Translate this page into:
Investigate the utilization of novel natural photosensitizers for the performance of dye-sensitized solar cells (DSSCs)
⁎Corresponding author at: Physics of Electronics Materials Research Division, Department of Physics, Faculty of Mathematics and Natural Sciences, Institut Teknologi Bandung, Bandung, 40132 Indonesia. achmad.nasyori1@gmail.com (Achmad Nasyori), fatimah@itb.ac.id (Fatimah Arofiati Noor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dye-sensitized solar cells (DSSCs) offer a promising route for sustainable energy conversion, with natural photosensitizers emerging as attractive alternatives to conventional synthetic dyes due to their abundant resources, cost-effectiveness, and eco-friendly materials. However, the efficiency of DSSC utilizing natural photosensitizer remains low. In this study, we investigate the utilization of novel natural photosensitizers extracted from gambier leaves, gambier branches, cinnamon, and petiole of tectona leaves, which contain flavonoids/tannins, chlorophyll, and anthocyanins, aiming to achieve high-performance DSSCs. Five different solvents—ethanol, isopropanol, distilled water, methanol, and Zamzam water—are explored to optimize the extraction process of the natural dyes. The doctor blade technique is employed to coat TiO2 nanomaterials onto ITO glass substrates. UV–Vis spectrophotometry and FTIR spectroscopy are used to characterize the optical properties and structural composition of the dyes, revealing that flavonoid/tannin groups are the primary compounds responsible for light harvesting. The DSSC performance is evaluated under a 30 W lamp, adjusted to light intensity of 10 mW/cm2. As a result, the DSSCs using gambier leaf extract as photosensitizer demonstrate the highest recorded efficiency of 4.71 %, with a Jsc of 2.95 mAcm−2 and a Voc of 0.64 V. These findings contribute to advancing DSSC technology by leveraging the potential of natural photosensitizers for sustainable energy conversion applications.
Keywords
Natural photosensitizers
Dye-sensitized Solar Cells
Flavonoid/tannis
Solvent variations
High recorded performance
1 Introduction
The growing urgency for renewable and sustainable energy sources has become paramount as the environmental threats posed by fossil fuels—particularly pollution and global warming—intensify. Among the renewable alternatives, solar energy emerges as a leading candidate due to its inexhaustible supply and diverse benefits, making it a key focus for widespread research and application. In this context, dye-sensitized solar cells (DSSCs), recognized as third-generation photovoltaic technology, have garnered significant global attention. Their appeal lies in key advantages such as low-cost production, environmental friendliness, semi-transparency, flexible design, and a straightforward manufacturing process. For more than two decades, Grätzel cells have been rigorously studied and optimized, driven by their unique characteristics, establishing DSSCs as a promising alternative to conventional photovoltaic devices (Grätzel, 2003). While DSSCs hold great promise for meeting the energy demands of the next generation, their commercialization requires further effort. Significantly effective research is currently underway to enhance each component of the DSSC, aiming to prolong its stability and boost its efficiency.
A typical DSSC consists of a dye-sensitized semiconductor photoanode (working electrode), photocathode (counter electrode), and an electrolyte containing the I3−/I− redox couple as shown in Fig. 1b. In principle upon photoexcitation, the photosensitizer undergoes a transition where an electron is promoted from its ground state to an excited state, enabling injection into the conduction band of TiO₂. This electron transfer is crucial for the generation of photocurrent in DSSCs. Subsequently, the oxidized dye is restored to its ground state through electron donation from the redox electrolyte, which typically consists of an organic solvent system containing the iodide/triiodide (I−/I3−) redox couple. The regeneration of the oxidized dye by iodide prevents the recombination of the conduction band electron with the oxidized sensitizer, thereby sustaining the charge separation essential for efficient device operation. The iodide itself is regenerated by the reduction of triiodide at the counter electrode, a process facilitated by electron migration through the external circuit. This electron flow completes the electrochemical cycle, ensuring continuous device functionality under illumination (Grätzel, 2003; Bella et al., 2015; Cruz et al., 2022;Yadav et al., 2023).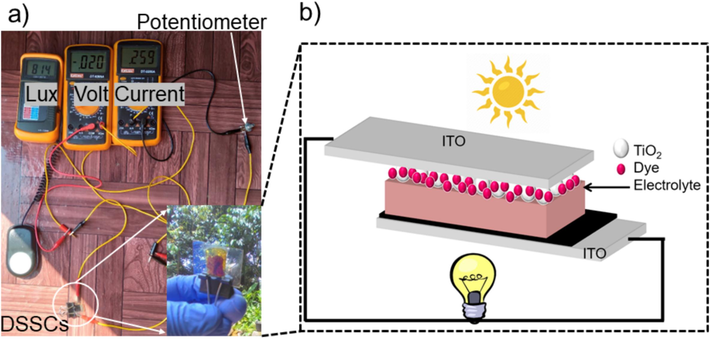
(a) Schematic of the I-V measurement under the direct sunlight and (b) Illustration of the components and working principle of DSSCs.
In DSSCs, the photosensitizer plays a pivotal role in capturing sunlight and converting it into electricity. While various inorganic dyes, organic dyes, and natural dyes have been synthesized and employed as sensitizers, ruthenium-based synthetic organic dyes have emerged as particularly effective. Notably, DSSCs sensitized with Ru-based N719 dye have achieved the highest reported efficiency to date, exceeding 12 % (Ji et al., 2020; Zhang et al., 2019). However, inorganic dyes face several challenges, including complex synthesis procedures, costly raw materials, environmental impacts, non-biodegradability, and regulatory constraints (Bella et al., 2015; Cruz et al., 2022; Yadav et al., 2023). Consequently, the use of natural dyes or metal-free organic sensitizers has emerged as a viable alternative due to their abundant resources and cost-effectiveness.
A variety of natural compounds, encompassing flavonoids (Seithtanabutara et al., 2023), anthocyanins (Ghann et al., 2017; Rajaramanan et al., 2023), chlorophyll (Al-Alwani et al., 2016; Chang et al., 2010; Conrad-Fletemeyer et al., 2023; Nan et al., 2017), tannins (Nasyori et al., 2024), carotenoid (Chumwangwapee et al., 2023; Prakash et al., 2023), and curcumin (Suyitno et al., 2018), derived from diverse botanical sources such as fruits (Nasyori et al., 2024), leaves (Al-Alwani et al., 2016; Hendi et al., 2023), flowers (Cruz et al., 2022; Patunrengi et al., 2019), roots (Chumwangwapee et al., 2023; Khammee et al., 2023), seeds (Gómez-Ortíz et al., 2010; Prakash et al., 2023), and wood (Nasyori et al., 2024), have been extensively extracted and employed as photosensitizers in DSSCs.
In a systematic investigation by Iswadi et al. (Patunrengi et al., 2019), three distinct DSSC devices were constructed utilizing natural photosensitizers extracted from leaves, flowers, and a combination of mimosa Linn, achieving a maximum efficiency of 0.16 %. Similarly, Seithtanabutara et al., (Seithtanabutara et al., 2023) employed a composite approach by combining photosensitizers derived from ivy gourd leaves and turmeric, resulting in an enhanced efficiency of 0.30 %. Further studies corroborated these findings, demonstrating the synergistic effects of combining anthocyanin and chlorophyll extracted from Troll flowers and Cypress leaves, resulting in an efficiency of 0.22 % (Nan et al., 2017). Additionally, Soosairaj et al. (Soosairaj et al., 2023) employed a photosensitizer composed of carotenoids extracted from Leucophyllum frutescens and Ehretia microphylla, achieving a conversion efficiency of 1.33 %. In another study, Ghann et al. (Ghann et al., 2017) utilized the anthocyanin extracted from pomegranate as a photosensitizer, reporting an efficiency of 2.0 %, an open circuit voltage of 0.39 V, and an open circuit current of 12.2 mA/cm2. Despite these advancements, the recorded efficiencies of DSSCs employing natural dyes as photosensitizers still fall short compared to those utilizing inorganic dyes. Therefore, there is a pressing need to explore and identify novel natural photosensitizers to enhance DSSC performance.
In our previous research (Nasyori et al., 2024), we successfully identified novel natural photosensitizers for DSSCs, specifically gambier fruit or seed extract and petiole of tectona leaves, achieving notable conversion efficiencies of 4.71 % and 4.09 %, respectively. Notably, gambier fruit/seed extract comprises a composite of leaves and stems obtained from gambier plants, processed to produce the gambier fruit or seed. Gambier, an indigenous plant of Kubang Balambak, West Sumatra, Indonesia, is traditionally utilized in medical treatments and as a natural dye source. The primary compound in gambier seed is tannin, belonging to the flavonoid or catechin group. In this work, we focus on the extraction and investigation of gambier leaves, gambier branches, cinnamon, date fruits, and petiole of tectona leaves as potential photosensitizers for DSSCs, encompassing variations in solvent usage. All photosensitizers are subsequently subjected to structural and optical property analysis, followed by the evaluation of their photovoltaic (PV) performance.
2 Material and fabrication
2.1 Materials
All solvents, including Isopropyl alcohol (IPA, 99 %), alcohol (99 %), methanol (99 %), and aquadest (99 %) were sourced locally from West Sumatra, Indonesia except the Zamzam solvent was purchased from Saudi Arabia. The raw natural dyes, such as gambier leaves, gambier branch, cinnamon, and petiole of tectona leaves were obtained and extracted from Indonesian natural resources, while the date fruits were procured from Saudi Arabia. Titanium dioxide (TiO2, 99 %, 5–10 nm) and Indium thin oxide (ITO, 7 – 10 /sq, 20 × 20 × 7 mm) were supplied by Titanos, China and Latech Scientific, Singapore, respectively. Potassium iodide (KI) and Iodine (I2) (99 %, solid) were obtained from ROFA Laboratorium Center, Indonesia.
2.2 Photosensitizer extraction processes
The extraction procedures begin with the raw natural materials (leaves of gambier, stem wood of gambier, cinnamon, date fruits, and petioles of tectona leaves). These materials were initially cleaned with water approximately 10 min, followed by washing with aquadest for ∼25 min. The cleaned materials were then left at room temperature for around 180 min. Each cleaned material was ground in a blender for approximately 25 min. Weighed samples, up to 250 g each, were then mixed with 300 mL of solvent (Isopropyl alcohol, alcohol, methanol, and aquadest) and macerated at room temperature for 24 h.
Following maceration, the dyes were evaporated for about 60 min and cooled at room temperature for approximately 130 min. The pure dyes were then filtered for 45 min and stored at room temperature. The filtered dyes were divided into two portions: one was kept at room temperature for future use, while the other was subjected to characterization. The optical and structural characterization was performed using an UV–Visible spectroscopy (wavelength range: 200–1200 nm and absorption range: 0–1 a.u) and Shimadzu IR Prestige-21 FTIR spectroscopy (wavenumber range: 500–4500 cm−1, with transmittance range: 0–50 %).
2.3 Fabrication of the photoanode and cathode of DSSCs
The fabrication of the primary components of DSSCs proceeded as follows: first, we prepared the photoanode, which comprises ITO glass, TiO2, and the photosensitizer extract. The ITO glasses were cleaned using the aquadest for approximately 5 min and IPA for about 15 min in an ultrasonic cleaner. After cleaning, the glasses were dried at room temperature for 15 min and their resistivity was measured. A 1 cm2 area on the positive side of the ITO glass was masked with tape as shown in Fig. 2a. Concurrently, 0.4 g of TiO2 was mixed with 0.6 mL of aquadest using ultrasonic cleaner until a paste was formed. This TiO2 paste was applied to the 1 cm2 ITO glass using the doctor blade techniques, resulting in a layer approximately 11 μm thick, as illustrated in Fig. 2a. The semi photoanode was then sintered at approximately 250 oC for 60 min and cooled at room temperature for 45 min. Following the step, semi-photoanode was soaked in photosensitizer for 12 h, resulting the final photoanode as depicted in Fig. 2b. Next, we fabricated the photocathode or counter electrode, which consists of ITO and carbon derived from candle soot (Toor and Sayyad, 2021). We used the cleaned ITO glass from previous process. The active area of the ITO glass was coated with carbon from candle soot, and the design was cleaned with cotton wool to define a 1 cm2 area and with a thickness of 11 μm (the same with photoanode) as shown in Fig. 2c. Finally, the electrolyte was prepared by dissolving 5 g of potassium iodide (KI) and 0.5 g of iodine (I₂) in 10 mL of distilled water (aquades) and mixing in an ultrasonic cleaner for 15 min.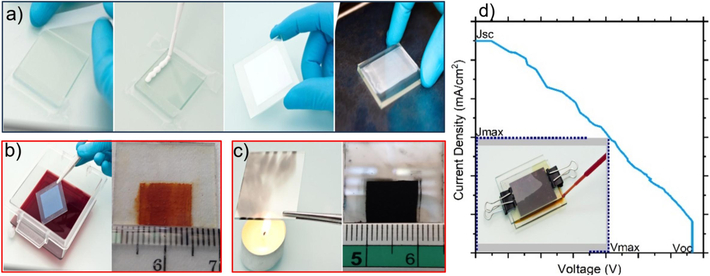
Illustration of DSSC fabrication (a) photoanode deposition process from left to rigth (b) deposite a photosensitizer on photoanode (c) cathode and (d) DSSC divice (Reproduced from ref. (Chebrolu and Kim, 2019) with permission from the Royal Society of Chemistry).
Upon fabricating all the DSSC core components, the next step was assembling the DSSC devices in a sandwich structure, as shown in Fig. 2d. Then, we measured the current and voltage (I-V) characteristics of the DSSC devices using a 30 W lamp set to an intensity of 10 mW/cm2. The setup included two multimeters to monitor the I-V flow, a lux meter to measure the input power, and a potentiometer to control the I-V flow as depicted in Fig. 1a. In this study, we fabricated twelve DSSC devices using photosensitizer extracted from gambier leaves, gambier branches, cinnamon, date fruits, and petioles of tectona leaves. We also considered to extract those photosensitizers using Isopropyl alcohol, alcohol, methanol, zamzam, and aquadest.
3 Result and Discussion
3.1 Optical and structural characterizations of natural photosensitizer extracts
UV–Vis spectroscopy results: The performance of DSSC is critically influenced by the light absorption capacity of the dye sensitizer and the efficient transfer of excited electrons through the mesoporous semiconductor layer. In this study, we collected gambier leaves, gambier branches, petioles of tectona leaves, and date fruits, and immersed them in various solvents including alcohol, distilled water, methanol, isopropyl alcohol, and Zamzam water (99 %). Altogether, we characterized twelve photosensitizer samples by examining their optical properties using UV–Visible spectroscopy across wavelengths ranging from 200–1200 nm.
Fig. 3a displays the absorbance results of gambier leaves dissolved by five solvents. The initial absorption peak, appearing between 350 – 400 nm, align with previous reports on extracted flavonoid/tannin compounds (Megala and Rajkumar, 2016; Nasyori and Noor, 2021; Rather et al., 2020). Additionally, secondary peaks at 655 and 666 nm were observed for samples immersed in alcohol and isopropyl alcohol, respectively. This indicates a transition from flavonoid to chlorophyll compounds (Rajaramanan et al., 2023). These results emphasize the dominance of flavonoid/tannin compounds in gambier leaves. Moving on to Fig. 3b, the optical properties of cinnamon, gambier branches, and petioles of tectona leaves were immersed in isopropyl alcohol and methanol. The Zamzam water was used for soaking date fruits and gambier branches. The prominent peaks between 300 – 375 nm across all solvents indicate the presence of flavonoid or tannin compounds (Rather et al., 2020). Furthermore, in Fig. 3b, the peaks at 394 nm and 666 nm for gambier branches immersed in isopropyl alcohol indicate a transition from tannin to chlorophyll compounds. Fig. 3c highlights the use of Zamzam water for the first time to extract compounds from date fruits and gambier branches. Interestingly, while immersion in isopropyl alcohol, alcohol, and methanol resulted in solidification of dates after 24 h, extraction using Zamzam solvent successfully yielded the active compounds from ajwa and sukkari dates. The absorbance peaks at 369 and 343 nm correspond to flavonoid/tannin compounds (Hammouda et al., 2014; Megala and Rajkumar, 2016; Nasyori and Noor, 2021; Rather et al., 2020).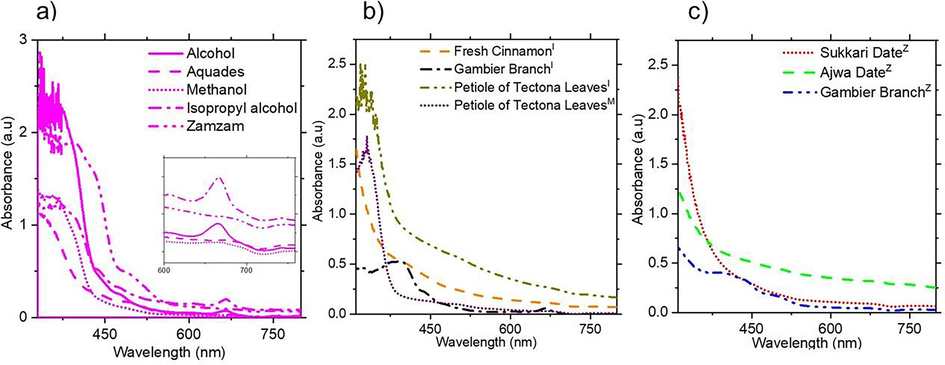
UV–Vis spectra of the natural photosensitiser extract with (a) gambier leaves using five solvents, (b) cinnamon, gambier branches, petioles of tectona leaves, using I=isopropyl alcohol, M=methanol and (c) sukkari date, ajwa date, and gambier branches using Z=Zamzam water.
FTIR spectra analysis: Each natural photosensitizer extract contains distinct pigment compounds, leading to varied electrical properties when exposed to sunlight. This pigment compounds can be categorized based on their characteristic absorbance spectra. As highlighted in the UV–Vis spectra results, we further explore the chemical composition of these natural photosensitizers, often classified within anthocyanin, flavonoid, curcumin, and chlorophyll groups. Fig. 4 presents the fundamental molecular structures of these pigment categories.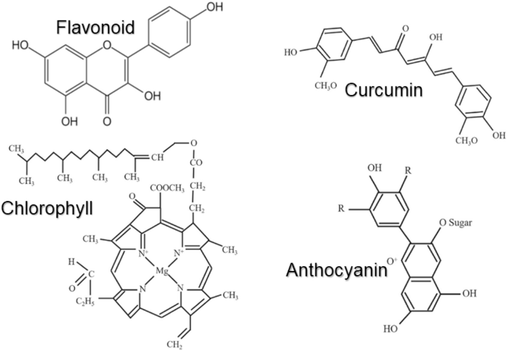
Chemical structures of key organic compound found in natural photosensitiser extracts, including flavonoid, curcumin, chlorophyll, and anthocyanin.
Flavonoids are characterized by a C6-C3-C6 carbon structure, facilitating extensive physiological activity. This structure includes a flavone or G-ring, that connects two benzena rings, as shown in Fig. 4, allowing efficient charge transfer from the highest-occupied molecule orbital (HOMO) to the lowest-unoccupied molecular orbital (LUMO), requiring minimal energy (Khammee et al., 2023; Mejica et al., 2022; Seithtanabutara et al., 2023). The electron injections from these dyes into the conduction band of a semiconductor enhances photoelectric conversion efficiency. Chlorophyll, one of the most commonly used photosensitizer in DSSCs, absorbs ultraviolet, red, and blue light, with absorbance peaks typically between 400 – 660 nm (Rajaramanan et al., 2023; Seithtanabutara et al., 2023). This pigment is abundant in plants, algae, and cyanobacteria. Another widely studied pigment is anthocyanin, responsible for blue, red, and yellow coloration in fruits and plants. Structurally similar to flavonoids, anthocyanins possess an oxygen atom and a C-ring, with absorbance peaks between 500–555 nm (Mejica et al., 2022; Seithtanabutara et al., 2023), whereas flavonoid peaks occur at 350–400 nm (Bella et al., 2015; Grätzel, 2003; Yadav et al., 2023). Curcumin, a deep yellow pigment derived from ginger, has a structure consisting of carbon double bonds, methoxy, and hydroxyl groups, with absorbance peaks in the 420–580 nm range (Seithtanabutara et al., 2023; Suyitno et al., 2018).
The chemical groups present in the natural photosensitizer extracts were characterized using FTIR spectroscopy in the range of 4000–500 cm−1. Fig. 5a shows the FTIR analysis of gambier leaves extracted with five different solvents. The spectra for gambier leaves extracted in alcohol, methanol, and isopropyl alcohol exhibit consistent peaks, including C=C stretching at 2152 cm−1, 1629 cm−1, and 1521 cm−1 (Gómez-Ortíz et al., 2010), C–H bending of methyl groups at 1466 cm−1 and 1379 cm−1(Gómez-Ortíz et al., 2010; Seithtanabutara et al., 2023), and C-O vibration at 1266 cm−1(Gómez-Ortíz et al., 2010). Additional peaks at 1200 cm−1, 1146 cm−1, 1106 cm−1, 1063 cm−1, 1050 cm−1, and 1035 cm−1 correspond to C–O–C stretching, while peaks at 992 cm−1 indicate =C–H, and those at 820 cm−1, 764 cm−1, 666 cm−1, and 630 cm−1 represent C–H bonds of alkene groups (Gómez-Ortíz et al., 2010; Seithtanabutara et al., 2023). For gambier leaves extracted in aquadest and Zamzam water, C=C stretching is observed at 2122 cm−1 and 1737 cm−1, with additional peaks for −C=C−stretching at 1629 cm−1, −C−C aromatic stretch at 1520 cm−1, C–H at 1466 cm−1 and 1394 cm−1, and −C−C−and −C−O stretching between 1285 cm−1 and 1107 cm−1. Peaks at 1052 cm−1, 945 cm−1, 821 cm−1, and 630 cm−1 represent C−O−C stretching and −COOH deformation (Rather et al., 2020). The region from 4000–2500 cm−1 indicates the presence of benzene rings, characteristic of flavonoid/tannin compounds forming intermolecular H-bonded frameworks and methyl groups, attributed to strong −OH stretching (Sampaio et al., 2019). Isopropyl alcohol shows a peak at 2930 cm−1 corresponding to alkane C–H stretching, consistent with weak absorption bands (Çakar et al., 2016).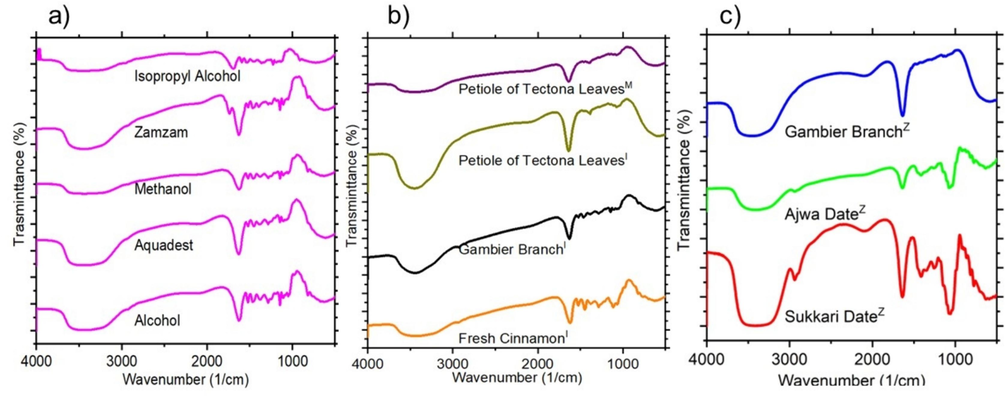
FTIR spectra of the natural photosensitiser extracts showing (a) gambier leaves using five different solvents (alcohol, methanol, isopropyl alcohol, aquadest, and Zamzam water), (b) cinnamon, gambier branches, petioles of tectona leaves, using I=isopropyl alcohol, M=methanol, and (c) sukkari date, ajwa date, and gambier branches using Z=Zamzam water.
Fig. 5b shows the petiole of tectona leaves, gambier branch, and cinnamon extracted in isopropyl alcohol and methanol. For tectona petioles in isopropyl alcohol, peaks at 1637 cm−1, 1346 cm−1, 1077 cm−1, and 576 cm−1 correspond to −C=C−stretching, −OH, C−O−C, and −COOH groups, respectively. Methanol extraction exhibits peaks at 1636 cm−1, 1456–1385 cm−1, 1154–1077 cm−1, and 613 cm−1, indicating − C=C−, −C−C−aromatic stretches, C−O−C stretching, and C–H bonds (Seithtanabutara et al., 2023). The gambier branch shows alkane C–H stretching at 2927 cm−1, C=O or carbonyl stretching at 1629 cm−1, C−O elongation at 1461 cm−1 and 1380 cm−1, and C–O vibrations at 1265 cm−1. Peaks at 1145 cm−1, 1072 cm−1, 919 cm−1, and 621 cm−1 indicate C−O−C stretching and C–H bonds (Gómez-Ortíz et al., 2010; Rather et al., 2020; Seithtanabutara et al., 2023). For cinnamon extracted with isopropyl alcohol, peaks at 1621 cm−1, 1446–1056 cm−1, and 977–628 cm−1 represent C=O, C−O, and C−H groups (Hirose et al., 2019).
The use of Zamzam water for extraction was tested on gambier branches, ajwa dates, and sukkari dates. Peaks for gambier branches include 2099 cm−1, corresponding to carbonyl stretching at 1636 cm−1, C−O elongation at 1461 cm−1, C−O−C stretching at 1145 cm−1, and C–H at 565 cm−1. For sukkari dates, strong − OH stretching peaks at 3904–3841 cm−1 indicate anthocyanin compounds (Sampaio et al., 2019). Ajwa and sukkari dates show peaks at 2938 cm−1, 2106 cm−1, 1636 cm−1, 1411–1059 cm−1, and 919–591 cm−1, corresponding to alkane C–H stretching, −C=C−stretching, C=O, C−O, and C−H groups (Gómez-Ortíz et al., 2010; Rather et al., 2020; Sampaio et al., 2019; Seithtanabutara et al., 2023).
It is worth noting that each natural dye exhibits a distinct compound profile, with transmittance values serving as indicative markers of predominant functional groups. The presence of carbonyl and hydroxyl groups significantly impacts the adherence of these dyes to semiconducting TiO₂ photoanodes, making them promising candidates for photosensitizers (Seithtanabutara et al., 2023). Strong anchoring between functionalized dye groups and TiO₂ is essential for efficient electron injection from the dye’s lowest energy levels into the TiO₂ conduction band, ultimately contributing to high-efficiency DSSCs (Rajaramanan et al., 2023).
3.2 Dye-Sensitizer solar cell performances
The next step of this study involved evaluating the performance of DSSC extracted from various natural photosensitizers. We fabricated twelve DSSC devices, each with an active area of 1 cm2 and immersed them in different natural dye extracts using a range of solvents. The devices were initially tested under the direct sunlight as illustrated in Fig. 1a. However, due to unpredictable weather conditions, we switched the measurement setup by using 30 W lamp, calibrated to an intensity of 10 mW/cm2 as illustrated in Fig. 1a. An I-V circuit was connected to enable accurate measurements.
Fig. 2d displays the key parameter such as Jmax, Vmax, Jsc, Voc, and FF, which are obtained directly. The measurements were recorded and calculated manually using two multimeters and a potentiometer. This manual approach facilitated straightforward calculation of the open-circuit voltage (Voc) and short-circuit current density (Jsc). Voc is defined as the voltage across the device when no current is flowing, while Jsc represents the current density when no external voltage is applied. The maximum power output (Pmax) and fill factor (FF) were subsequently calculated using Eq. (1).
Gambier leaves extracted from five different solvents: Our initial study focused on extracting Gambier leaves using five different solvents and evaluating their effectiveness as photosensitizers in DSSC devices. Notably, the highest efficiency of 4.71 %, with a Jsc of 2.95 mA/cm2 and a Voc of 0.64 V, was achieved with the photosensitizer extracted using aquadest, as shown in Fig. 6a and Table 1. This result aligns with our FTIR transmittance analysis, which revealed that aquadest extraction led to a strong presence of C=C stretching, −C=C−, and −C−C aromatic stretching, indicating a higher extraction yield of flavonoid/tannin compounds (Rather et al., 2020). Interestingly, the consistency of Voc values across solvents such as alcohol, aquadest, and zamzam suggests that solvent choice has a minimal impact on Voc, likely due to their similar absorbance peak ranges. This observation is in line with previous literature findings (Grätzel, 2003). It is important to note that the choice of solvent can significantly influence other performance parameters and should be carefully considered during optimization.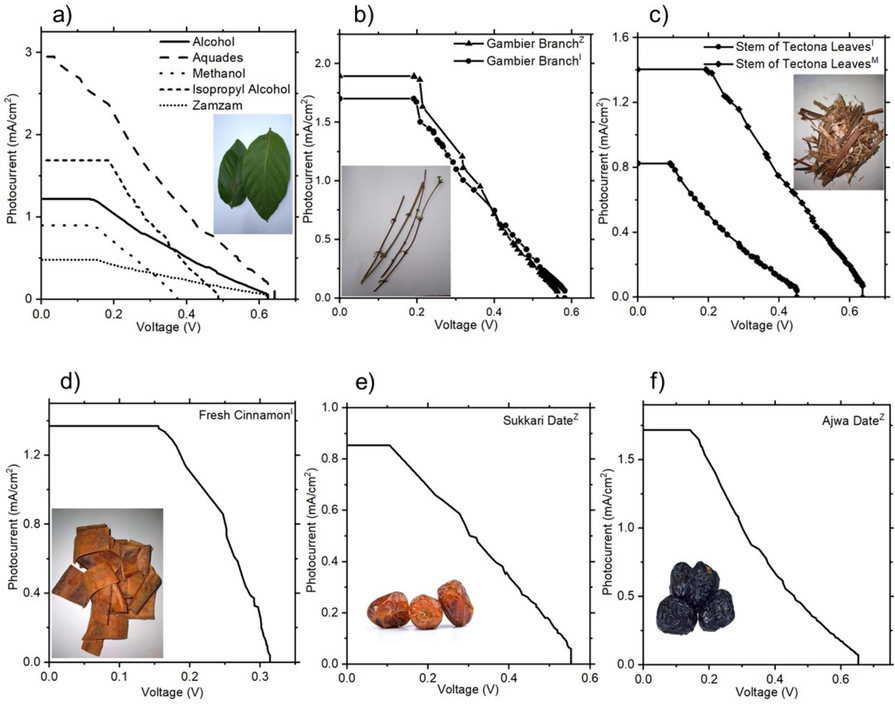
Performance curves of DSSCs with photoanode sensitized using extract from various natural materials and different solvents. (a) Gambier leaves, (b) Gambier branches, (c) Petiole of tectona leaves, (d) Cinnamon, and (e-f) Date fruits.
Natural Dyes
Solvent
Jsc
mAcm−2
Voc
V
FF
Pin
mWm−2
η
%
Gambier LeavesAlcohol
1.22
0.62
0.30
10
2.33
Methanol
0.89
0.37
0.42
10
1.42
Isopropyl alcohol
1.69
0.48
0.38
10
3.15
Aquadest
2.95
0.64
0.24
10
4.71
Zamzam
0.48
0.61
0.32
10
0.97
The second-highest efficiency was obtained with the photosensitizer extracted in isopropyl alcohol, yielding an efficiency of 3.15 %, with a Jsc of 1.69 mA/cm2 and a Voc of 0.48 V. Our analysis, supported by UV–Vis and FTIR data, indicates that the combination of flavonoid and chlorophyll compounds in this extract may contribute to the enhanced performance of the DSSCs. This transition between flavonoid and chlorophyll components highlights the complex interplay between different photosensitizer compounds and their effects on device performance (Rajaramanan et al., 2023; Seithtanabutara et al., 2023).
Isopropyl alcohol and methanol solvents: The second study investigated the performance of DSSCs sensitized with natural extracts from cinnamon, gambier branch, and petioles of tectona leaves, extracted in isopropyl alcohol (IPA) and methanol solvents. The devices exhibited promising efficiencies, with recorded values above 1 %, demonstrating the potential of these natural extracts as viable photosensitizers for DSSCs, as shown in Fig. 6b-e and Table 2. Particularly noteworthy were the efficiencies achieved with the gambier branch extracted in IPA and petioles of tectona leaves in methanol, reaching 3.46 % (with Jsc = 1.70 mAcm−2 and Voc = 0.58 V) and 3.31 % (with Jsc = 1.40 mAcm−2 and Voc = 0.63 V), respectively. These results underscore the efficacy of natural extracts as viable alternatives to traditional synthetic dyes in DSSCs. The superior performance of DSSCs sensitized with gambier branch extracted in IPA can be attributed to several factors. Spectral analysis revealed a high peak in absorbance spectra at 666 nm, signifying robust light absorption. Additionally, the presence of alkane C–H stretching at 2927 cm−1 suggests a synergistic interaction between flavonoid and chlorophyll compounds. These findings are consistent with prior studies (Grätzel, 2003; Rajaramanan et al., 2023; Rather et al., 2020; Seithtanabutara et al., 2023). Similarly, DSSCs sensitized with petioles of tectona leaves in methanol also demonstrated notable efficiency, highlighting the potential of this natural extract as a photosensitizer. The observed performance may be attributed to the unique chemical composition of tectona leaves, which may enhance efficient light absorption and charge transfer processes.
Natural Dyes
Solvent
Jsc
mAcm−2
Voc
VFF
Pin
mWm−2
η
%
Fresh Cinnamon
Isopropyl alcohol
1.37
0.31
0.52
10
2.26
Gambier Branch
Isopropyl alcohol
1.70
0.58
0.35
10
3.46
Petioles of Tectona Leaves
Isopropyl alcohol
0.82
0.45
0.27
10
1.01
Petioles of Tectona Leaves
Methanol
1.40
0.63
0.37
10
3.31
Zamzam water solvent: Our next study investigated the utilization of Zamzam water as a solvent for natural photosensitizers extracted from date fruits and gambier branches in DSSCs. Remarkably, the gambier branch extract in Zamzam water yielded the highest recorded conversion efficiency in this series, achieving an η of 3.85 %, a Jsc of 1.89 mA/cm2, and a Voc of 0.53 V, as shown in Table 3. This result is particularly noteworthy as it marks the first successful application of Zamzam water as a solvent in DSSCs. The promising performance observed suggests that Zamzam water could be a valuable candidate for further investigation, especially regarding its potential effects on DSSC performance. The unique properties of Zamzam water, which is revered in Islamic tradition and believed to possess beneficial qualities, make it an intriguing solvent for DSSC technology. The high conversion efficiency achieved with the gambier branch extract in Zamzam water highlights the critical role of solvent choice in optimizing DSSC performance. The enhanced light absorption and charge transfer efficiency observed may be attributed to specific interactions between the natural photosensitizers and the unique composition of Zamzam water. These results underscore the need for further studies to better understand the underlying mechanisms and to refine the use of Zamzam water in DSSC fabrication.
Natural Dyes
Solvent
Jsc
mAcm−2
Voc
VFF
Pin
mWm−2
η
%
Ajwa Date
Zamzam
1.71
0.65
0.27
10
3.12
Sukari Date
Zamzam
0.46
0.32
0.72
10
1.08
Gambier Branch
Zamzam
1.89
0.53
0.37
10
3.85
From Tables 1 to 3, it is evident that the fill factor (FF) of all-natural photosensitizers is inconsistent. This variability is likely due to the complex compositions and unstable carbonyl and hydroxyl functional groups present in natural dyes. Such factors may lead to inconsistent dye adsorption on the TiO₂ surface, resulting in weak adhesion, poor electron transfer, and increased recombination losses, ultimately impacting the FF negatively (Mishra et al., 2009; Seithtanabutara et al., 2023).
Overall, our DSSCs utilizing natural photosensitizers demonstrate superior performance compared to those reported in previous studies (Al-Alwani et al., 2016; Chang et al., 2010; Chumwangwapee et al., 2023; Conrad-Fletemeyer et al., 2023; Cruz et al., 2022; Ghann et al., 2017; Gómez-Ortíz et al., 2010; Hendi et al., 2023; Khammee et al., 2023; Nan et al., 2017; Patunrengi et al., 2019; Prakash et al., 2023; Rajaramanan et al., 2023; Seithtanabutara et al., 2023; Suyitno et al., 2018). The highest efficiencies achieved in our work were 4.71 % with gambier leaves, 3.85 % with gambier branches, 3.31 % with Tectona petioles, and 3.12 % with Ajwa dates, highlighting the significant potential of these natural photosensitizers for further exploration in DSSC applications. Additionally, we emphasize the critical role of solvent selection, as it directly influences the interaction between dye molecular configurations and solvents, thereby affecting the optical and structural properties of the dyes. Notably, the novel use of Zamzam water as a solvent in DSSC technology points to alternative and sustainable approaches for enhancing solar energy conversion. Future research should delve deeper into the mechanisms behind the performance enhancements observed with mixtures of different dyes, as these insights could be key to advancing more efficient and sustainable solar cell technologies.
4 Conclusion
Our investigation highlights the potential of natural photosensitizers as viable alternatives to synthetic dyes in Dye-Sensitized Solar Cells for sustainable energy conversion. By exploring novel natural compounds extracted from various plant sources and optimizing extraction techniques, we have demonstrated the efficacy of gambier leaves as a promising photosensitizer, achieving a notable efficiency of 4.71 %. Furthermore, we emphasize the critical role of selecting appropriate solvents, as the interaction between the solvent and dye molecules significantly influences the molecular configuration and, consequently, the spectral and structural properties of the dye. Additionally, our study underscores the importance of flavonoids and tannins as key compounds for effective light harvesting. These findings pave the way for further advancements in DSSC technology, promoting a shift toward more environmentally friendly and efficient solar energy conversion methods.
Funding
This research was generously supported by the “Riset Desentralisasi PDUPT DIKTI” Research Grant from the Ministry of Research, Technology, and Higher Education of Indonesia for the 2021 fiscal year.
CRediT authorship contribution statement
Achmad Nasyori: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Iswadi I. Patunrengi: Review & editing. Fatimah Arofiati Noor: Writing – review & editing, Supervision, Project administration, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Optimization of dye extraction from Cordyline fruticosa via response surface methodology to produce a natural sensitizer for dye-sensitized solar cells. Results Phys.. 2016;6:520-529.
- [CrossRef] [Google Scholar]
- Investigation of vegetable tannins and their iron complex dyes for dye sensitized solar cell applications. Electrochim. Acta. 2016;209:407-422.
- [CrossRef] [Google Scholar]
- Dye-sensitized solar cell using natural dyes extracted from spinach and ipomoea. J. Alloys Compd.. 2010;495:606-610.
- [CrossRef] [Google Scholar]
- Recent progress in quantum dot sensitized solar cells: An inclusive review of photoanode, sensitizer, electrolyte, and the counter electrode. J. Mater. Chem. C. 2019;7:4911-4933.
- [CrossRef] [Google Scholar]
- Investigation of bi-colour natural dyes potential for dye sensitized solar cell. Energy Rep.. 2023;9:415-421.
- [CrossRef] [Google Scholar]
- Synthesis of donor substituted chlorophyll derivatives for applications in dye sensitized solar cells. European J. Org. Chem.. 2023;26:1-14.
- [CrossRef] [Google Scholar]
- Natural and synthetic flavylium-based dyes: the chemistry behind the color. Chem. Rev.. 2022;122:1416-1481.
- [CrossRef] [Google Scholar]
- Fabrication, optimization and characterization of natural dye sensitized solar cell. Sci. Rep.. 2017;7:1-12.
- [CrossRef] [Google Scholar]
- Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Sol. Energy Mater. Sol. Cells. 2010;94:40-44.
- [CrossRef] [Google Scholar]
- Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev.. 2003;4:145-153.
- [CrossRef] [Google Scholar]
- Tissue and cellular localization of tannins in tunisian dates (Phoenix dactylifera L.) by light and transmission electron microscopy. J. Agric. Food Chem.. 2014;62:6650-6654.
- [CrossRef] [Google Scholar]
- Dye-sensitized solar cells constructed using titanium oxide nanoparticles and green dyes as photosensitizers. J. King Saud Univ. - Sci.. 2023;35:1-7.
- [CrossRef] [Google Scholar]
- Direct one-step synthesis of a formally fully bio-based polymer from cellulose and cinnamon flavor. Green Chem.. 2019;21:4927-4931.
- [CrossRef] [Google Scholar]
- 14.2% Efficiency dye-sensitized solar cells by co-sensitizing novel Thieno[3,2-b]indole-based organic dyes with a promising porphyrin sensitizer. Adv. Energy Mater.. 2020;10:1-12.
- [CrossRef] [Google Scholar]
- Natural dyes extracted from Inthanin bok leaves as light-harvesting units for dye-sensitized solar cells. Appl. Nanosci.. 2023;13:391-403.
- [CrossRef] [Google Scholar]
- Theoretical study of anthoxanthin dyes for dye sensitized solar cells (DSSCs) J. Comput. Electron.. 2016;15:557-568.
- [CrossRef] [Google Scholar]
- Maejo International Journal of Energy and Environmental Communication photosensitizer for dye-sensitized solar cell : A review. Maejo Int. J. Energy Environ. Commun.. 2022;1:12-22.
- [Google Scholar]
- Metal-Free organic dyes for dye-Sensitized solar cells: From structure: Property relationships to design rules. Angew. Chemie - Int. Ed.. 2009;48:2474-2499.
- [CrossRef] [Google Scholar]
- Studies on the optical and photoelectric properties of anthocyanin and chlorophyll as natural co-sensitizers in dye sensitized solar cell. Opt. Mater. (Amst). 2017;73:172-178.
- [CrossRef] [Google Scholar]
- The effects of the concentration of red and yellow gambier fruit dyes on the short-circuit photocurrent in dye-sensitised solar cells. J. Phys. Conf. Ser.. 2021;1811:1-7.
- [CrossRef] [Google Scholar]
- Improved the DSSCs performance by sensitizing eight natural dyes and varying the annealing process on the TiO2. Mol. Cryst. Liq. Cryst.. 2024;768:32-46.
- [CrossRef] [Google Scholar]
- A new study of dye-sensitized solar cell from the extract of leaf, fruit, and mix of Mimosa pudica Linn. Proc. Int. Conf. Sci. Technol. ICOST. 2019;2:1-7.
- [CrossRef] [Google Scholar]
- Optimization, fabrication, and characterization of anthocyanin and carotenoid derivatives based dye-sensitized solar cells. J. King Saud Univ. - Sci.. 2023;35:1-11.
- [CrossRef] [Google Scholar]
- Natural sensitizer extracted from Mussaenda erythrophylla for dye-sensitized solar cell. Sci. Rep.. 2023;13:1-13.
- [CrossRef] [Google Scholar]
- Valorization of natural dyes extracted from mugwort leaves (folium artemisiae argyi) for wool fabric dyeing: optimization of extraction and dyeing processes with simultaneous coloration and biofunctionalization. ACS Sustain. Chem. Eng.. 2020;8:2822-2834.
- [CrossRef] [Google Scholar]
- Investigation of nanostructured TiO2 thin film coatings for DSSCs application using natural dye extracted from jabuticaba fruit as photosensitizers. Ionics (Kiel).. 2019;25:2893-2902.
- [CrossRef] [Google Scholar]
- Potential investigation of combined natural dye pigments extracted from ivy gourd leaves, black glutinous rice and turmeric for dye-sensitised solar cell. Heliyon. 2023;9 1–15
- [CrossRef] [Google Scholar]
- Synergetic effect of Leucophyllum frutescens and Ehretia microphylla dyes in enhancing the photovoltaic performance of dye-sensitized solar cells. Environ. Sci. Pollut. Res.. 2023;30:52895-52905.
- [CrossRef] [Google Scholar]
- Effect of light and temperature on the efficiency and stability of curcumin-dye-sensitized solar cells. Int. Energy J.. 2018;18:53-60.
- [Google Scholar]
- Candle soot based carbon counter electrode for cost-effective dye sensitized solar cells. Proc. 18th Int. Bhurban Conf. Appl. Sci. Technol. IBCAST. 2021;2021:87-92.
- [CrossRef] [Google Scholar]
- A brief review on natural dyes, pigments: Recent advances and future perspectives. Results Chem.. 2023;5:1-17.
- [CrossRef] [Google Scholar]
- 13.6% Efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state. ACS Energy Lett.. 2019;4:943-951.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103423.
Appendix A
Supplementary material
The following are the Supplementary material to this article:







