Translate this page into:
Investigation of heavy metal exposure and trace element levels in acute exacerbatıon of COPD
⁎Corresponding authors at: Department of Emergency Medicine, Bozok University Faculty of Medicine, Yozgat, Turkey (V.A. Türksoy) and Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, and Department of Pharmacology & Toxicology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran (A. Eftekhari). v.aliturksoy@bozok.edu.tr (Vugar Ali Türksoy), eftekharia@tbzmed.ac.ir (Aziz Eftekhari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The study aims to evaluate the levels of trace and heavy metals among chronic obstructive pulmonary disease (COPD) patients with acute exacerbation and their impact on the severity and mortality of the disease.
Methods
100 healthy volunteers and 114 patients with acute exacerbation participated in this work. According to Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, COPD patients are divided into 4 groups. Analysis of heavy metals (lead, cadmium, arsenic, cobalt, nickel, mercury, aluminum, calcium, and manganese) and trace metals (copper, chromium, and zinc) was performed using a plasma mass spectrometer.
Results
Body mass index was lower in COPD exacerbation patients (p < 0.05) in comparison to the control. In comparison with the control group, the levels of heavy metals were greater in COPD cases (p < 0.001). Al, Ca, Co, Ni, Cu, As, Cd, and Hg levels of GOLD group 4 were found to be higher compared to GOLD Group 1 (p < 0.001). Likewise, the Mn level was found higher in GOLD Group 1 (p < 0.05). However, the level of Zn was lower in GOLD group 4 in comparison with GOLD 1 cases (p < 0.001). The factors for the prediction of the disease in the COPD patient group were determined using multivariate regression analysis. Al, Ca, Mn, Co, As, and Hg was determined to be independent risk factors in predicting COPD exacerbations (p < 0.05). Al, Co, Pb, Ni, Hg, and Cd levels were higher in COPD exacerbations that resulted in mortality (p < 0.05). Co, Cd, Hg, and Pb were determined to be independent risk factors for mortality in COPD exacerbation cases (p < 0.05).
Conclusions
Our study showed that serum heavy metal levels are linked with the harshness and mortality of acute COPD attacks. These findings may indicate that changes in serum heavy metal levels can be used to determine the severity of a COPD exacerbation.
Keywords
COPD
Heavy metal
Trace elements
Mortality
1 Introductıon
Due to its high prevalence, increasing incidence, and severe personal, social, and economic costs around the world, chronic obstructive pulmonary disease (COPD) is a serious public health concern (Vikjord et al., 2022; Halpin et al., 2019). When the disease burden and mortality due to COPD are examined, great differences are observed between countries and even between different social groups within the same country (Marmot et al., 2019). COPD has become a disease that can be seen in young people and women due to environmental exposures such as the health effects of air and environmental pollution (Agusti et al., 2020; Agustí et al., 2019). Despite the rapid advances in technology, our knowledge of the underlying pathobiological mechanisms of COPD is still limited (Dransfield et al., 2019). Biomarkers with potential benefits are needed in the prognosis of COPD exacerbation (Corradi et al., 2009). Prevıous works showed that heavy metals induce the pathogenesis of COPD through uncontrolled oxidative stress and chronic inflammation (Bertin et al., 2006; Cohen et al., 2002). It should also be noted that DNA repair and disruption of barrier mechanisms may also contribute to this process (Kirschvink et al., 2006). In one study in Korea, researchers found a notable link between obstructive lung disease and lead and cadmium concentrations in serum (Kim et al., 2015). In this work, we assessed the potential relationship between heavy metal levels (Al, Cd, Pb, Cr, Ca, Mn, Co, Ni, Cu, As, Hg, Zn) and the severity of attacks and lung function using the COPD GOLD classification. We also examined the relationship between mortality in COPD patients and heavy metal levels.
2 Materıal and method
2.1 Study design
The research is a descriptive study to evaluate the 114 patients with acute exacerbation form of COPD and 100 healthy subjects with no COPD with acute exacerbation (control group) who applied to Yozgat Bozok University Research Hospital Emergency Department. The participants aged between 18 years and over with COPD with acute exacerbation were included in the experimental group. The patient group was divided into 4 groups according to GOLD classification. 30-day mortality was determined through hospital records and the e-pulse system. The co-morbidities, active smoking, biochemical blood values , and heavy metal levels of the patients were recorded. COPD staging is divided into GOLD 1, GOLD 2, GOLD 3, and GOLD 4 classes according to the postbronchodilator (GOLD) FEV1 system classification, presenting with COPD exacerbation. An expected FEV1 in GOLD 1 after postbronchodilator, FEV1 ≥ 80 % indicates mild airflow limitation. In GOLD 2, the expected FEV1 50 % ≤ FEV1 < 80 % indicates moderate airflow limitation. In GOLD 3, a 30 % ≤ FEV1 < 50 % indicates severe airflow limitation, and in GOLD 4, an FEV1 < 30 % indicates serious airflow limitation. Control group includes participants without any COPD with acute exacerbation and any chronic disease. Medical histories, age, and gender were recorded. All subjects filled out a consent form to participate in this study. The permission for our work was received from the committee of clinical research ethics of Yozgat Bozok University (decision number 2017-KAEK-189_2021.12.29_01). Descriptive and sociodemographic characteristics such as age and gender were used as a data collection method.
2.2 Sample collection
Blood samples were collected for Lead (Pb), Mercury (Hg), Arsenic (As), Cadmium (Cd), Cobalt (Co), Nickel (Ni), Zinc (Zn), Copper (Cu), Aluminium (Al), Calcium (Ca), Manganese (Mn) and Chrome (Cr) levels measurements. 1 mL of each sample was moved to polypropylene tubes and we added 5 mL of nitric acid (Suprapur®, 65 %), 2 mL of hydrogen peroxide, and 3 mL of ultrapure water to samples, respectively. The tubes hold 24 h at room temperature for digestion samples and are completed with 20 mL of ultrapure water. We used Turksoy et al. developed method and optimized it for preparing the samples for analysis (Turksoy et al., 2019).
Laboratory analysis.
We used mass spectrometry with inductively coupled plasma (Thermo Scientific, USA) using 1550 W power, 0.86 L/min plasma gas, 0.95 L/min nebulizer gas, 2.99 bar nebulizer pressure, 3.4 °C spray chamber, and 0.01 ms dwell time to the measurement of twelve metals (Pb, Cd, As, Co, Ni, Hg, Zn, Cu, Al, Ca, Mn and Cr) in samples. The sampler probe was washed with the three steps between injections: (1) ultrapure water for 30 s (rinsing) and (2) nitric acid (2 %) for 45 s (washing), (3) ultrapure water for 45 s (rinsing). To determine the level of each metal, we used an 11-point calibration curve (0.1–250 µg/L). 0.9990 (for all metals) was found as the minimum r2 value in the calibration curves.
3 Validation of methods
We repeated the standard and sample measurements five times to increase the accuracy of the results and lower the relative standard deviation (<5%). We used Whole Blood L-1 Standard Reference Material (Seronorm™ Trace Elements, Norway) for the validation method. The 100 µg/L of Hafnium was used for the internal standard. The intra and inter-day precision of Standard Reference Materials based on the standard deviation of replicates was utilized for the quality control method.
3.1 Statistical analysis
The SPSS Statistics software was applied for data analysis (Chicago, IL, USA). We used descriptive statistics (frequencies, ratios, mean and median) and measures of distribution (standard deviation and minimum–maximum) for the demographic characteristics of the participants. Kolmogorov-Smirnov test was used to detemine normal distribution. We used Spearman or Pearson correlation analysis for relationships between groups. For anormal distributed data, we performed non-parametric tests (Mann-Whitney U and Kruskal Wallis). We used multivariate logistic regression analysis to specify the severity and mortality in COPD cases related risk factors and determine of type of heavy metals for independent risk factors.
4 Results
The study consisted of 100 healthy volunteer controls and 114 patients with acute exacerbation of COPD. Table 1 shows the demographic information of the COPD cases. BMI in COPD patients was lower in comparison with control group (p < 0.05). The levels of As, Al, Cd, Co, Ca, Cr, Mn, Ni, Cu, Hg, and Pb were higher in COPD patients (p < 0.001) (Table 2). The level of Zn was same with control (p = 0.489). The distribution of age, BMI, FEV, and vital values in COPD cases according to the GOLD classification is shown in Table 3. The distribution of heavy metal levels in COPD cases according to the GOLD classification is shown in Table 4. Especially, Al, Ca, Co, Ni, Cu, As, Cd and Hg levels of Group 4 were higher compared to Group 1 (p < 0.001). Likewise, the Mn level was found to be increased (p < 0.05). However, Zn levels in group 4 were lower than group 1 (p < 0.001). The predicting factors in the COPD patient group were determined using multivariate regression analysis. Al, Ca, Mn, Co, As, and Hg heavy metals were determined to be independent risk factors in predicting COPD exacerbations (p < 0.05) (Table 5, Fig. 1). The correlation analysis of heavy metals with mortality is shown in Table 6. Cd, Al, Co, Ni, Pb, and, Hg levels were increased in COPD exacerbations that resulted in mortality (p < 0.05). Co, Pb, Cd, and, Hg were determined to be independent risk factors for mortality in COPD cases (p < 0.05) (Table 7, Fig. 2). Data are presented as mean ± SD. p < 0.05 is significant. Hgb, hemoglobin; BMI, body mass index. Data are presented as mean ± SD. P < 0.05 is significant. Al, aluminum; Ca, calcium; Cr, chromium, Mn, manganese, Co, cobalt; Ni, nickel; Cu, copper; Zn, zinc, As arsenic; Cd cadmium; Hg, mercury; Pb, lead. There is no significant difference between groups containing the same letter (a, b and c). Data are presented as mean ± SD. p < 0.05 is significant. Hgb, hemoglobin; BMI, body mass index. There is no significant difference between groups containing the same letter (a, b and c). Data are presented as mean ± SD. p < 0.05 is significant. Al, aluminum; Ca, calcium; Cr, chromium,Mn, manganese, Co, cobalt; Ni, nickel; Cu, copper; Zn, zinc, As arsenic; Cd cadmium; Hg, mercury; Pb, lead. Data are presented as mean ± SD. p < 0.05 is significant. Al, aluminum; Ca, calcium; Cr, chromium, Mn, manganese, Co, cobalt; Ni, nickel; Cu, copper; As arsenic; Cd, cadmium; Hg, mercury; Pb, lead. Data are presented as mean ± SD. P < 0.05 is significant. Al, aluminum; Ca, calcium; Cr, chromium, Mn, manganese, Co, cobalt; Ni, nickel; Cu, copper; Zn, zinc, As arsenic; Cd cadmium; Hg, mercury; Pb, lead. Data are presented as mean ± SD. P < 0.05 is significant. Al, aluminum; Co, cobalt; Ni, nickel; Cd cadmium; Hg, mercury; Pb, lead.
Case
Control
n = 114
n = 100
Mean ± Std.
Mean ± Std.
p
Age
59.68 ± 7.07
59.4 ± 7.34
0.748
BMI
23.8 ± 2.07
24.87 ± 0.88
0.002
Hgb
11.96 ± 0.97
12.09 ± 1.19
0.41
n (%)
n (%)
p
Urban/ Rural
Urban
70 (61.4)
49 (49)
0.068
Rural
44 (38.6)
51 (51)
Case
Control
Mean ± Std.
Mean ± Std.
p
Al
5.01 ± 2.98
3.53 ± 0.58
< 0.001
Ca
60.15 ± 26.80
27.64 ± 11.18
< 0.001
Cr
9.34 ± 2.63
5.98 ± 4.24
< 0.001
Mn
10.62 ± 2.01
8.47 ± 0.94
< 0.001
Co
0.75 ± 0.22
0.53 ± 0.16
< 0.001
Ni
6.31 ± 4.11
3.98 ± 2.18
< 0.001
Cu
87.20 ± 33.61
40.01 ± 23.31
< 0.001
Zn
88.99 ± 61.47
106.34 ± 70.75
0.489
As
11.78 ± 4.67
6.54 ± 1.42
< 0.001
Cd
2.62 ± 0.93
1.81 ± 0.50
< 0.001
Hg
0.52 ± 0.52
0.30 ± 0.16
< 0.001
Pb
3.89 ± 1.78
3.08 ± 1.00
< 0.001
Group
G1
G2
G3
G4
Mean ± Std.
Mean ± Std.
Mean ± Std.
Mean ± Std.
p
Age
59.89 ± 7.88
59.57 ± 5.53
59.45 ± 7.5
59.83 ± 7.48
0.962
BMI
25.25 ± 0.67a
24.8 ± 0.61a
23.54 ± 1.25b
21.68 ± 2.67b
<0.001
Hgb
11.96 ± 0.85
11.95 ± 1.03
11.97 ± 1.07
11.97 ± 0.94
0.811
FEV
105.21 ± 12.9a
64.46 ± 7.35b
39.21 ± 5.19c
27.14 ± 3.5d
<0.001
Hearth Rate
94.36 ± 24.62
97.25 ± 25.13
101.72 ± 32.42
103.66 ± 28.12
0.367
Resp. Rate
19.75 ± 8.03a
21.61 ± 8.15a
24.24 ± 9.06a.c
29.79 ± 9.54b.c
<0.001
Group
G1
G2
G3
G4
Mean ± Std.
Mean ± Std.
Mean ± Std.
Mean ± Std.
p
Al
3.87 ± 1.18a
4.75 ± 2.6a,c
4.81 ± 1.03b,c
6.56 ± 4.79b
<0.001
Ca
35.85 ± 13.3a
59.21 ± 25.42b
71.01 ± 32.37b,c
73.67 ± 12.41c
<0.001
Cr
9.05 ± 3.21
9.64 ± 2.65
8.98 ± 2.68
9.67 ± 1.91
0.434
Mn
9.98 ± 1.35a
10.04 ± 1.1a,b
10.7 ± 1.24a,b
11.71 ± 3.12b
0.014
Co
0.56 ± 0.15a
0.69 ± 0.14b
0.8 ± 0.16b,c
0.96 ± 0.19c
<0.001
Ni
3.38 ± 1.59a
5.64 ± 3.12b
7.43 ± 5.77b
8.66 ± 2.68c
<0.001
Cu
60.17 ± 24.46a
87.1 ± 82.77b
91.93 ± 30.36b,c
108.67 ± 28.19c
<0.001
Zn
131.3 ± 54.1a
90.67 ± 62.98b
77.86 ± 70.32b
57.65 ± 26.77b
<0.001
As
7.6 ± 2.84a
11.44 ± 3.8b
13.08 ± 4.18b,c
14.86 ± 4.43c
<0.001
Cd
2.15 ± 0.6a
2.22 ± 0.58a
2.75 ± 0.78b,c
3.35 ± 1.13c
<0.001
Hg
0.39 ± 0.24a
0.46 ± 0.24a
0.59 ± 0.93a
0.64 ± 0.26b
<0.001
Pb
4.09 ± 2.51a,b
3.6 ± 1.9a
3.49 ± 0.87a,b
4.37 ± 0.94b
0.094
Mutlivariate regression
B
S.E.
P
OR
95 % C.I.for EXP(B)
Lower
Upper
Al
1.169
0.588
0.047
3.219
1.017
10.188
Ca
0.155
0.076
0.042
1.168
1.005
1.356
Cr
0.269
0.228
0.237
1.309
0.837
2.046
Mn
2.443
0.612
< 0.001
21.512
3.470
38.191
Co
−12.224
4.426
0.006
20.3
14.4
119
Ni
−0.208
0.128
0.104
0.812
0.632
1.044
Cu
0.010
0.042
0.817
1.010
0.930
1.096
As
1.139
0.410
0.006
3.122
1.397
6.978
Cd
1.087
0.816
0.183
2.964
0.599
14.670
Hg
7.277
3.317
0.028
4.400
2.174
9.600
Pb
0.711
0.397
0.073
2.036
0.936
4.430
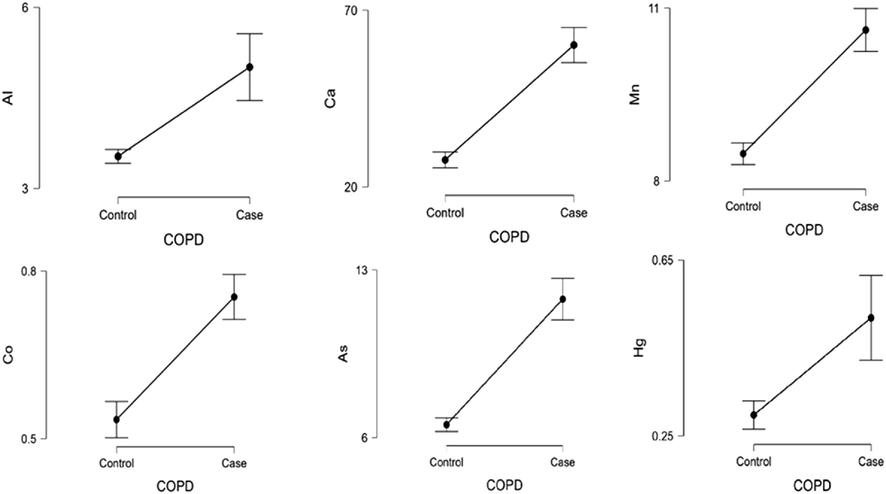
Mutlivariate regression plots in terms of predicting disease in COPD exacerbation patients.
Mortality
No
Yes
Mean ± Std.
Mean ± Std.
P
Al
4.72 ± 1.92
8.02 ± 7.62
0.013
Ca
59.72 ± 27.36
64.61 ± 20.61
0.258
Cr
9.19 ± 2.56
10.88 ± 3.04
0.090
Mn
10.41 ± 1.55
12.77 ± 4.15
0.109
Co
0.74 ± 0.21
0.94 ± 0.23
0.004
Ni
6.11 ± 4.21
8.39 ± 2.15
0.001
Cu
86.62 ± 32.46
93.19 ± 45.69
0.703
Zn
90.56 ± 62.10
72.64 ± 54.62
0.718
As
11.71 ± 4.47
12.52 ± 6.61
0.406
Cd
2.55±0.88
3.38 ± 1.14
0.013
Hg
0.50 ± 0.53
0.68 ± 0.37
0.042
Pb
3.80 ± 1.77
4.86 ± 1.68
0.019
B
S.E.
P
OR
95 % C.I.for EXP(B)
Lower
Upper
Al
0.52
0.27
0.054
1.7
1.0
2.8
Co
3.07
1.49
0.039
21.6
1.2
398.3
Ni
0.00
0.08
0.971
1.0
0.9
1.2
Cd
1.13
0.37
0.002
3.1
1.5
6.3
Hg
2.82
1.14
0.014
16.7
1.8
156.0
Pb
0.61
0.19
0.001
1.8
1.3
2.7
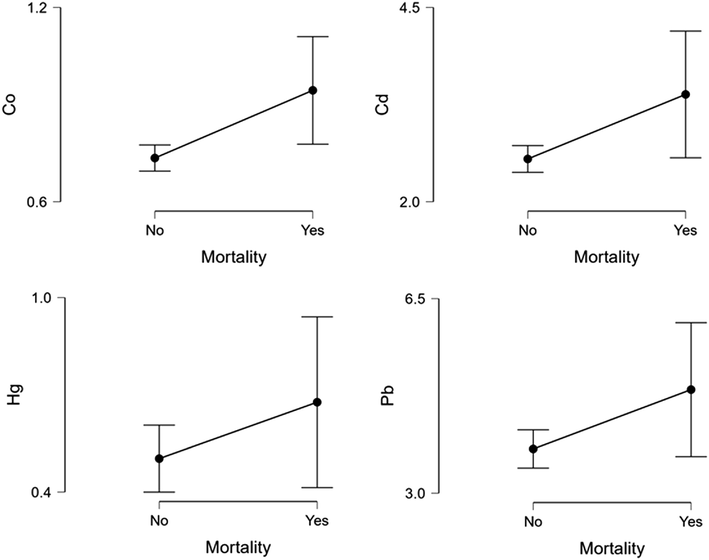
Mutlivariate regression analysis graph in terms of mortality.
5 Dıscussıon
In this work, we found that the levels of heavy metals (Pb, Cr, Al, As, Ca, Ni, Hg, Mn, Co, Cu, and Cd) were higher in the serum of individuals with acute exacerbation of COPD in comparison to the control group. At the same time, we found that serum heavy metal (Al, Ca, Mn, Co, Ni, Cu, As, Cd, Hg) levels increased with the severity of the disease. With a reduction of Zn levels in serum, the severity of the disease shows an increased manner. Moreover, in COPD patients with acute exacerbation, higher levels of Co, Cd, Hg, and Pb in serum are independent risk factors for mortality.
Cellular toxicity due to cadmium has been investigated under different headings such as DNA and membrane-functional changes, metalloenzyme interference, thiol protein changes, energy metabolism inhibition, and increased oxidative damage (Chen et al., 2009; Kirmizi et al., 2020). It is also well known that cadmium, an environmentally toxic substance, causes many respiratory diseases in humans (Leem et al., 2015; Zeng et al., 2016). A previous study showed that high concentrations of serum cadmium were correlated with reduced pulmonary function (Oh et al., 2014). Different investigations on the possible connection between cadmium exposure and COPD have produced mixed findings. In another study based in China, no correlation was found between serum cadmium levels and lung function in healthy children without COPD (Pan et al., 2020). In comparison with the control group, we observed higher serum cadmium levels in COPD cases. Again, in our study group, the serum cadmium levels of the patients in the gold 3 and gold 4 groups were found to be significantly higher than those of gold 1 and 2. These results we obtained to support the inverse relationship between serum cadmium levels and lung functions. In addition, in the study of Ya-Lin Jiang et al., they argued that serum cadmium concentration in COPD patients showed a positive correlation with inflammation (Jiang et al., 2022). Considering that the severity of inflammation increases with the severity of the disease, the higher serum cadmium levels of the patients in the gold 3 and gold 4 groups in our study support this result.
Glutathione (GSH) depletion is one of the important determinant of organ toxicities (Ahmadian et al., 2017; Eftekhari et al., 2016) and previous studies showed that mercury depletes GSH and causes oxidative stress and severe endothelial cell dysfunction, and leads to different lung diseases, such as bronchitis and pulmonary fibrosis (Tchounwou et al., 2003). In a study, it was observed that FEV 1 after bronchodilator decreased with increasing mercury concentrations (Heo et al., 2017). In our study, higher serum mercury levels were detected in the patient population in the acute COPD exacerbation gold 4 group. At the same time, one of the independent mortality risk factors was mercury levels. These results may indicate that mercury exposure may have severe clinical consequences. Arsenic (As) is a metalloid commonly found in soil and groundwater (Fatoki et al., 2022; Roy et al., 2020). Arsenic exposure, though not directly with COPD, has been strongly associated with decreased lung function and respiratory disease mortality in adults (Parvez et al., 2013; Sanchez, et al., 2018). Exposure to lead is linked to reduced lung function and a high risk of COPD (Leem et al., 2015, Gogoi et al., 2019). Chromium is also a metal associated with adverse effects on respiration, which is known to cause lung damage and cancer (Novey et al., 1983). The data obtained from your study show that high serum As levels increase with the severity of the disease, but Pb and Cr levels are not associated with the severity of the disease.
Cu is an important metal for many cellular functions such as antioxidant activity, iron transport, and collagen synthesis (Robinson et al., 2013). Higher levels of Cu increase inflammation and oxidative stress (Guo et al., 2013). Conversely, it is documented that in inflammation-related Peyronie's disease the level of serum Cu is low (Gunes et al., 2013). In another study conducted on patients with COPD, high serum Cu levels were found in the patient group with acute COPD attacks (Tanrikulu et al., 2011). Similarly, in cases with acute exacerbation, the levels of Cu in serum are high in our work. In addition, according to the gold classification, we found an increase in the serum Cu levels of the patients as the clinical severity of COPD exacerbation increased. In light of our findings, elevated serum copper levels may be an indicator of inflammation resulting from clinical aggravation of the disease. Again, in a study conducted on rheumatoid arthritis (inflammatory disease) patients, it was observed that copper levels were higher in serum (Önal et al., 2011).
It was reported that in patients with a critical situation the level of Zn decreased, especially in patients with sepsis (Mertens et al., 2015). In another work, Zn levels were low in critically ill patients with COPD (Karadag et al., 2004). In our investigation, the levels of Zn in serum are the same in both groups. However, in our patient group, levels of serum Zn were lower in patients with a more severe clinical picture (grade 2–3-4) according to the gold classification compared to milder patients (grade 1). This may indicate that zinc deficiency may cause more severe COPD exacerbations. In previous studies, it is known that the antioxidant enzyme superoxide dismutase contains Zn in its structurally active part (Chuapil et al., 1976, Huang et al., 1977). In line with the findings of our study, the possible excessive use of oxidant-antioxidant systems in patients with clinically severe acute COPD may have been effective in the reduction of zinc due to the use of zinc by those systems.
6 Limitations
This study has some limitations. First of all, the clarification of the cause-effect relationship between lung function and serum heavy metal levels is hard, since it is a case-control study. Secondly, no information about exposure (high dietary intake, smoking history, occupation, etc.) was obtained from the patients. We tried to examine the association between serum levels of heavy metal and pulmonary functions, regardless of any exposure. The relatively low number of patients can be counted as one of the limitations of this study.
7 Conclusion and future perspectives
Our study showed that serum heavy metal levels are linked with the harshness and mortality of acute COPD attacks. These findings may indicate that changes in serum heavy metal levels can be used to determine the severity of a COPD exacerbation. However, future studies with larger patient groups will be useful in clarifying the predictive role of heavy metals levels in serum as markers of disease status.
Acknowledgment
The authors are thankful for moral support of Yozgat Bozok University, Yozgat, Turkiye.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chronic obstructive pulmonary disease pathogenesis. Clin. Chest Med.. 2020;41(3):307-314.
- [Google Scholar]
- Update on the pathogenesis of chronic obstructive pulmonary disease. N. Engl. J. Med.. 2019;381(13):1248-1256.
- [Google Scholar]
- In vitro and in vivo evaluation of the mechanisms of citalopram-induced hepatotoxicity. Arch. Pharm. Res.. 2017;40(11):1296-1313.
- [Google Scholar]
- Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie.. 2006;88(11):1549-1559.
- [Google Scholar]
- Effect of zinc on cells and biomembranes. Med. Clin. North Am.. 1976;60(4):799-812.
- [Google Scholar]
- Effect of inhaled chromium on pulmonary A1AT. Inhalation Toxicol.. 2002;14(7):765-771.
- [Google Scholar]
- Metallic elements in exhaled breath condensate and serum of patients with exacerbation of chronic obstructive pulmonary disease. Metallomics.. 2009;1(4):339-345.
- [Google Scholar]
- Towards eradication of chronic obstructive pulmonary disease: a Lancet Commission. The Lancet.. 2019;393(10183):1786-1788.
- [Google Scholar]
- Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica. 2016;46(4):369-378.
- [Google Scholar]
- Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. Journal of Hazardous Materials. Advances.. 2022;100052
- [Google Scholar]
- Circulatory heavy metals (cadmium, lead, mercury, and chromium) inversely correlate with plasma GST activity and GSH level in COPD patients and impair NOX4/Nrf2/GCLC/GST signaling pathway in cultured monocytes. Toxicol. In Vitro. 2019;54:269-279.
- [Google Scholar]
- Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. International Journal of Medical Sciences. 2013;10(1):79.
- [Google Scholar]
- It is time for the world to take COPD seriously: a statement from the GOLD board of directors. Eur. Respir. J.. 2019;54(1)
- [Google Scholar]
- Serum heavy metals and lung function in a chronic obstructive pulmonary disease cohort. Toxicology and Environmental Health Sciences. 2017;9(1):30-35.
- [Google Scholar]
- Serum cadmium positively correlates with inflammatory cytokines in patients with chronic obstructive pulmonary disease. Environ. Toxicol.. 2022;37(1):151-160.
- [Google Scholar]
- Trace elements as a component of oxidative stress in COPD. Respirology.. 2004;9(1):33-37.
- [Google Scholar]
- Candidate genes for COPD: current evidence and research. International journal of chronic obstructive pulmonary disease. 2015;10:2249.
- [Google Scholar]
- Are heavy metal exposure and trace element levels related to metabolic and endocrine problems in polycystic ovary syndrome? Biol. Trace Elem. Res.. 2020;198(1):77-86.
- [Google Scholar]
- Airway inflammation in cadmium-exposed rats is associated with pulmonary oxidative stress and emphysema. Free Radical Res.. 2006;40(3):241-250.
- [Google Scholar]
- Relationship between blood levels of heavy metals and lung function based on the Korean National Health and Nutrition Examination Survey IV–V. International journal of chronic obstructive pulmonary disease.. 2015;10:1559.
- [Google Scholar]
- Social determinants and non-communicable diseases: time for integrated action. BMJ. 2019;364
- [Google Scholar]
- Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. BJA: British Journal of Anaesthesia.. 2015;114(6):990-999.
- [Google Scholar]
- Asthma and IgE antibodies induced by chromium and nickel salts. Journal of allergy and clinical immunology.. 1983;72(4):407-412.
- [Google Scholar]
- Blood cadmium levels are associated with a decline in lung function in males. Environ. Res.. 2014;132:119-125.
- [Google Scholar]
- Effects of different medical treatments on serum copper, selenium and zinc levels in patients with rheumatoid arthritis. Biol. Trace Elem. Res.. 2011;142(3):447-455.
- [Google Scholar]
- Effects of lead, mercury, and cadmium co-exposure on children’s pulmonary function. Biol. Trace Elem. Res.. 2020;194(1):115-120.
- [Google Scholar]
- Arsenic exposure and impaired lung function. Findings from a large population-based prospective cohort study. Am. J. Respir. Crit. Care Med.. 2013;188(7):813-819.
- [Google Scholar]
- Robinson, S.D., Cooper, B., Leday, T.V., 2013, October. Copper deficiency (hypocupremia) and pancytopenia late after gastric bypass surgery. In Baylor University Medical Center Proceedings. 26(4). 382-386.
- Arsenic methyltransferase and methylation of inorganic arsenic. Biomolecules.. 2020;10(9):1351.
- [Google Scholar]
- A meta-analysis of arsenic exposure and lung function: is there evidence of restrictive or obstructive lung disease? Current environmental health reports.. 2018;5(2):244-254.
- [Google Scholar]
- Coenzyme Q10, copper, zinc, and lipid peroxidation levels in serum of patients with chronic obstructive pulmonary disease. Biol. Trace Elem. Res.. 2011;143(2):659-667.
- [Google Scholar]
- Environmental exposure to mercury and its toxicopathologic implications for public health. Environmental Toxicology: An International Journal.. 2003;18(3):149-175.
- [Google Scholar]
- Changing levels of selenium and zinc in cadmium-exposed workers: probable association with the intensity of inflammation. Mol. Biol. Rep.. 2019;46(5):5455-5464.
- [Google Scholar]
- The HUNT study: association of comorbidity clusters with long-term survival and incidence of exacerbation in a population-based Norwegian COPD cohort. Respirology.. 2022;27(4):277-285.
- [Google Scholar]
- Heavy metals in PM2. 5 and in blood, and children's respiratory symptoms and asthma from an e-waste recycling area. Environ. Pollut.. 2016;210:346-353.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102422.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







