave, Akbou, Algeria, and study the antimicrobial activity.
2 Materials and methods
2.1 Isolation of actinomycetes
For the isolation of Streptomyces sp. GLD25, different sediments samples have been collected aseptically and randomly from various locations of the cave regions of Algeria. The collected samples were suspended using sterile distilled water and further the diluted samples were spread on ISP-2 medium (pH = 7 ± 0,2) contained 30 μg/ml of nystatin, 50 μg/ml of cycloheximide, and 30 μg/ml nalidixic acid and incubated at 30° C for 1–4 weeks in a humidity and dark.
2.2 Morphological, physiological and biochemical characterization of actinomycetes
To study the morphological characteristics of the strain, the strain was cultivated in ISP1, ISP2, ISP 3, ISP 4, ISP 5, ISP6, ISP7, ISP9 and GYP media, the incubation was at 30 °C for 14 day. The Gram staining was performed using Gram's reaction as described by (Coico, 2006). The detailed information of the media composition is presented in the supplementary files. Further to determine the growth pattern of the strain using different temperature (25, 35, 45 and 55 °C), NaCl tolerance [3, 5, 7 and 10% (w/v)] and different pH (5, 7, 9, 10) was noted by growing on Bennett’s medium for 14 days. The ability of the strain to use starch, casein, gelatin, glucose, lactose, saccharose, citrate, the coagulation of milk, nitrate reduction, the production of urease, indole and H2S have been examined as described in Bergey’s manual of bacteriology (Goodfellow et al., 2012).
2.3 Molecular identification of the isolate
2.3.1 DNA extraction, amplification and sequencing
According to GenJET Genomic DNA purification kit manufacturer’s instructions, total genomic DNA of the isolate has been extracted. The amplification of the 16S rRNA gene was carried out using PCR using bacterial universal primers revers and forward 27F-5′AGAGTTTGATCMTGGCTCAG3′ and 1492R-5′TACGGYTACCTTGTTACGACTT3′. The thermal conditions of PCR reaction were as follows: initial denaturation at 94 °C (5 min), followed by 35 cycles at 94 °C (30 s), annealing at 52 °C (30 s), elongation at 72 °C (40 s), and 72 °C (10 min) as final extension (Al-Dhabi et al., 2019a).
2.4 In vitro antimicrobial activity
2.4.1 Preliminary screening and optimization of media and metabolites production
For the preliminary screening, bacterial and fungal suspension were prepared with final concentrations as 108 CFU/mL and 106 CFU/mL respectively. The isolate was inoculated on Petri plates containing Bennett’s medium (7 ± 0,2) for 8 days at 28 ± 2 °C. (Messaoudi et al., 2015).
For media optimization the strain has been inoculated on Petri plates containing GLM, M2 (Wiliams medium), AF and Bennett’s media with pH adjusted at 7 ± 0.2 then incubated for 8 days at 28 ± 2 °C. After incubation, a cylinders of the strain from each medium with diameter of 6 mm have been made using cork borer and placed immediately on Mueller Hinton inoculated with bacterial suspension (108 CFU/ml) and Sabouraud with fungal suspension (106 CFU/ml), as positive control Streptomycin (10 µg/disc) and Nystatin (100 μg/disc) were used.
2.4.2 Extraction of antimicrobial metabolites
The procedure used to obtain the crude extract was solid state fermentation (SSF). The GLD25 strain has been grown on Bennett’s agar medium selected previously based on the results of optimization media. After incubation at 28 ± 2 °C for 8 days, small parts of the media containing mycelium were cut and mixed with ethyl acetate, the mixture subjected a maceration overnight then filtration. The obtained filtrate was evaporated using rotavaporator at 45 °C to give a crude extract, which was stored at 4 °C for antioxidant, antimicrobial and chemical profiling of the extract (Leulmi et al., 2019).
2.4.3 Well diffusion method
Sixty mili grams of the crude extract was suspened in 500 μL of DMSO. Further, the suspension of bacteria with 108 CFU/mL as a final concentration and fungi with 106 CFU/mL) was prepared and swabbed on Mueller Hinton agar and Sabouraud agar respectively (Al-Dhabi et al., 2016).
2.4.4 Minimum inhibitory concentration (MIC)
In 96 well microplates, the MIC values have been carried out via the micro-dilution process (Al-Dhabi et al., 2019b; Al-Dhabi et al., 2020).
2.5 Antioxidant potential of the crude extract
2.5.1 DPPH free radical scavenging assay
The antioxidant activity of the crude extract of the GLD25 was determined by DPPH (2,2-diphenyl-1-picrylhydrazyl) assay following the method described by (Sanchez-Moreno et al., 1998). The assay was done in triplicate and the percentage of DPPH radical scavenging activity was calculated according to the following formula:
The value of the concentration providing 50% inhibition (IC50) was graphically determinate using a logarithmic regression extrapolated to 50% inhibition unit.
2.5.2 Ferric reducing assay (FRAP)
The ferric reducing is method using for the determination of the antioxidant power of compounds. It is based to the ability of the drugs to reduce the Fe3+ to Fe2+. The FRAP assay was performed as described by (Rezanejad et al., 2020). The IC50 of the extract was determined by a linear regression extrapolated to 0.5 absorbance unit.
2.6 Identification of major metabolites using (GC/MS) analysis
The crude extract subjected analysis by GC–MS for the characterization of the major metabolites using Shimadzu GC MS-QP2010 with capillary column. The helium gas has been used to eluate the sample. The injection temperature was 260 °C and temperature program was 60–280 °C, the injection volume was 1 µL with the flow rate of 1 ml/1 min, and the linear velocity: 36,5cm/sec. NIST 11 library was used to compare the compounds (Al-Dhabi et al., 2019b).
2.7 Statistical analysis
All the experiments were performed in triplicate. The experimental data were calculated by mean ± standard deviation (SD).
3 Results
3.1 Isolation and identification of Streptomyces sp. GLD25
3.1.1 Morphological, physiological and biochemical characterizations
The strain Streptomyces sp. GLD25 was isolated on ISP2 medium at 28 ± 2 °C in a dark and humidity. It was Gram-positive and recognized with formation of areal and vegetative mycelium, sporulation, and dry texture of the colonies. The strain contained spores and had generally abundant growth on all media except on ISP9 which was moderate, the color of aerial and substrate mycelium was generally white or beige and did not produce pigments Table 1, Fig. 1. However, physiological and biochemical characters exhibited that the optimal conditions for the growth of the strain were 25 °C, pH = 7 ± 0, 2, and 5% NaCl (W/V). The strain was catalase positive and had the ability to use: citrate, starch, casein, gelatin, urea and glucose as described on Table S1. -: absent; +: moderate; ++: good; +++: significant.
Character
ISP1
ISP2
ISP3
ISP4
ISP5
ISP6
ISP7
ISP9
GYP
Growth
+++
+++
++
+++
+++
+++
+++
+
+++
Color of substrate mycelium
White
Beige
Grey
White
Olive green
Beige
Beige
White
Beige
Color of aerial mycelium
White
Beige
Beige
Beige
White
Beige
Beige
White
White
Soluble pigments produced
–
–
–
–
–
–
–
–
–
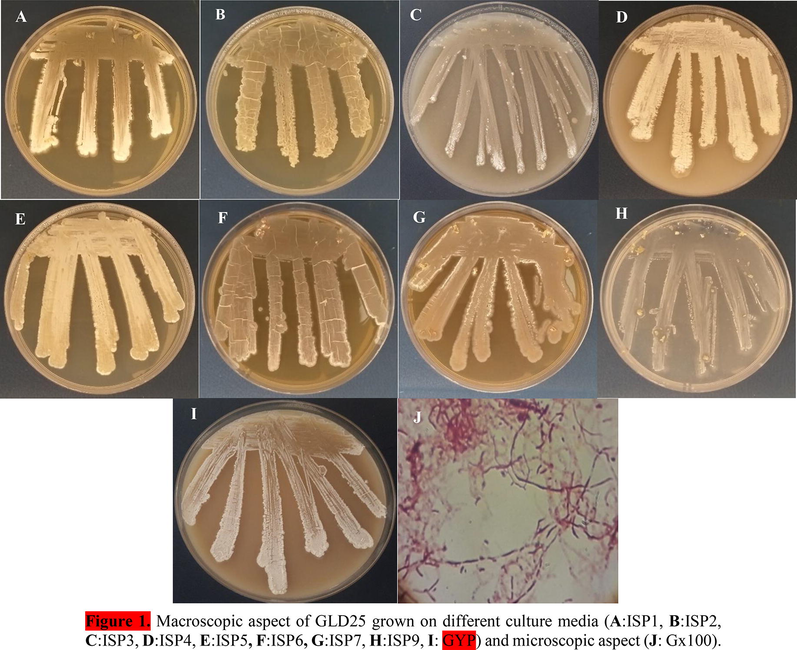
Macroscopic aspect of GLD25 grown on different culture media (A:ISP1, B:ISP2, C:ISP3, D:ISP4, E:ISP5, F:ISP6, G:ISP7, H:ISP9, I: GYP) and microscopic aspect (J: Gx100).
3.1.2 Phylogenetic analysis of the isolate
The 16S rRNA gene sequence of the strain was deposited in Gene bank under the accession number MW375071.1 belong to Streptomyces and present highest similarity towards (99.71%) Streptomyces cyaneofuscatus strain WKFF7 (Accession number KY630731.1) and 99.64% with Streptomyces pratensis strain TU32 (Accession number MH482882.1) and Streptomyces lavendulae strain T10 (Accession number KY213666.1) (Fig. 2).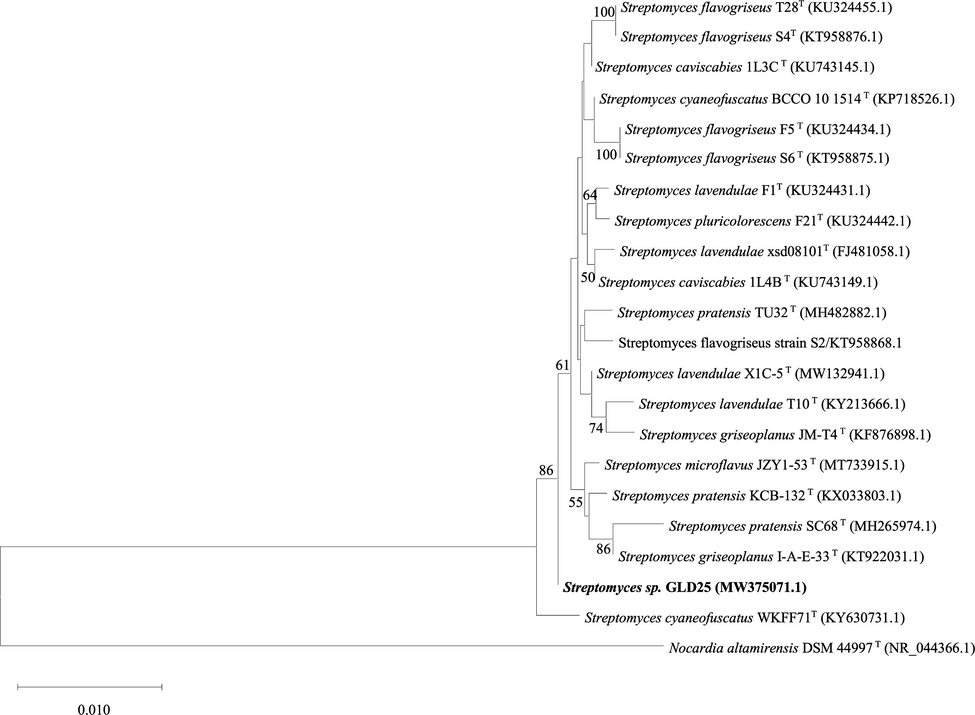
The 16S rRNA gene sequence analysis showing the relationship of Streptomyces sp. GLD25 with other closest species using MEGA X.
3.2 Antimicrobial activity
Preliminary screening results exhibited that the strain had a significant antimicrobial potential. The optimization of media showed that Bennett is the most appropriate medium to produce antimicrobial compounds (Table S2). However, the ethyl acetate extract obtained by solid state fermentation (SSF) had a good activity against: Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, Candida albicans and Fusarium oxysporum (Table S3 and Fig. 3). The minimum inhibitory concentration of the active extract revealed that the significant value was 0,015 mg/mL facing Bacillus subtilis and Staphylococcus aureus whereas the value was >2 mg/mL facing Escherichia coli, Proteus vulgaris, Salmonella typhimurium, Klebsiella pneumoniae and Escherichia coli ESBL as mentioned in Table 2. On the other hand, the strain did not exhibit any antifungal activity.
Antimicrobial activity using well diffusion method of the extract against: A: Staphylococcus aureus, B: Bacillus subtilis, C: Bacillus cereus.
Tested microbes
MIC of crude extract mg/mL
MIC of Streptomycin µg/well
Staphylococcus aureus (ATCC 6538P)
0,015
>60
Staphylococcus micron
>2
1.87
Bacillus subtilis
0,015
1.87
Escherichia coli (ATCC 10536)
>2
>60
Klebsiella pneumoniae (ATCC 13882)
>2
0.46
Proteus vulgaris (ATCC 33420)
>2
3.75
Salmonella typhimurium (ATCC 13311)
>2
30
Escherichia coli ESBL (DR4345)
>2
>60
3.3 Antioxidant activity of the extract
Scavenging activity of the ethyl acetate extract was 65,87 ± 2,55 % at 30 mg/mL (Fig. 5.) and ascorbic acid as positive control with 98,75 ± 0% at 30 mg/mL. The IC50 value corresponding to the concentration required for 50% inhibition of the extract was 20,12 ± 2,05 mg/mL with 0,083 ± 0,011 mg/mL for the ascorbic acid (Fig. 4).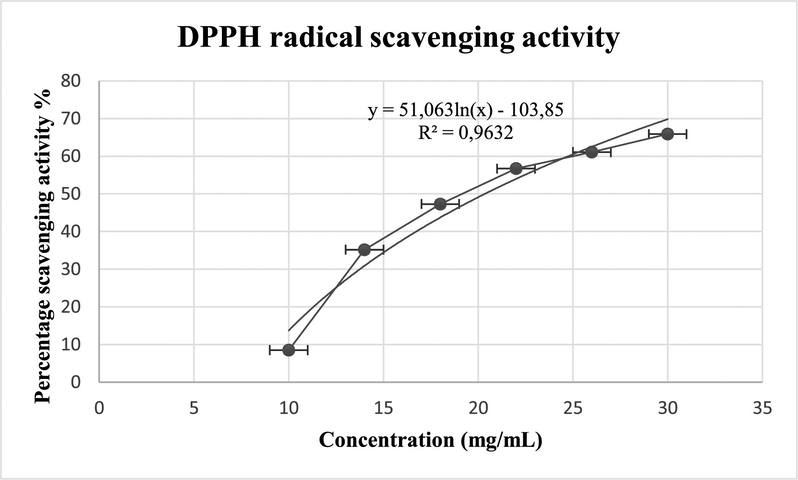
DPPH radical scavenging activity of the extract.
The ferric reducing results showed that the significant absorbance was 2, 75 ± 0 at 30 mg/mL, the IC50 of the extract is 8,80 ± 0,2mg/mL, and IC50 = 0,030 ± 0,008 mg/mL for the ascorbic acid. The results are presented in Fig. 5.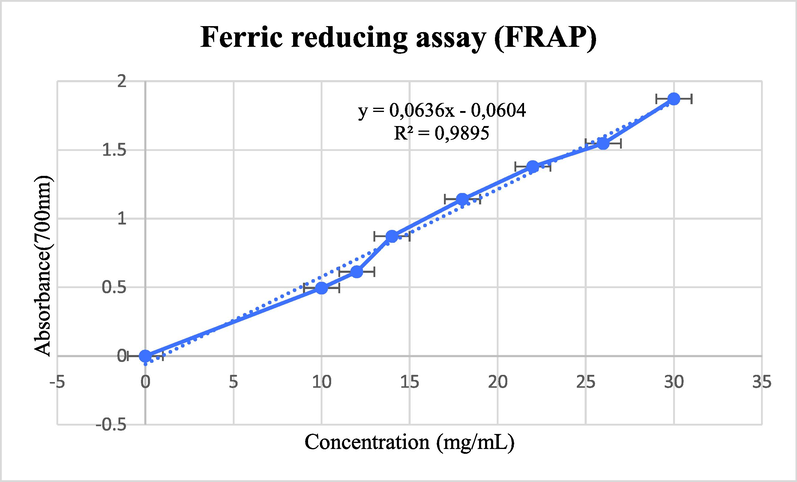
Ferric reducing activity (FRAP) activity of the extract.
3.4 GC–MS profile of crude extract
The gas chromatography-mass spectrometer analysis has shown the chemical profiling of ethyl acetate crude extract of Streptomyces sp. GLD25. The result showed that the extract contained twenty nine constituents which may be responsible to the biological activities. The major compounds were Diisooctyl-phthalate, 6-hydroxy-heptanoic acid, Hexadecanoic acid, Benzeneacetic acid, Stearinic acid and 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid respectively Table S4 and Fig S1.
4 Discussion
Karst caves have always been considered as distributed subsurface ecosystems, they are known to be oligotrophic environments, which are humid, dark with annual temperature relatively stable. However, their microbiomes have always been suggested as drivers for biogeochemical cycles and cave evolution since their diversity and their metabolites (Zhu et al., 2021). Hence, several studies have been carried out on the isolation of cave actinobacteria. The genus Streptomyces is the most producer of interesting secondary metabolites. Besides, the exploitation of unexplored environments as source of isolation of this genus is the most efficient approaches to discover bioactive drugs (Tan et al., 2017).
The selective strain was isolated on ISP2 with pH = 7 ± 0.2 at 28 ± 2 °C in humidity and dark. For the isolation of actinomycetes, a diversity of media was reported in several researches. ISP2 was efficiently used as an isolation medium for novel actinomycetes (Terra et al., 2018). The selective strain was isolated on ISP2, However, the isolation of actinobacteria depends not only on the media used, but also on other factors such as the time of incubation (Song et al., 2015), the factor of dilution (Pathom-aree et al., 2017), air-dried of samples which might be a basic step for selective isolation of actinomycetes since it helps to reduce unwanted (Rashad et al., 2015). Furthermore, the incubation of the actinomycetes in the dark is an intriguing factor to allow the production of metabolites and their diffusion into the agar (El‐tarabily et al., 1997).
The biological potentials of cave Actinobacteria have been studied by several works (Ghosh et al., 2020; Zhu et al., 2021). The active extract of the strain GLD25 showed a good antibacterial activity against Gram-negative and Gram-positive bacteria. In addition, a remarkable antifungal activity was noted. Therefore, antioxidant properties of the extract were also important as describes previously. However, the results of this study are consistent with previous results. The extract of Streptomyces sp. MUM212 demonstrated significant antioxidant activity through DPPH, ABTS and superoxide radical scavenging assays (Tan et al., 2017). Several complications in the body are associated with reactive oxygen species and different strategies have been used to discover potent natural antioxidants drugs. Further, it has been stated that Streptomyces sp. JS520 isolated from cave in Serbia exhibit antioxidant and antimicrobial activities (Stankovic et al., 2012).
GC–MS is a method used to detect and separate a volatiles elements present in small amounts, the active extract subjected a GC–MS has shown a variety of compounds some of them have been demonstrated for their biological activities by literature. Therefore, Diisooctyl phthalate (1,2-benzenedicarboxylic acid diisooctylester) which is a major compound of the extract with antibacterial activities against Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans (Chen et al., 2009). Additionally, Indole-2-carboxylic acid and its derivatives were reported to their biological activities especially antifungal (Kipp and Young, 1999), antioxidant and anticancer activity (Wang et al., 2017). Furthermore, Tetradecanoic acid is demonstrated for its repellent and larvicidal activity (Sivakumar et al., 2011). Erucylamide possess promising insecticidal activity, it can be used as fiber softener, pigment dispersant and in dyes (Ahmed et al., 2020). Moreover, Oleamide has been notified by histamine secretion attenuation, interleukin-4 and tumor necrosis factor production (Yang et al., 2016).
5 Conclusion
The present study highlighted the antimicrobial, antioxidant potential of Streptomyces sp. strain GLD25 isolated from cave and contributes to expanding our understanding of cave microorganisms. However, the active extract contains compounds to be further investigated. In conclusion, caves actinomycetes might be an imperative and promising source for the discovery of natural bioactive drugs.
Acknowledgements
Université Abou Bekr Belkaid Tlemcen, Algeria for its funding. Authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/20), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.) Sci. Rep.. 2020;10:522.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi. J. Biol. Sci.. 2019;26(4):758-766.
- [Google Scholar]
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles.. 2016;20(1):79-90.
- [Google Scholar]
- Chemical constituents of Streptomyces sp. strain Al-Dhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J. Infect. Public. Health.. 2020;13(2):235-243.
- [Google Scholar]
- Optimizing the Management of Cadmium Bioremediation Capacity of Metal-Resistant Pseudomonas sp. Strain Al-Dhabi-126 Isolated from the Industrial City of Saudi Arabian Environment. Int. J. Environ. Res. Public. Health.. 2019;16(23):4788.
- [Google Scholar]
- Identification and antibacterial activity of secondary metabolites from Taxus endophytic fungus. J. Biotechnol.. 2009;25(3):368.
- [Google Scholar]
- The potential for the biological control of cavity-spot disease of carrots, caused by Pythium coloratum, by streptomycete and non-streptomycete actinomycetes. New. Phytol.. 1997;137(3):495-507.
- [Google Scholar]
- Microbial ecology: caves as an extreme habitat.In. In: Cheeptham N., ed. Cave microbiomes: A novel resource for drug discovery. Springer Briefs in Microbiology; 2013. p. :85-108.
- [Google Scholar]
- Culture dependent analysis of bacterial diversity in Canada’s Raspberry Rising Cave revealed antimicrobial properties. Int. J. Speleol.. 2020;49(1):43-53.
- [Google Scholar]
- The Extremophilic Actinobacteria: From Microbes to Medicine. Antibiotics.. 2021;10(6):682.
- [Google Scholar]
- The soluble eumelanin precursor 5, 6-dihydroxyindole-2-carboxylic acid enhances oxidative damage in human keratinocyte DNA after UVA irradiation. Photochem. Photobiol.. 1999;70(2):191-198.
- [Google Scholar]
- Streptomyces colonosanans sp. nov., A Novel Actinobacterium Isolated from Malaysia Mangrove Soil Exhibiting Antioxidative Activity and Cytotoxic Potential against Human Colon Cancer Cell Lines. Front. Microbiol.. 2017;8:877.
- [Google Scholar]
- Enhanced production and quantitative evaluation of nigericin from the algerian soil-living Streptomyces youssoufiensis SF10 strain. Fermentation.. 2019;5(1):13.
- [Google Scholar]
- Identification and preliminary characterization of non-polyene antibiotics secreted by new strain of actinomycete isolated from sebkha of Kenadsa. Algeria. Asian. Pac. J. Trop. Biomed.. 2015;5(6):438-445.
- [Google Scholar]
- Dermacoccus abyssi sp. nov., a piezotolerant actinomycete isolated from the Mariana Trench. Int. J. Syst. Evol. Microbiol.. 2017;56(6):1233-1237.
- [Google Scholar]
- Actinomycetes: Isolation, characterization and screening for antimicrobial activity from different sites of Chitwan. Nepal. J. Microbiol. Biotechnol.. 2018;3(1):25.
- [Google Scholar]
- Cave actinobacteria as producers of bioactive metabolites. Front. Microbiol.. 2019;10:387.
- [Google Scholar]
- Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiol. Res.. 2015;175:34-47.
- [Google Scholar]
- Values of antioxidant activities (ABTS and DPPH) and ferric reducing and chelating powers of gamma-irradiated rosemary extract. Radiochim. Acta.. 2020;108(6):477-482.
- [Google Scholar]
- A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food. Agric.. 1998;76(2):270-276.
- [Google Scholar]
- Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera: Culicidae) Asian. Pac. J. Trop. Med.. 2011;4:706-710.
- [Google Scholar]
- Cytotoxic and antibacterial Angucycline- and Prodigiosin- analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar. Drugs.. 2015;13(3):1304-1316.
- [CrossRef] [Google Scholar]
- Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl. Microbiol. Biotechnol.. 2012;96:1217-1231.
- [Google Scholar]
- Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front.. 2017;pharmacol. 8:276.
- [Google Scholar]
- A Novel Alkaliphilic Streptomyces Inhibits ESKAPE Pathogens. Front. Microbiol.. 2018;9:2458.
- [Google Scholar]
- In Vitro DNA-Binding, Anti-Oxidant and Anticancer Activity of Indole-2-Carboxylic Acid Dinuclear Copper(II) Complexes. Molecules.. 2017;22(1):171.
- [Google Scholar]
- Antiallergic activity of ethanol extracts of Arctium lappa L. undried roots and its active compound, oleamide, in regulating FcεRI-mediated and MAPK signaling in RBL-2H3 cells. J. Agric. Food. Chem.. 2016;64(18):3564-3573.
- [Google Scholar]
- Zhu, H.Z., Zhang, Z.F., Zhou, N., Jiang, C.Y., Wang, B.J., Cai, L., Wang, H.M., Liu, S.J. 2021. Intensive Bacterial Cultivation and Genome Assembly Reveal Previously Unknown Bacteria and Metabolic Potential in Karst Caves. Appl. Environ. Microbiol.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101719.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







