Translate this page into:
Investigating the colon toxicity and carcinogenic role of monosodium glutamate compared with Dimethylhydrazine in male Wistar rats: Exploring the link to childhood colon cancer risk
⁎Correspondence author at: Department of Biochemistry, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu 641 021, India. rathi.muthaiyan@kahedu.edu.in (Rathi Muthaiyan Ahalliya)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background
Colon cancer is rising among younger population than elder people. About 50% of colon cancer cases attributed to dietary factors. Monosodium glutamate (MSG) widely used taste enhancer prevalent in fast foods and processed items.
Objectives
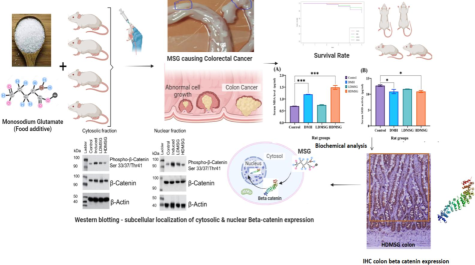
The study investigated into the potential toxic and carcinogenic role of MSG in male Wistar rats of 1–3 months old and compared the effects with the Dimethyl hydrazine (DMH): by observing survival probability, estimation of serum biochemical parameters, and analysis for colon beta catenin protein expression.
Methods
Rats were grouped into control, DMH (s.c), low- and high dose MSG (LDMSG, HDMSG) (p.o). Survival rate statistically calculated using Kaplan-Meier plots and Log-rank tests. Biochemical analyses were done using standard protocols and one-way ANOVA were performed for data analysis. Beta catenin protein expressions were studied using immunohistochemistry and western blotting.
Results
Our study emphasizes that high dose MSG consumed male Wistar rats cause high decline in survival rate compared to low dose MSG and DMH. Estimated serum biochemical parameters showed significantly increased oxidative stress, altered liver and kidney function markers, alongside elevated serum sodium, total cholesterol, triglycerides, LDL, and inflammatory markers. Observed, colon polyps formed in DMH and MSG rats. Rat’s colon immunohistochemistry study expressed β- catenin whereas Western blotting results confirmed the altered β- catenin and β-catenin phosphorylation ratios in cytosol and nuclear region were elevated in DMH-induced colon cancer (p value of 0.0002), MSG low dose (p value of 0.003) and high dose (p value of 0.01) statistically significant. These findings highlights declined survival probabilities and pronounced oxidative stress markers, organ function changes, disrupted lipid profiles, and increased nuclear β-catenin expression reveals the potential toxic and carcinogenic role of MSG is influencing colon cancer development in male Wistar rat models.
Conclusion
Based on the results, the study underscores the potential toxic and carcinogenic role of MSG, particularly at neoplastic stages of colon cancer in male Wistar rat models.
Keywords
Monosodium glutamate
Dimethylhydrazine
Colon cancer
Kaplan Meier Plot
Serum Biochemical parameters
β–Catenin
1 Introduction
Modern research highlights a surge in food additives to enhance taste and appeal. A study revealed that about a quarter of packaged foods and beverages contain six or more additives, suggesting potential health risks (Montera et al., 2021). Monosodium glutamate (MSG) is a commonly used additive, and its consumption has increased due to frequent intake of processed foods and bakery items (Beyreuther et al., 2007). Although MSG intake up to 16.0 mg/kg body weight is generally considered safe (Kazmi et al., 2017), its usage and consumption vary by individual. Recent research indicates that rats exposed to MSG during pregnancy gave birth to male offspring with autism (Soltani et al., 2024). Increased toxicity studies suggest MSG may harm the brain, liver, kidney, and colon in Wistar rat models (Rajendran et al., 2023). Diet significantly impacts intestinal health and colon cancer risk, with around 50 % of cases linked to dietary changes (Kune et al., 1992). In India, 2010 data projected 13,700 childhood cancer deaths, with a national rate of 37 deaths per million (Gupta et al., 2016), highlighting the need to investigate dietary factors contributing to severe diseases like cancer. Although colon cancer rates have declined in older adults, there is a troubling increase among younger individuals (Willyard, 2021). While rare, childhood colorectal cancer has been observed in ages 11 to 14 (Kim et al., 2013), emphasizing the need to recognize its potential impact on children.
Colon cancer involves decreased survival rates, biochemical alterations, and disruptions in molecular signaling. Even minor biochemical changes can affect molecular pathways (Tripathi et al., 2022; Saudagar and Tripathi, 2022), increasing risks and offering markers for treatment. For example, lipid peroxidation and reactive oxygen species are linked to colon cancer progression. Recent studies on ethanol-related colorectal cancer (ER-CRC) showed that dietary sesaminol reduced CRC pathogenesis in mice by mitigating ethanol-induced oxidative stress and inflammation in the colon and rectum (Ohira et al., 2022). This demonstrates the potential of targeting these pathways for cancer prevention and treatment.
Colon cancer stem cells are thought to initiate tumor growth and contribute to treatment resistance (Gopan et al., 2023). The progression of colon cancer involves molecular alterations, including Wnt, KRAS, TGF-β, and p53, in a sequential manner commonly known as the “Vogelgram model” (Fearon, 2011). The Wnt pathway is implicated in the early stages of cancer (adenoma to carcinoma), while p53 is associated with later stages (carcinoma to metastasis) (Al Hargan et al., 2021). The Wnt/β-catenin pathway plays a pivotal role in colon carcinogenesis, primarily through the regulation of two key genes: 1. Adenomatous polyposis coli (APC), a tumor suppressor gene, and 2. β-catenin (CTNNB1), an oncogene. In the presence of APC, β-catenin undergoes ubiquitination in the cytosol, preventing its oncogenic effects. However, defective APC leads to the accumulation of β-catenin in the nucleus, which drives colon carcinogenesis (Gopan et al., 2023).
Present, study investigated into the potential toxic and carcinogenic role of MSG in male Wistar rats of 1–3 months old and compared the effects with the Dimethylhydrazine (DMH): by observing survival probability, serum biochemical estimations, observing colon polyp formation and colon beta catenin protein expression analysis.
2 Materials and methods
2.1 DMH and MSG preparation
1,2-Dimethylhydrazine (DMH) was sourced from TCI Chemicals and prepared as described by Nirmala and Ramanathan (2011). MSG, with 99.9 % purity, was purchased from a local Pollachi market (the same brand used in restaurants). Fresh MSG preparations were used (Banerjee et al., 2020; Das et al., 2024).
2.2 Ethical Approval
The study was approved by the Institutional Animal Ethical Committee (reference number: KAHEAAECt2021/11-09J0).
2.3 Experimental design
Male Wistar rats aged 1 to 3 months, equivalent to human adolescents aged 9 to 15 years (Zhang et al., 2019), were used. Twenty-four rats were divided into four groups and individually housed in plastic cages in the KAHE animal house, with standard temperature, humidity, and a 12-hour light/dark cycle. They had unrestricted access to a standard diet and water for five weeks. The groups were: Control, DMH-induced colon cancer (positive control), Low-Dose MSG (LDMSG), and High-Dose MSG (HDMSG). Summary of dosage and administration for colon cancer treatment study was shown in Table 1.
Animal group
Dose and route of administration
Treatment period
Control
Water oral
Daily
DMH induced colon cancer
20 mg/kg b.w., s.c. (Nirmala & Ramanathan, 2011)
Once a week for 5 weeks
LDMSG
20 g/kg b.w., oral (Rajendran et al., 2023)
Daily for 5 weeks
HDMSG
30 g/kg b.w., oral (Rajendran et al., 2023)
Daily for 5 weeks
2.4 Survival monitoring
All animals were closely monitored until the end of their natural lifespan or ethically euthanized. Overall survival was recorded from the conclusion of the 5-week experimental period.
2.5 Sample collection and processing
After the 5-week treatment duration, rats underwent an overnight fast before euthanasia. Euthanasia was performed by a humane method (Avma, 2020). The animals were sacrificed by cervical dislocation under mild chloroform (Hickman and Johnson, 2011). Blood samples collected in three separate serum tubes from each group for triplicate measurements. Colon tissue samples (Pooled) were processed immediately.
2.6 Serum biochemical analysis
Serum samples from the grouped rats were analyzed for various biomarkers using assay kits purchased from Abbkine. Lipid peroxidation (LPO) was assessed by measuring Malondialdehyde (MDA) (Catalog No: KTB1050-EN) levels at OD532 nm with a microplate reader. Antioxidant enzyme activities were measured as follows: catalase (CAT) (Catalog No: KTB1040) at OD540 nm, superoxide dismutase (SOD) (Catalog No: KTB1030) at OD450 nm, and glutathione peroxidase (GSH-Px) (Catalog No: KTE101141) at OD450 nm, all using a microplate reader. Liver enzyme levels were determined using a standard UV kinetic method, with serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) measured at 340 nm. Kidney function was evaluated by measuring serum urea with the GLDH method at OD340 nm, uric acid with the Uricase-POD method at OD540 nm, and creatinine with the Jaffe’s method at OD520 nm, while sodium levels were assessed using a colorimetric method at OD530 nm. Lipid profiles included low-density lipoprotein (LDL) measured by the LDL-Direct method at OD600 nm, and total cholesterol (TC) and triglycerides (TG) measured using the GPO-POD method at OD510 nm. Inflammatory markers were quantified for interleukin-1 β (IL-1 β) (Catalog No: KTE9001), tumor necrosis factor-α (TNF-α) (Catalog No: KTE9007), and C-reactive protein (CRP) (Catalog No: KTE101074) using a microplate reader at OD450 nm.
2.7 Immunohistochemistry
Paraffin-embedded colon tissue sections (3 µm) were used in the study and placed on positively charged Bio SB Hydrophilic Plus Slides (BSB 7028). The slides were air-dried for 2 h at 58 °C, then deparaffinized in xylene and rehydrated through graded alcohols. For heat-induced epitope retrieval, the slides were placed in a Coplin jar containing ImmunoDNA Retriever with Citrate and set on a trivet in a pressure cooker with 1–2 in. of distilled water. The heat was turned to high and the slides were incubated for 15 min. Afterward, the pressure cooker was opened, and the slides were immediately transferred to room temperature and allowed to stand for 15–20 min. Immunohistochemical staining for β-catenin was performed manually according to the manufacturer’s protocol (Doc# PI3756). After staining, the slides were washed with deionized water, mounted with DPX, covered with a cover slip, and visualized using a CILIKA digital microscope. Immunoreactivity of the colon tissues was scored as either positive or negative, quantified β catenin expression using imageJ software (version 1.43, NIH, USA).
2.8 Western blotting
Colon sample preparation followed the methodology described by Baghirova et al. (2015). Briefly, 25 μg of protein extract (cytosolic and nuclear fractions) was separated by SDS-PAGE, and the resolved proteins were transferred to nitrocellulose membranes. The membranes were blocked with a blocking solution, then incubated overnight with specific primary antibodies: Phospho-β-catenin (Ser33/37/Thr41) (Catalog No: 9561, Cell Signaling Technology), Non-Phospho-β-catenin (Catalog No: mab1329, R&D Systems), and β-Actin (Catalog No: A3854, Sigma). After washing, the membranes were treated with secondary HRP-conjugated antibodies (Catalog Nos: 7074, 7075, 7076, Cell Signaling Technology) and incubated with ECL reagent (Catalog No: 6883, Cell Signaling Technology). The relative abundance of each band was quantified using ImageJ software (version 1.43, NIH, USA).
2.9 Statistical analysis
Each experimental condition was independently repeated three times, with each sample obtained from three animals per group. Biochemical analyses were performed using separate tubes for each sample to avoid cross-contamination. Kaplan-Meier (KM) survival analysis and Log-rank tests were conducted using R Software (version 4.1.2) to assess survival curves and compare differences between groups. Western blot experiments were performed using pooled colon samples from the animals to consolidate the data. Data were expressed as mean ± SD with n = 3, where n refers to the number of independent experiments. Comparisons were made between the control group and the treatment groups (DMH and MSG), as well as between the DMH and MSG groups. Statistical significance was assessed using the Student's t-test for comparisons between two groups and one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests for multiple group comparisons. Significance levels were defined as follows: p < 0.05, *p < 0.01, **p < 0.001, and ***p < 0.0001. GraphPad Prism® statistical software version 6 (GraphPad, San Diego, CA) was used for statistical analysis and graph production.
3 Results
3.1 Survival rate
Rat models were administered at both low and high doses of MSG (LDMSG, and HDMSG) compared to among Control and DMH groups survival rates using KM plot. No deaths were recorded in the control group, while survival probabilities between the LDMSG and DMH groups appeared similar (n = 4). However, rats exposed to HDMSG exhibited lower survival probabilities (n = 3) compared to the others. This suggests that exposure to high doses of MSG may heighten negative health effects, increase childhood colon cancer risk. Despite observed trends in the data (Fig. 1), the reported p-value of 0.7 indicates that the differences in survival probabilities did not reach statistical significance.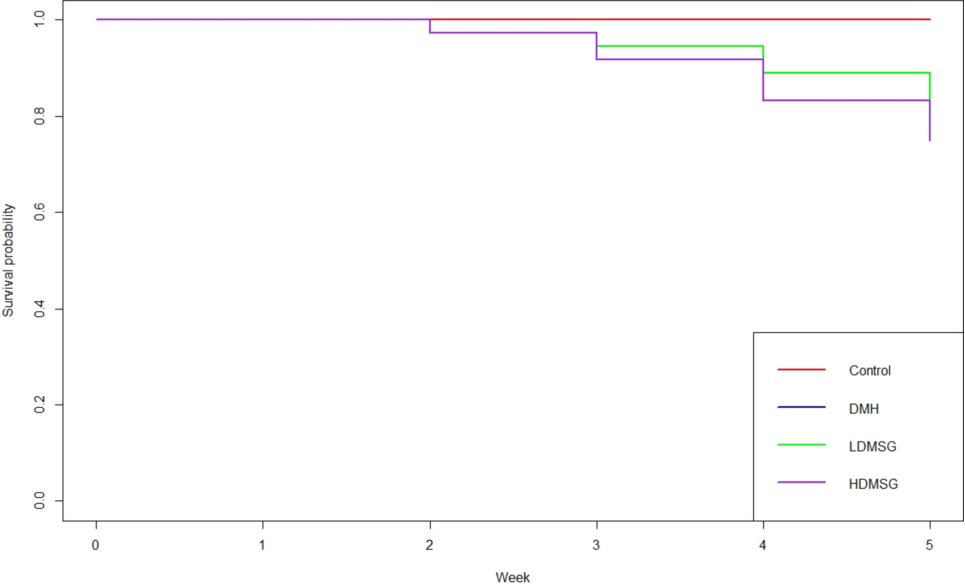
Effects of MSG on Survival Rate: Survival analysis of experimental rat groups using Kaplan-Meier (KM) plots along with a Log-rank test. The x-axis represents time in weeks, and the y-axis indicates survival probability (p-value). The number of animals alive at the end of the experiment is as follows: Control (n = 6), DMH (n = 4), LDMSG (n = 4), and HDMSG (n = 3).
3.2 Biochemical analysis
Serum biochemical profiles were estimated for all grouped animals, and mean values were graphically plotted and compared with the control (Fig. 2.& Fig. 3).The present research investigated serum MDA levels and the activity of AO enzymes, including CAT, SOD, and GSH-Px. Fig. 2a-d presents the results, indicating a significant increase (p < 0.0001 to 0.001) in MDA concentration among the DMH and HDMSG groups, suggesting a notable rise in LPO potentially due to oxidative stress, while the increase was insignificant for LDMSG rats (Fig. 2a). In Fig. 2b, SOD enzyme activity was decreased significantly in DMH and HDMSG rats but showed non-significant decreases in the LDMSG group. Notably, Fig. 2c indicates a decreased catalase activity in both DMH and LDMSG animals, significantly higher in the HDMSG group (p < 0.001 to 0.01). Conversely, GSH-Px activity (Fig. 2d) showed a significant increase (p < 0.0001 to 0.001) in both MSG groups (LDMSG and HDMSG), and DMH rats exhibited a higher significant increase (p < 0.001 to 0.01). Moreover, the study evaluated SGOT and SGPT activities and serum concentrations of urea, uric acid, creatinine, and sodium to analyse liver and kidney functions in the grouped animals. Fig. 2e indicates a significant increase (p < 0.0001) in serum SGOT (U/L) in all three test groups (DMH, LDMSG, and HDMSG), while Fig. 2f reports decreased serum SGPT (U/L) levels with significance (p < 0.0001 to 0.001) in DMH and LDMSG groups and more significantly (p < 0.0001) in HDMSG rats reveals altered liver function. The study measures the serum levels of sodium (Fig. 2g), with a focus on the MSG rat groups and the DMH-induced colon cancer rat models. The results indicate significant increase (p < 0.0001) in sodium levels among the MSG rat groups due to the ingestion of monosodium glutamate elevated sodium levels in the blood. The sodium levels in the DMH-induced colon cancer rat models also exhibit significant increases (p < 0.01 to 0.05), indicates that there is an impact on sodium levels, to a lesser extent compared to the MSG rat groups. The assessment of kidney functions revealed increased urea levels in all three experimental rat groups (Fig. 2h) with statistical significance (p < 0.0001), indicating altered kidney function. Fig. 2i depicts increased creatinine levels with significance (p < 0.0001) in both MSG rat groups, while Fig. 2j shows increased uric acid levels with significance (p < 0.0001 to 0.001) in DMH rats and with significance (p < 0.001 to 0.01) in LDMSG and HDMSG rat groups.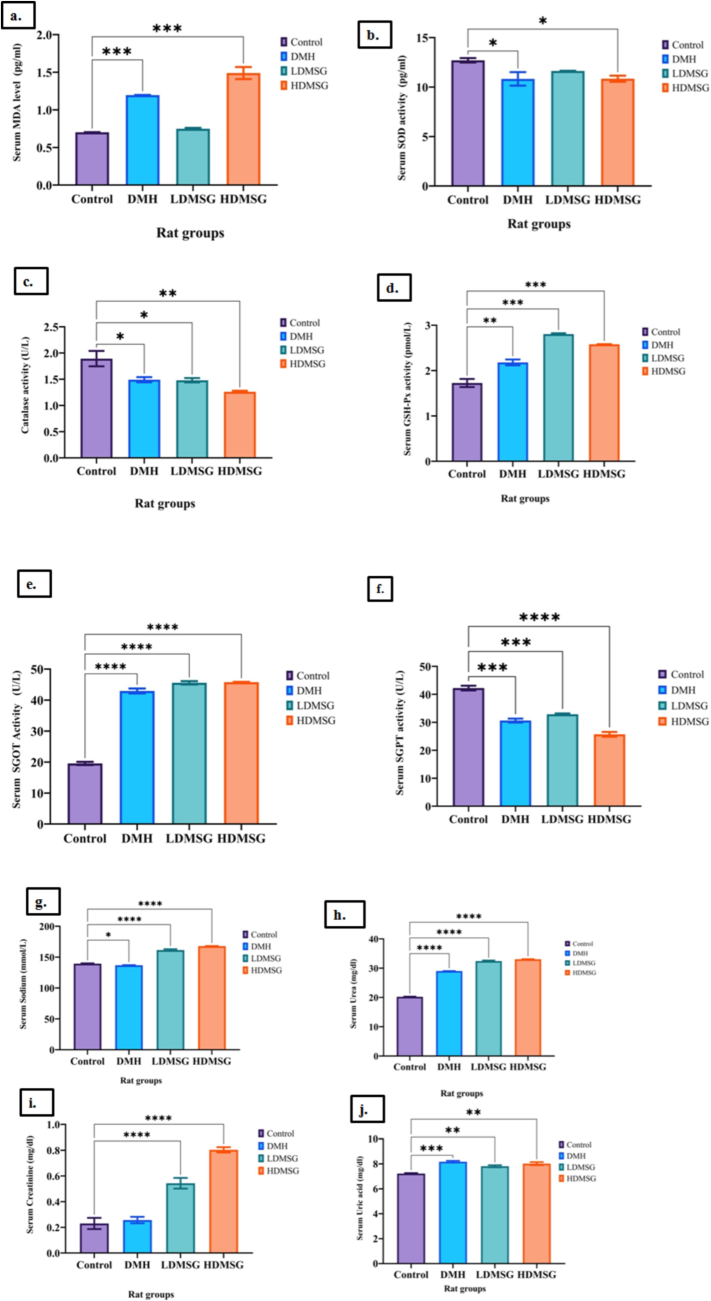
a-j: Effects of MSG on oxidative stress markers and antioxidant enzymes. 2a-Malondialdehyde (MDA). 2b- Super oxide dismutase (SOD). 2c-Catalase (CAT). 2d-GSH-Px. 2e-Serum glutamic oxaloacetic transaminase (SGOT). 2f-Serum glutamic pyruvate transaminase (SGPT). 2 g-Sodium. 2 h-Urea. 2i-Creatinine. 2j-Uric acid. Data are expressed as mean ± SD with n = 3, where n refers to the number of independent experiments. Error bars represent standard deviation. Statistical significance is indicated by asterisks: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
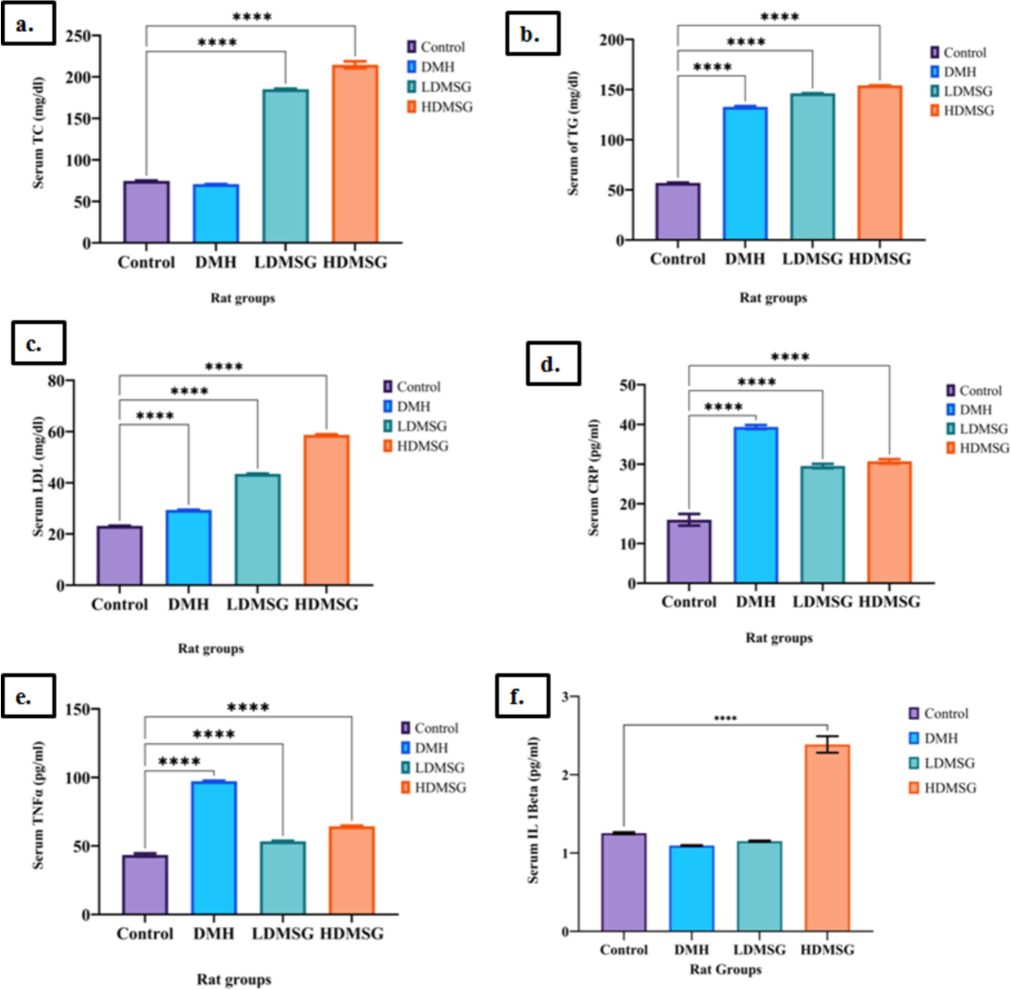
a-f:Effects of MSG lipid biomarkers and proinflammatory markers. 3a-Total cholesterol (TC). 3b-Triglycerides (TG). 3c-Low density lipoprotein (LDL). 3d-c-reactive protein (CRP). 3e-Tumor necrosis factor-α (TNF-α). 3f- Interleukin 1β (IL-1β). Data are expressed as mean ± SD with n = 3, where n refers to the number of independent experiments. Error bars represent standard deviation. Statistical significance is indicated by asterisks: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Analysis on lipid profile, TC levels (Fig. 3a) were found significantly increased (p < 0.0001) exclusively for MSG rat groups (LDMSG and HDMSG) but not for DMH-induced colon cancer rats. Conversely, TG (Fig. 3b) and LDL levels (Fig. 3c) exhibited significant increases (p-value < 0.0001) across all three DMH, LDMSG, and HDMSG compared to the control.
Examining inflammatory markers, the study reports significant elevations in serum CRP (Fig. 3d) and TNF-α level (Fig. 3e) (p-value < 0.00001) for the experimental rat groups (DMH, LDMSG, HDMSG) compared to the control rats. Furthermore, serum IL-1β levels (Fig. 3f) increased significantly (p-value < 0.00001), specifically in the HDMSG rat group.
3.3 Colon polyps
The investigation observed and confirmed the development of colon polyps in rats from the DMH and MSG groups (Fig. 4 b-d). The number of polyp formations was greater in the DMH group compared to the MSG group. These findings confirm that MSG has the potential to induce colon polyp formation in rats, though to a lesser extent compared to DMH, which is used as a positive control for colon cancer.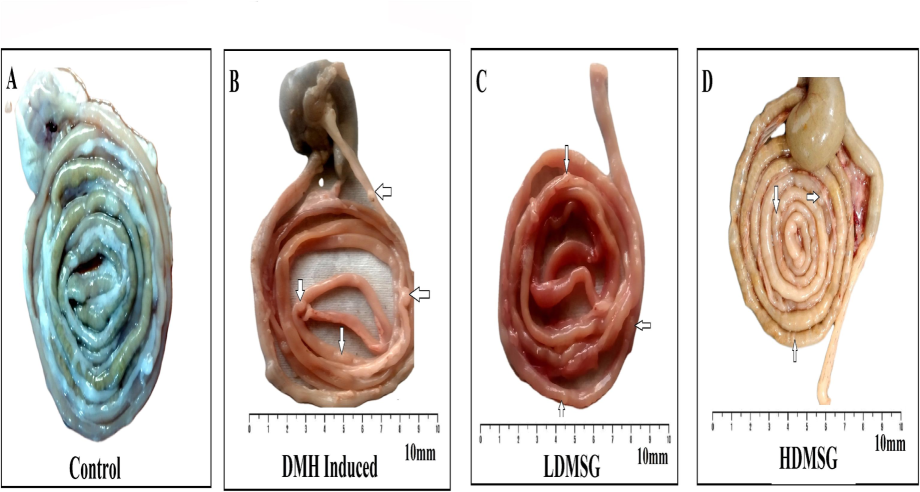
A-D: Effects of MSG on colon polyp/tumor formation in rats. (A-D) Representative images of colon tissue sections from different groups. White arrows indicate colon polyp formations. (A) Control: No polyp formations observed. (B) DMH-induced colon cancer group: Shows the presence of colon polyps. (C) LDMSG: Shows colon polyp formations. (D) HDMSG: Shows colon polyp formations. Scale bar denotes 10 mm.
3.4 Immunohistochemistry
The impact of MSG on colon β-catenin expression was analysed through immunohistochemistry (IHC). The findings depicted in Fig. 5 revealed positive staining for β-catenin expression in the colon cells of the experimental rats (B-D), except for the control group (A). Quantified β-catenin expression represented in Fig. 5E.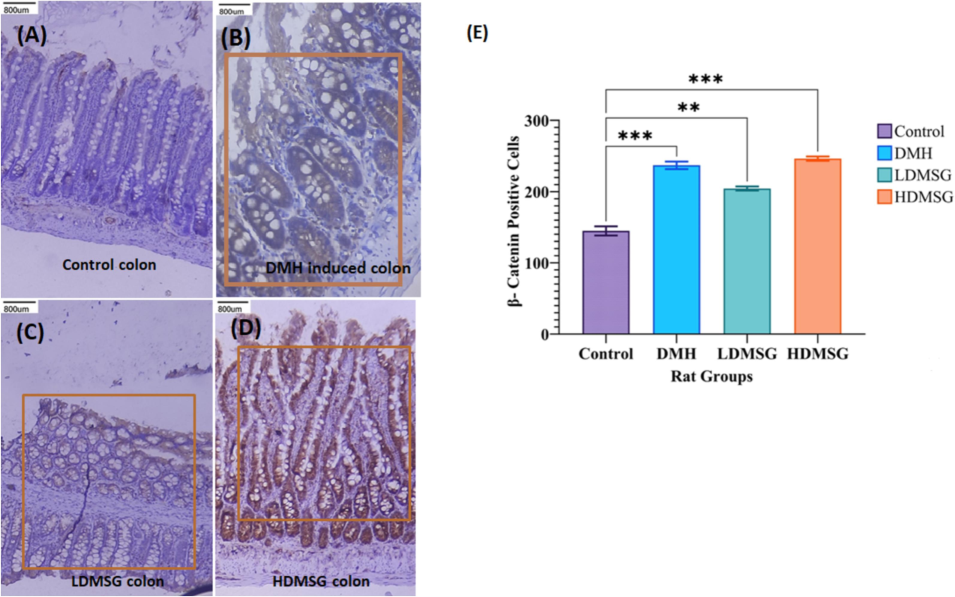
Immunohistochemistry for β-catenin Expression in Colon Tissues. (A) Control Colon: Negative staining. (B) DMH-Induced Colon Cancer Rat Group: Positive immunohistochemical staining of β-catenin, indicating its expression associated with cancer. (C) LDMSG Rat Colon: Positive staining for β-catenin expression. (D) HDMSG Rat Colon: Positive staining for β-catenin in the colon of rats treated with a high dose of MSG. The orange rectangles in panels B, C, and D indicate the selected regions for β-catenin quantification. (E) Quantification of β-catenin IHC Expression: Results are presented as mean ± SD (n = 3). Error bars represent standard deviation. Statistical significance is indicated by asterisks: **p < 0.01, ***p < 0.001.
3.5 Western blotting
Western blotting of phosphorylated β-catenin (ser 33/35 and Thr41 sites) and the free active β-catenin in both the cytosol (Fig. 6a) and nuclear (Fig. 6b) results in the subcellular localization. The densitometry analysis reports (Fig. 6c) on the phosphorylation ratio of cytosolic and nuclear β-catenin revealed significant bands in the colons of DMH-induced colon cancer rats (p-value of 0.0002), the LDMSG group (p-value of 0.003), and the HDMSG group (p-value of 0.01).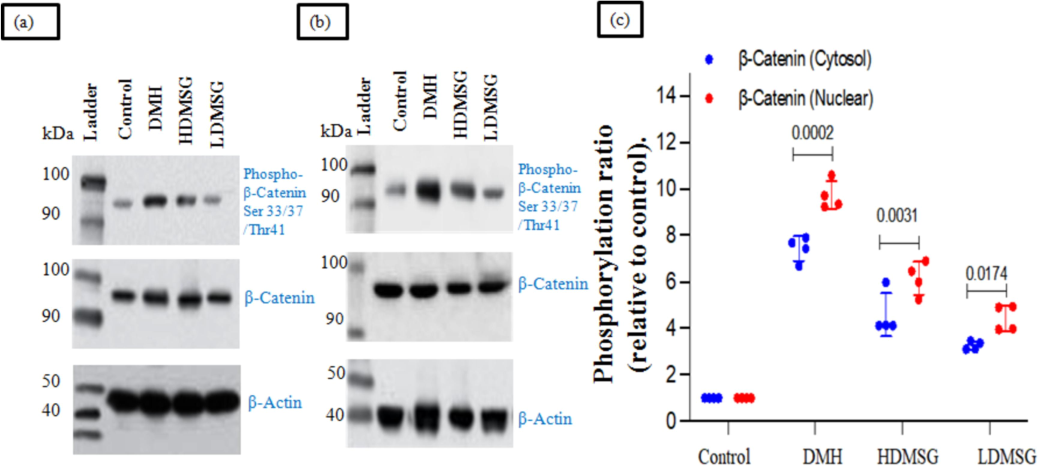
a-c: Role of MSG in modifying WNT/β catenin molecular signaling by subcellular localization in cytosol and nuclear region of colon β-catenin (Pooled), mean ± SD with (n = 3) by Western blotting. (a) Cytosol: Western blot analysis showing β-catenin expression in the cytosolic fraction of colon tissue. The blot demonstrates the distribution of β-catenin within the cytosol. (b) Nuclear Region: Western blot analysis showing β-catenin expression in the nuclear fraction of colon tissue. The blot reveals the distribution of β-catenin within the nucleus. (c) Phosphorylation Ratio of Cytosolic and Nuclear β-Catenin: Quantitative analysis of the phosphorylation ratio of β-catenin in both cytosolic and nuclear fractions, normalized to β-Actin as a loading control. This panel shows the relative phosphorylation levels and their impact on β-catenin signaling.
4 Discussion
Previously, Sajjaviriya et al. (2019) reported that MSG-treated zebra fish embryos (100 mg/L) had a significantly lower survival rate compared to controls. Histological analysis revealed higher lesion scores (p = 0.05) in the liver and kidneys and reduced reproductive performance after 60 days of MSG exposure. In a recent study, Gigola et al. (2021) investigated the effects of capecitabine, a probiotic treatment, on DMH-induced colon cancer in rats. They found that capecitabine improved overall survival and life quality in these models. To our knowledge, our study is the first to compare the survival rates of MSG-treated versus DMH-treated male Wistar rats, focusing on childhood colon cancer risk (Fig. 1). Interestingly, in our study, survival rates were compared across the Control, DMH, LDMSG, and HDMSG groups. Animals in the DMH group and LDMSG group showed similar survival rates, with four animals alive in each group. In contrast, the HDMSG group had a lower survival rate, with only three animals remaining. However, the difference in survival rates between the DMH and HDMSG groups was not statistically significant (p = 0.7). This lack of significance may be attributed to limitations in the study design, including a small sample size and short trial duration. Therefore, the findings should be interpreted with caution.
Sahin et al. (2023) reported that exposure to MSG in male Sprague-Dawley rats increased LPO and ROS production, as evidenced by higher MDA levels and decreased SOD activity, consistent with our findings. Similarly, Banerjee et al. (2020) found that rats fed MSG and a high-lipid diet had increased ROS and reduced levels of SOD, catalase, and glutathione, aligning with our results. Our study indicates that MSG consumption elevates LPO, leading to increased free radicals and cell damage. The decreased catalase activity suggests heightened ROS levels, though these are partially mitigated by increased GSH-Px activity. A similar pattern was observed in the DMH-induced colon cancer groups. Higher MSG intake results in colon cell damage due to free radicals, which are partially scavenged by GSH-Px.
Our findings, Fig. 2e-j, are consistent with earlier studies investigating the chronic toxicity of MSG in Wistar albino rats (Nnadozie et al., 2019). We observed significant elevation of SGOT and SGPT enzyme activities and increased levels of urea, creatinine, uric acid, and sodium in MSG-treated rats. These results corroborate recent research highlighting MSG’s impact on hepatic and renal systems as well as the gut microbiome (Nahok et al., 2019; 2021).A significant increase in creatinine was observed in the HDMSG group compared to the DMH group (p < 0.0001). In contrast, no significant difference in creatinine levels was found between the DMH and control groups. This suggests that MSG has a more pronounced effect on renal function than DMH. SGOT and SGPT elevation were comparable between the MSG and DMH groups, highlighting that both treatments affect liver function similarly. Significant increases in urea, uric acid, and sodium levels were observed in the MSG group, with trends that were consistent between MSG and DMH groups. This suggests that while MSG and DMH both impact renal function, MSG may induce more severe alterations. The significant differences in creatinine levels between MSG and DMH groups suggest that MSG has a greater impact on renal function. These findings underscore the potential adverse effects of MSG on liver and kidney health, particularly when compared to DMH.
An altered lipid profile can lead to dyslipidemia, a risk factor for colon cancer. Activation of MAPK and ROS signaling pathways by LDL can induce intestinal inflammation and promote colon cancer progression (Wang et al., 2017). Dyslipidemia markers, such as reduced HDL and increased LDL, TG, and TC levels, are associated with higher colon cancer risk (Li et al., 2020). In this study, elevated LDL and TC levels were observed in the LDMSG and HDMSG rat groups, while increased TG levels were seen in DMH, LDMSG, and HDMSG groups. These alterations in lipid profiles, resulting from deregulated lipid metabolism, contribute to a cancer-promoting environment in both DMH-induced colon cancer and MSG rat models.
CRP, TNF-α, and IL-1β are key indicators of systemic inflammation and cancer severity. Elevated levels of these markers reflect the connection between inflammation and cancer growth. In this study, serum levels of CRP, TNF-α, and IL-1β were measured in experimental rats to assess their role in systemic inflammation. Banerjee et al. (2020) demonstrated that MSG increases serum CRP, TNF-α, and IL-1β levels in rats, indicating potential liver and heart pathology. Our study compared the effects of MSG with the carcinogenic chemical DMH and found that serum CRP and TNF-α levels were significantly higher in both LDMSG and HDMSG groups, as well as in the DMH group (p-value < 0.0001). IL-1β levels were significantly increased only in the HDMSG group (p-value < 0.01 to 0.05), but decreased in the LDMSG and DMH groups. Elevated levels of these markers are associated with chronic inflammation and cancer.
Although the study observed the formation of colon polyps (Fig. 4), the tumor incidence rate could not determined due to limitations including small sample size, declining survival rate and short study period.
Wnt pathway, oncogenic mutations are predominant in colon cancer. About > 80 % mutation in regulatory component (APC) of Wnt occurs 10 % in beta catenin involved in early colon carcinogenesis (Fearon, 2011). In presence of Wnt ligand APC forms destruction complex phosphorylates beta catenin (ser33, ser37 and Thr41) enters ubiquitination act as tumor suppressor (Novellasdemunt et al., 2015). Either point mutations at these amino acid sites or in absence of “Wnt” activates low density lipoprotein (LRP) receptor encourage β-catenin nuclear translocation an oncogenic process (Novellasdemunt et al., 2015).
Present study established that consumption of MSG could alter the expression of β-catenin at subcellular level comparably to DMH. Recently, Bhattacharya et al. (2020) reported a cross section study of colorectal cancer patients to demonstrate the role of β-catenin expression in cell membrane, cytoplasm and nuclear region and established a statistically significant (p < 0.0001) result showed that IHC beta-catenin expression subsequent presence of a higher nuclear beta-catenin score in the malignant neoplasms due to the gradual shift of beta-catenin from the membranous to cytoplasm to the nucleus. Al Hargan et al. (2021) conferred that MSG increased APC, BECN1 gene expressions and decreased TP53 gene expression in colon cancer cell lines which decisively supports the current findings on higher MSG consumption with childhood colon cancer risk. β-catenin protein coded by CTNNB1gene is the key transcription modulator of Wnt signaling pathway and the hotspot mutation of this gene change the regulatory serine residues (S45F) and threonine (T41A) at NH2-terminals are established in MSI CRC (Morin et al., 1997). Our study quantified colon immunohistochemistry for β-catenin expression, revealing positive β-catenin immunostaining in the DMH and MSG groups (Fig. 5E), while the normal control showed negative staining (Fig. 5A). Additionally, Western blotting results indicated β-catenin nuclear translocations due to phosphorylation at Ser33/37/Thr4 (Fig. 6C), which aligns with the aforementioned findings.
The present study reveals that intake of MSG at specified doses in rat models resulted in decreased survival rates and changes in serum biochemical measures associated with the development of colon polyps. These alterations contributed to the progression of colon cancer by affecting β-catenin protein expression and further complicating the Wnt/β-catenin molecular pathway (Fig. 7), thereby increasing the risk of colon cancer. These findings suggest the potential for MSG to induce changes in the Wnt/β-catenin signaling pathway, highlighting the need for caution regarding the use of MSG as a food additive. The present study results were summarized in Fig. 7.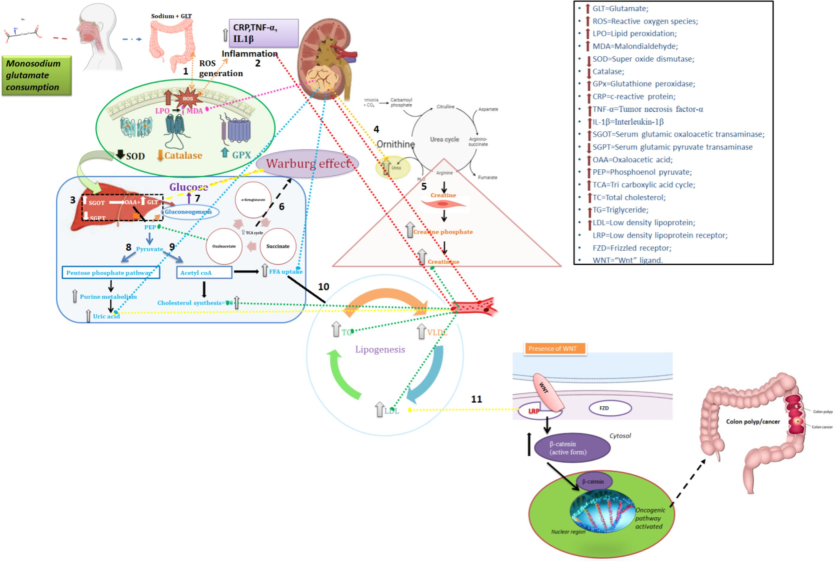
Summarizing the impact of MSG consumption on biochemical changes andWnt/ β-catenin pathways linked to colon cancer development. 1. Increased MSG consumption increases lipid peroxidation by increased MDA which increase ROS generation and antioxidant enzyme GPX, decreased antioxidant enzyme activity of SOD and catalase; 2. Increased ROS generation and defective antioxidant enzyme action provokes inflammation resulting in increased serum inflammatory markers namely CRP,TNFα,IL-1β; 3.increased ROS influence the defective organ functions there by altering the liver and kidney metabolism: Increased SGOT increases glutamate and oxaloacetate; 4. Due to defective kidney function glutamate metabolise with ornithine and increase the urea level; 5. Creatinine increase due to Creatine defect metabolism;6. Glutamate is metabolised through TCA cycle where the higher ATP production supplies energy to the cancer cell progression; 7. If glutamate go through the production of glucose via gluconeogenesis again the glucose enters TCA cycle; this triangular effect of glutamate, glucose and TCA cycle is well defined by the Warburg effect for cancer cell initiation and progression; 8.another metabolite namely oxaloacetate converts to PEP and then to pyruvate enters to pentose phosphate pathway and increase uric acid; 9. Pyruvate converts to acetyl-coA which enter to cholesterol synthesis and 10. Lipogenesis where the synthesis of TG, VLDL and LDL occurs and get increased 11. At this point, our hypothetical pathway focuses on the LDL increase; where the LDL increase could alter the lipid modifiable protein namely Wnt/β catenin. Assuming, from the obtained research results of biochemical and β catenin expression and subcellular localization and propose that LDL binds with LRP receptor in the presence of which is involved in the activation of β catenin mediated oncogenic pathway of colon cancer.
5 Conclusion
In conclusion, based on the results, the study underscores the potential toxic and carcinogenic role of MSG, particularly at neoplastic stages of colon cancer in male Wistar rat model.
CRediT authorship contribution statement
Meenakshi Sundari Rajendran: Writing – original draft, Methodology, Investigation, Conceptualization. Selvaraj Jayaraman: Writing – review & editing, Methodology, Investigation. Javed Masood Khan: Conceptualization. Sharmila Jasmine: Writing – review & editing, Formal analysis. RajKumar Prabhakaran: Formal analysis, Data curation. Manikandan Vani Raju: Data curation. Meenakshi Kaniyur Chandrasekaran: Conceptualization. Rathi Muthaiyan Ahalliya: Supervision, Conceptualization. Poornima Kannappan: Conceptualization. Chella Perumal Palanisamy: Writing – review & editing. Gopalakrishnan Velliyur Kanniappan: Writing – review & editing, Formal analysis.
Acknowledgements
The authors are grateful to the Researchers Supporting Project number (RSP2024R360), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alterations in APC, BECN1, and TP53 gene expression levels in colon cancer cells caused by monosodium glutamate. Braz. J. Biol.. 2021;83
- [Google Scholar]
- AVMA guidelines for the euthanasia of animals: 2020 edition. Members of the Panel on Euthanasia AVMA Staff Consultants. Schaumburg, IL: AMVA; 2020.
- Sequential fractionation and isolation of subcellular proteins from tissue or cultured cells. MethodsX. 2015;2:440-445.
- [CrossRef] [Google Scholar]
- Mechanistic study of attenuation of monosodium glutamate mixed high lipid diet induced systemic damage in rats by Coccinia grandis. Sci. Rep.. 2020;10(1):15443.
- [CrossRef] [Google Scholar]
- Assessment of beta-catenin expression by immunohistochemistry in colorectal neoplasms and its role as an additional prognostic marker in colorectal adenocarcinoma. Med. Pharmacy Rep.. 2019;92(3):246.
- [Google Scholar]
- Quercetin counteracts monosodium glutamate to mitigate immunosuppression in the thymus and spleen via redox-guided cellular signaling. Phytomedicine. 2024;126:155226
- [CrossRef] [Google Scholar]
- Survival effect of probiotics in a rat model of colorectal cancer treated with capecitabine. World Journal of Gastrointestinal Oncology. 2021;13(10):1518.
- [CrossRef] [Google Scholar]
- Gopan S, P., Das, A., Esmeeta, A., Deka, D., Duttaroy, A.K., Pathak, S. and Banerjee, A., 2023. An Insight on Colon Cancer Stem Cells and Its Therapeutic Implications. In Handbook of Oncobiology: From Basic to Clinical Sciences, 1-23. Singapore: Springer Nature Singapore. DOI: 10.1007/978-981-99-2196-6_63-1.
- Childhood cancer mortality in India: direct estimates from a nationally representative survey of childhood deaths. J. Global Oncol.. 2016;2(6):403-411.
- [Google Scholar]
- Evaluation of the aesthetics of physical methods of euthanasia of anesthetized rats. J. Am. Assoc. Lab. Anim. Sci.. 2011;50(5):695-701.
- [Google Scholar]
- Monosodium glutamate: Review on clinical reports. Int. J. Food Prop.. 2017;20(sup2):807-1815.
- [Google Scholar]
- Colon carcinoma in childhood: review of the literature with four case reports. Int. J. Colorectal Dis.. 2013;28:157-164.
- [Google Scholar]
- Kune, G.A., Bannerman, S. and Watson, L.F., 1992. Attributable risk for diet, alcohol, and family history in the Melbourne Colorectal Cancer Study.
- The association and joint effect of serum cholesterol, glycemic status with the risk of incident cancer among middle-aged and elderly population in china cardiometabolic disease and cancer cohort (4C)-study. Am. J. Cancer Res.. 2020;10(3):975. PMID: 32266104
- [Google Scholar]
- Distribution and patterns of use of food additives in foods and beverages available in Brazilian supermarkets. Food Funct.. 2021;12(17):7699-7708.
- [Google Scholar]
- Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275(5307):1787-1790.
- [Google Scholar]
- Monosodium glutamate (MSG) renders alkalinizing properties and its urinary metabolic markers of MSG consumption in rats. Biomolecules. 2019;9(10):542.
- [CrossRef] [Google Scholar]
- Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients. 2021;13(6):1865.
- [CrossRef] [Google Scholar]
- Effect of kaempferol on lipid peroxidation and antioxidant status in 1, 2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur. J. Pharmacol.. 2011;654(1):75-79.
- [Google Scholar]
- Chronic toxicity of low dose monosodium glutamate in albino Wistar rats. BMC. Res. Notes. 2019;12:1-7.
- [CrossRef] [Google Scholar]
- Targeting Wnt signaling in colorectal cancer. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol.. 2015;309(8):C511-C521.
- [Google Scholar]
- Suppression of colonic oxidative stress caused by chronic ethanol administration and attenuation of ethanol-induced colitis and gut leakiness by oral administration of sesaminol in mice. Food Funct.. 2022;13(18):9285-9298.
- [CrossRef] [Google Scholar]
- Investigation of a child-equivalent dose of monosodium glutamate toxicity in Wistar rats. Journal of Excipients and Food Chemicals. 2023;14(2):59-70.
- [Google Scholar]
- The Effects of Apocynin on Monosodium Glutamate Induced Liver Damage of Rats. Heliyon 2023
- [CrossRef] [Google Scholar]
- Chronic toxicity of monosodium glutamate on the reproductive system and some internal organs of zebrafish (Danio rerio) The Thai Journal of Veterinary Medicine. 2019;49(4):335-342.
- [CrossRef] [Google Scholar]
- Saudagar, P. and Tripathi, T. eds., 2022. Advanced spectroscopic methods to study biomolecular structure and dynamics. Elsevier.
- Investigating the effect of exposure to monosodium glutamate during pregnancy on development of autism in male rat offspring. Food Chem. Toxicol.. 2024;114464
- [CrossRef] [Google Scholar]
- Advances in protein molecular and structural biology methods. Academic Press; 2022.
- Cholesterol enhances colorectal cancer progression via ROS elevation and MAPK signaling pathway activation. Cell. Physiol. Biochem.. 2017;42(2):729-742.
- [CrossRef] [Google Scholar]
- The effect of age, sex and strains on the performance and outcome in animal models of stroke. Neurochem. Int.. 2019;127:2-11.
- [CrossRef] [Google Scholar]







