Translate this page into:
Indigenous plant extracts as novel antimicrobial arsenal: Unveiling the potential of Bismarckia nobilis, Choysia ternata, Chamaedora cataractarum, and Beaucarnea recurvate
⁎Corresponding authors. Amin.ullah@abasyn.edu.pk (Amin Ullah), tariq.nadeem@cemb.edu.pk (Tariq Nadeem)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction and Aim
Amid the rapid advancements in contemporary medicine, the resurgence of phytomedicine as a therapeutic avenue has garnered substantial attention. Nearly 30% of FDA-approved pharmaceuticals trace their origins to botanical sources. Phytomedicine has been shown to hold promising applications to attenuate bacterial virulence and source new of bioactive compounds to battle multidrug resistant pathogens. In this context, the current investigation delves into the antimicrobial potential of four indigenous plant species
Methodology
This study is primed to unveil the antibacterial and antifungal potential of n-hexane and methanolic extracts of Bismarckia nobilis, Choysia ternata, Chamaedora cataractarum, and Beaucarnea recurvate against strains of S. aureus and C. neoformans. These plant extracts' Minimum Inhibitory Concentration (MIC) was discerned via the agar well diffusion assay, microbroth dilution assay, and MTT reduction assay.
Results
Notably, the n-hexane extracts of B. nobilis and C. ternata exhibited robust activity against S. aureus strains, with 100 mg/mL concentrations yielding inhibition zones measuring 12.1–13.1 mm and 13.1–15.1 mm, respectively. Correspondingly, the methanolic extracts (100 mg/mL) of B. nobilis, C. ternata, C. cataractarum, and B. recurvata presented notable antifungal activity against Cryptococcus neoformans, as evidenced by zones of inhibition measuring 14.25 mm, 13.25 mm, 16.25 mm, and 17.35 mm, respectively. Microbroth dilution assays revealed that the MIC of CT and BN plants against S. aureus ranged from 0.78 to 3.125 mg/mL and 1.56–12.5 mg/mL, respectively, with a consequential MIC index of 0.1248 for BN and CT plants against S. aureus. The n-hexane extract of B. nobilis and C. ternata showed antibacterial activity against pathogenic S. aureus. Similarly, the methanolic extracts of B. nobilis, C. ternata, C. cataractarum, and B. recurvata exhibited potent antifungal activity against C. neoformans.
Conclusion
This study postulates indigenous plant-derived extracts as potent and multifaceted antifungal and antibacterial resources for antimicrobial development.
Keywords
Antifungal activity
Minimum inhibitory concentration
Agar well diffusion assay
Antibiotics
Staphylococcus aureus
Cryptococcus neoformans
- S. aureus
-
Staphylococcus aureus
- MRSA
-
Methicillen-resistant Staphylococcus aureus
- AIDS
-
Acquired Immune Deficiency Syndrome
- NMR
-
Nuclear magnetic resonance
- B. nobilis
-
Bismarckia nobilis
- C. ternata
-
Choysia ternata
- B. recurvata
-
Beaucarnea recurvata
- C. cataractarum
-
Chaemodorea cataractarum
- MSA
-
Mannitol Salt Agar
- MHA
-
Mueller Hinton Agar
- PCR
-
Polymerase chain reaction
- DMSO
-
Dimethyl Sulfoxide
- MIC
-
Minimum inhibitory concentration
- MBC
-
Minimum bactericidal concentration
- CFU
-
Colony forming unit
- MTT
-
3-(4,5-Dimethylthiazol-2-yl)-2.5-Diphenyltetrazolium Bromide
- SDA
-
Sabouraud Dextrose Agar
- C. neoformans
-
Cryptococcus neoformans
- µl
-
Micro-liter
- mg/ml
-
Milli-gram per milli-liter
- mm
-
Milli-meter
- RNA
-
Ribonucleic acid
- DNA
-
Deoxyribonucleic acid
- H2O2
-
Hydrogen peroxide
- dNTP's
-
Deoxy nucleotide triphosphates
- SE
-
Standard error
- nm
-
nanometer
- GC–MS
-
Gas chromatography-mass spectrometry
- HPLC-MS
-
High-performance liquid chromatography
Abbreviations
1 Introduction
Staphylococcus aureus is a gram-positive, facultative anaerobic bacterium that appears in grape-like clusters. It causes community and hospital-acquired infections, such as bacteremia, osteoarticular, and skin infections. The treatment of S.aureus related infections is becoming challenging due to the emergence of multidrug resistant strains such as Methicillin-resistant S. aureus (MRSA). As a result, S.aureus strains have become resistant to various antibiotics such as Methicillin, nafcillin, oxacillin, and cephalosporin (Liu et al., 2005). Similarly, Cryptococcus neoformans is a pathogenic unicellular yeast that causes cryptococcal meningitis in AIDS patients. Normally, it infects one million people each year around the world. Previously, cryptococcosis was treated efficiently with the help of many commercially available antifungal agents, such as Amphotericin B, Flucytosine, and Fluconazole. However, over time and due to extensive use, the fungus has developed resistance to these antifungals, rendering them ineffective (Almeida et al., 2015).
Plant extracts have been used since the dawn of human civilization to treat various ailments because they are a rich source of many phytochemicals such as alkaloids, phenols, and tannins. So, many plant-derived phytochemicals can be used as a harmless and cheaper alternative strategy for the treatment of S.aureus associated infections. Bismarckia nobilis is an evergreen dioecious palm with a single thick trunk and unique silver-blue, rounded, and fan-shaped leaves (Mitchell, 2012). It is generally known as a silver palm due to its color. Only limited data is available regarding the medicinal use of Bismarckia in history. Some studies have shown that the methanolic extract of Bismarckia nobilis has smooth muscle relaxant potential in diarrhea, hypertension, and asthma (Saqib et al., 2019). Bismarckia plant has also been used by the people of Madagascar for oral health care (Ranjarisoa et al., 2016). The antioxidant potential of B.nobilis fiber was examined by Schauss and Voon (Schauss and Voon, 2006). Choysia ternata is a species of flowering plant belonging to the genus Rutaceae, commonly known as Mexican orange blossom or Mexican orange. Radulović et al., (2013a) studied the effect of methyl and isopropyl N-methyl anthranilate from C.ternata on experimental anxiety and depression in mice. The anti-inflammatory potential of a hybrid plant of C.ternata, Choisya Aztec pearl, was assessed by Carvalho et al. (2014). Leitão et al. (2017) isolated quinolinic alkaloids from Choysia species by high-speed countercurrent chromatography and studied their antioxidant activity. Beaucarnea recurvata, also known as ponytail palm, is an evergreen perennial plant belonging to the Asparagaceae family. It is a native Mexican plant grown in Europe and many other parts of the world as an ornamental plant. Any medicinal use of this plant has not been reported so far, but Eskander et al. (2011) isolated thirteen steroidal components from the leaves of B.recurvata using NMR and mass spectroscopy. Lastly, Chamaedora cataractarum, also known as cat palm, is a small palm native to Central America and southern Mexico. To date, no data is available to suggest the medical importance of Chamaedora cataractarum plant.
The current study aims to prepare plant extracts in n-hexane and 70 % methanol to determine the antibacterial and antifungal activity against isolated S. aureus and C. neoformans strains, respectively.
2 Methodology
2.1 Collection and identification of plants
All plants selected for research were collected from the Botanical Garden of Punjab University. The plants were authenticated by the Department of Botany, University of The Punjab, Lahore, where a voucher number was assigned to each plant.
2.2 Preparation of plant extracts
n-hexane and 70 % methanol were used as solvents for preparing plant extracts by a slight modification of maceration described by Odey et al. (2012). Plant material was air-dried at room temperature and ground to form a fine powder. Pant powder was then added to the solvents in 1:4 and placed at 4 °C for three days with frequent agitation. After three days, the solution was filtered, and the solvent from the filtrate was evaporated in a rotary vacuum evaporator. After evaporation, a semisolid pellet of plant extracts was obtained in a 1.5 ml tube.
2.3 Determination of antibacterial activity of plant extracts
2.3.1 Bacteria selected for the study
Eight S.aureus strains were isolated from blood and pus samples. All samples were serially diluted in normal saline, and 10–5 dilution was spread onto mannitol salt agar (MSA) plate under aseptic conditions and allowed to incubate at 37 °C for 24 h. After frequent rounds of quadrant streaking, eight strains were subjected to identification based on their morphological, biochemical, and molecular characteristics. The morphological identification involved examining the colony morphology of isolated S. aureus strains on a selective medium, MSA, and microscopic examination by Gram staining. Moreover, the catalase and oxidase tests were performed to identify S. aureus. Lastly, the isolated S. aureus strains were identified at the molecular level by PCR amplifying the V3 region of 16S rRNA.
2.3.2 Antibacterial activity by agar well diffusion assay
The agar well diffusion method is widely used to evaluate the antimicrobial activity of plant extracts. 38 g Mueller-Hinton (MH) agar powder was weighed and dissolved in 1 L of distilled water. It was heated with frequent agitation and boiled for one minute to dissolve the medium completely. The medium was autoclaved at 121 °C for 15 min and cooled the agar to 45–50 °C before pouring it into sterile Petri dishes. MH agar was allowed to solidify. 100 mg/ml stock solution of plant extracts was used to determine the antibacterial activity of plant extracts by agar well diffusion assay. After that, 0.5 McFarland bacterial suspension was used to make a lawn of bacteria onto MHA plates with sterile cotton swabs under aseptic conditions. Wells of about 6 mm in diameter were punched on MHA plates with a sterile cork borer, and each well was filled with 100 µl of different plant extracts under sterile conditions. DMSO (Dimethyl sulfoxide) was used as a negative control. All plates were then incubated at 37 °C for 24 h. The volume of agar used in the well was fixed to ensure reproducible extract distribution.
2.4 Determination of MIC of plant extracts
The MIC of the plant extracts was determined using the agar well diffusion assay, microbroth dilution assay, and MTT reduction assay.
2.4.1 Agar well diffusion assay
The surface of MHA plates was inoculated with the bacterial suspension under sterile conditions. Eleven wells were punched into two plates, and each received a different plant concentration in descending order (50–0.05 mg/ml). After filling the agar wells with different concentrations of plant extracts, all plates were allowed to incubate at 37 °C for 24 h. The zone of inhibition of each well was measured the next day. The zone diameters were compared to standard interpretive criteria to determine the susceptibility of the pathogenic strains. This method allowed the determination of the susceptibility of S. aureus to various extracts based on the size of the inhibition zones. The size of the zone correlates with the susceptibility of the bacteria to the respective extract.
2.4.2 Micro broth dilution assay
Micro broth dilution assay was used to determine the MIC of plant extracts using liquid media and spectroscopic technique. It is a widely used method for determining antimicrobial agents' minimum inhibitory concentration (MIC) against bacterial or fungal strains. This assay involves the serial dilution of the antimicrobial agent in a microtiter plate containing a standardized inoculum of the target microorganism. The MIC is then determined as the lowest concentration of the antimicrobial agent that completely inhibits the visible growth of the microorganism after incubation.0.5 McFarland bacterial suspension was prepared in normal saline by picking up a single colony of bacteria from an agar plate. The first row of 96-well plates received only 200 µl MHA broth, serving as a sterility control. The second row of 96 well plates served as growth control, and it received 100 µl MHA broth and 100 µl bacterial suspension. Whereas the other rows (C to F) received 100 µl MHA. 100 µl of 100 mg/ml plant extract (CT) was added to the 1st well of rows C and D, and it was serially diluted till well 11. Similarly, the 1st well of rows E and F received the 100 mg/ml solution of the other plant extract (BN), and it was serially diluted until well 11. After that, 100 µl bacterial suspension was added to all the test wells. The plates were then incubated at 37 °C for 24 h. After 24 h, the absorbance of each plate was measured using a spectrophotometer. The lowest concentration of plant extract whose absorbance is the same or closely related to the sterility control and lower than that of growth control was considered the MIC of plant extract.
2.4.3 MTT reduction assay
The MIC of plant extracts was also evaluated by slightly modifying the MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide) reduction method as described by Malekinejad et al. (2012). It is a colorimetric assay that relies on color change for measurement. The 1st row of 96 well plate served as a sterility control, receiving only 200 µl of MHA broth. The second row served as growth control, containing 100 µl of MHA broth and 100 ul of the bacterial suspension. Rows C and D received the different concentrations of one plant (BN), while rows E and F received the different concentrations (50–0.05 mg/ml) of the other plant (CT). After that, 100 µl of bacterial suspension was added to all the rows C, D, E, and F wells, which previously contained MHA broth and plant extracts. The plates were allowed to incubate at 37 °C for 24 h. After 24 h of incubation, 22 µl of MTT solution prepared in PBS was added to all the wells, including sterility and growth control. The plates were wrapped in aluminium foil and placed in the dark for about 1 h at 37 °C. After that, 160 µl was removed from each well of the plate, and the remaining crystals were dissolved in 200 µl of DMSO. The MTT assay protocol measured the mitochondrial function of cells. The color of formazan crystals was compared with that of growth control, and the lowest concentration of plant extract that greatly reduces the color of formazan crystals was considered MIC (Gomez-Flores et al., 1995).
2.5 Bactericidal /bacteriostatic effect
After the determination of MIC of B. nobilis and Choysia. ternata plants by microbroth dilution assay, 25 µl aliquotes were removed from wells (MIC and above concentrations of plant extracts), serially diluted in normal saline, and then spread onto MHA plates. These plates were then allowed to incubate at 37 °C for 24 h. CFU/ml (colony forming units/ml) was calculated after 24 h of incubation. The bacterial concentration that caused 99.9 % inhibition in bacterial growth compared to growth control was considered the MBC of plant extract (Krishnan et al., 2010). To find the bacteriostatic or bactericidal effect of plant extract, MICindex was calculated by using the following formula.
MICindex = MIC/MBC.
2.6 Determination of antifungal activity of plant extracts
2.6.1 Fungus selected for the study
C. neoformans strains were isolated from the sputum sample by colony morphology and microscopic appearance. The samples were diluted in normal saline and spread on Sabouraud dextrose agar (SDA) medium. The culture plates were allowed to incubate at room temperature for 1 week. Moreover, C. neoformans strains were spread on Urease agar and incubated at 30 °C for 24 h.
2.6.2 Antifungal activity by agar well diffusion assay
Antifungal activity of all plant extracts (Bismarckia nobilis, Choysia ternata, Beaucarnea recurvata, Chamaedora cataractarum) was investigated against Cryptococcus neoformans by agar well diffusion assay. The sterilized SDA was poured into Petri dishes and allowed to solidify.SDA plates were then inoculated with 0.5 McFarland fungal suspension with sterile cotton swabs to make a lawn of fungal growth. Four wells of about 6 mm in diameter were punched into SDA plates, and each well was filled with 100 µl of plant extract (100 mg/ml). These plates were then incubated at 30 °C for about 48 h. After incubation, each well's fungal growth inhibition zone was measured in mm. The zone diameters were compared to standard interpretive criteria to determine the antifungal activity of the tested substances.
3 Results
3.1 Collection and identification of plants
The plants were authenticated by the Department of Botany, University of The Punjab, Lahore, and a voucher number was assigned to each (Supplementary data Table 1).
3.2 Characterization of bacterial strains
3.2.1 Biochemical characterization of bacterial strains
All strains formed rounded yellow-coloured colonies on MSA plates (Fig. 1). These colonies were purified by quadrant streaking until well-isolated colonies were obtained. All bacterial strains were gram-positive cocci in the form of grape-like clusters when viewed under the 100X lens of the compound microscope. Moreover, all strains were catalase-positive and oxidase-negative (Supplementary data Table 2).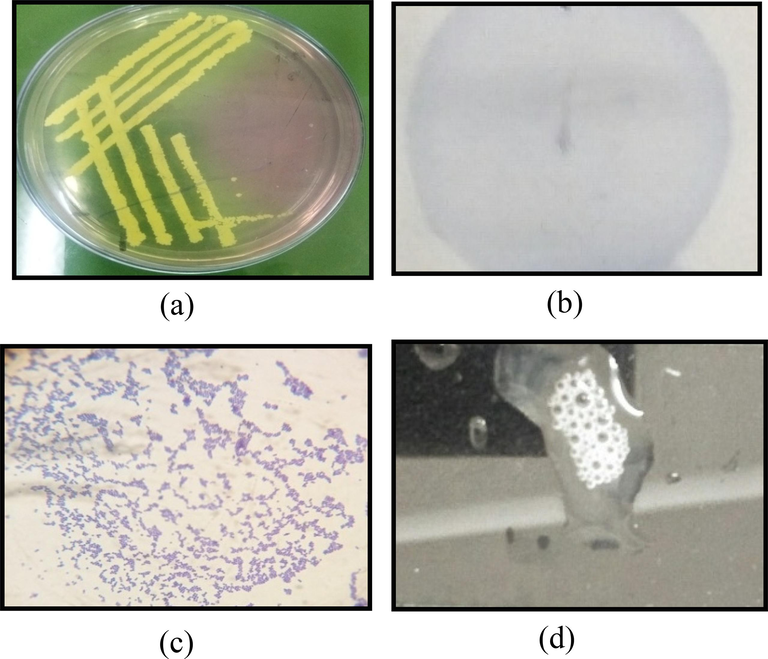
Biochemical characterization of S. aureus. (a) Growth of bacterial isolates on Mannitol salt agar (MSA) as rounded yellow-coloured colonies; (b) Appearance of bacterial isolates as clusters of purple-stained gram-positive cocci; (c) No purple coloration in oxidase negative, indicating absence of cytochrome C oxidase enzyme; (d) Bubble formation at the addition of 3% H2O2 indicated the presence of catalase.
3.2.2 PCR amplification of 16S rRNA gene of S.aureus
The reaction mixture used for PCR amplification consisted of 2.5U of Taq polymerase, 1X buffer, 0.15 mM dNTPs, 1.5 Mm MgCl2, primers (785F and 907R), and 1–2 µg of template DNA. The PCR reaction was allowed to run under optimized conditions (Supplementary data Figure 1).
3.2.3 Analysis of PCR products
PCR products were run on 1.5 % agarose gel along with a 100 bp ladder. DNA bands of about 144 bp were observed between 200 and 100 bp ladder segments (Fig. 2).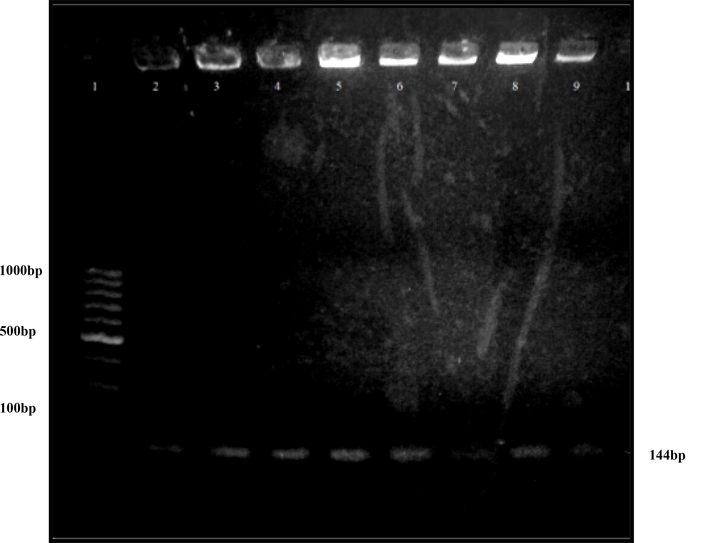
Analysis of V3 region of eight S.aureus strains by agarose gel electrophoresis. Lane 1 represents a 100 bp DNA ladder. Whereas lane 2–8 represents PCR products of S.aureus strains.
3.2.4 Antibacterial activity by agar well diffusion assay
After 24 h of incubation, the size of the zone of inhibition of different concentrations of plant extracts was measured in mm. Two plant extracts, BN and CT, formed clear zones of inhibition against the isolates of S. aureus tested (Fig. 3). At 100 mg/mL concentration, BN and CT yielded inhibition zones measuring 12.1–13.1 mm and 13.1–15.1 mm, respectively (Table 1).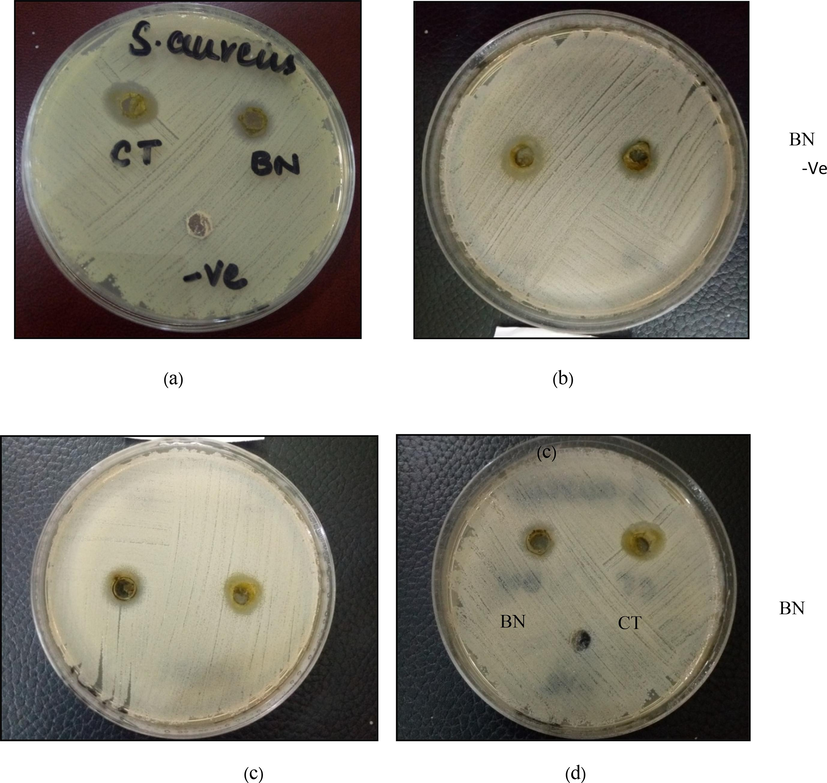
Antibacterial activity of (B. nobilis) BN and (C. ternata) CT plants against isolated S.aureus strain. DMSO was used as a negative control. (a) BN and CT inhibited bacterial growth and formed zones of inhibition measuring 15 ± 0.1 mm and 12.25 ± 0.25 mm, respectively, each at a 100 mg/ml concentration. (b) BN and CT inhibited bacterial growth and formed zones of inhibition measuring 13.1 ± 0.1 mm and 13.1 ± 0.1 mm, respectively, each at a 100 mg/ml concentration. (c) BN and CT inhibited bacterial growth and formed zones of inhibition measuring 14.1 ± 0.1 mm and 12.05 ± 0.25 mm, respectively, each at a 100 mg/ml concentration. (d) BN and CT inhibited bacterial growth and formed zones of inhibition measuring 15.1 ± 0.1 mm and 12.1 ± 0.1 mm, respectively, each at a 100 mg/ml concentration.
S.aureus strains
Zone of inhibition (mm)
C.ternata
B.nobilis
ASH3
15.1 ± 0.1
12.25 ± 0.25
ASH4
15 ± 0.0
12.5 ± 0.5
ASH5
13.1 ± 0.1
13.1 ± 0.1
ASH6
14.1 ± 0.1
12.05 ± 0.05
ASH7
14.05 ± 0.05
13.1 ± 0.1
ASH8
15.1 ± 0.1
12.1 ± 0.1
ASH2
15 ± 0.0
12.2 ± 0.0
ASH1
13.1 ± 0.1
12.1 ± 0.1
3.3 Determination of MIC of plant extracts
Three methods were used to determine the MIC of plant extracts, such as agar well diffusion assay, microbroth dilution assay, and MTT reduction assay.
3.3.1 Agar well diffusion assay
After 24 h of incubation, the size of the zone of inhibition of different concentrations of plant extracts was measured in mm. It was found that the zone of inhibition gradually decreased with decreasing concentrations of plant extracts (Fig. 4a, 4b). MIC of the CT plant determined by agar well diffusion assay was in the range of 1.56–3.125 mg/ml, whereas the MIC of the BN plant was in the range of 3.125–6.25 mg/ml (Tables 2a and 2b). All experiments were performed in duplicates, and results were displayed as mean diameter ± SE, where SE represents standard error. See (Fig. 5). – sign indicates no inhibition.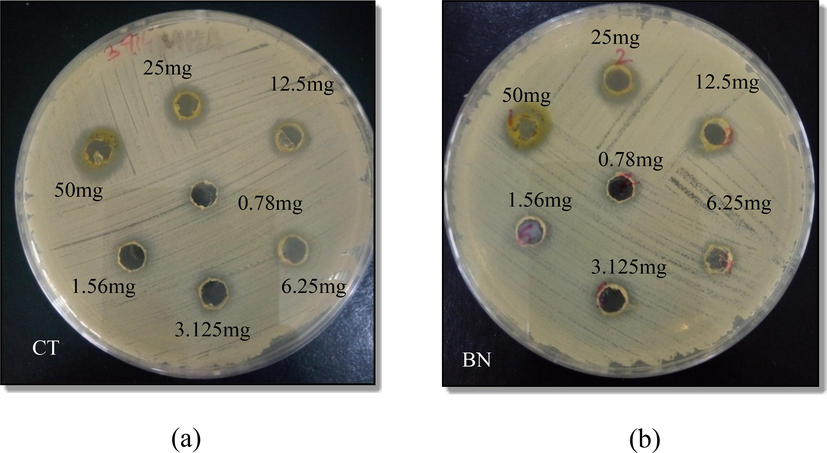
Effect of increasing concentration, from 0.78 mg/ml, 1.56 mg/ml, 3.125 mg/ml 6.25 mg/ml, 12.5 mg/ml, 25 mg/ml, to 50 mg/ml, of Choysia ternata extract (CT) and Bismarckia nobilis extract (BN) against S.aureus. (a) CT inhibited bacterial growth and formed zones of inhibition measuring 0 mm, 7 ± 0.0 mm, 8 ± 0.0 mm, 8.5 ± 0.0 mm, 11 ± 0.0 mm, and 12.05 ± 0.15 mm at the concentrations of 0.78 mg/ml, 1.56 mg/ml, 3.125 mg/ml, 6.25 mg/ml, 12.5 mg/ml, 25 mg/ml, and 50 mg/ml, respectively. The size of the zone of inhibition increased with the increasing concentration of plant extracts. (b) BN inhibited bacterial growth and formed zones of inhibition measuring 0 mm, 0 mm, 0 mm, 7.05 ± 0.05 mm, 8.1 ± 0.1 mm, 11.2 ± 0.1 mm, and 12 ± 0.0 mm at the concentrations of 0.78 mg/ml, 1.56 mg/ml, 3.125 mg/ml, 6.25 mg/ml, 12.5 mg/ml, 25 mg/ml, and 50 mg/ml, respectively. The size of the zone of inhibition increased with the increasing concentration of plant extracts.
Bacterial strains
Concentration of plant extract mg/ml
100
50
25
12.5
6.25
3.125
1.56
0.78
ASH3
13.15 ± 0.05
12.05 ± 0.15
11 ± 0.0
8.5 ± 0.0
8 ± 0.0
7 ± 0.0
–
–
ASH4
13.15 ± 0.05
11.1 ± 0.1
7.1 ± 0.1
7.1 ± 0.1
7 ± 0.0
–
–
–
ASH5
13.2 ± 0.1
12.1 ± 0.1
11.05 ± 0.05
10 ± 0.0
9 ± 0.0
8 ± 0.0
7 ± 0.0
–
ASH6
12.15 ± 0.05
11 ± 0.0
10.15 ± 0.05
9.25 ± 0.05
9 ± 0.1
8.0 ± 0.0
–
–
ASH7
13.1 ± 0.1
12.25 ± 0.25
11 ± 0.0
8.1 ± 0.1
7.1 ± 0.1
–
–
–
ASH8
12.05 ± 0.15
11.1 ± 0.1
10.1 ± 0.1
8.0 ± 0.0
–
–
–
–
ASH2
12.1 ± 0.1
12.05 ± 0.05
10.2 ± 0.2
8.05 ± 0.05
–
–
–
–
ASH1
12.1 ± 0.0
11.1 ± 0.1
10 ± 0.0
8.1 ± 0.1
–
–
–
–
Bacterial strains
Concentration of plant extract mg/ml
50
25
12.5
6.25
3.125
1.56
0.78
0.39
ASH3
12.5 ± 0.5
12.1 ± 0.1
12 ± 0.0
7.1 ± 0.1
7.05 ± 0.05
–
–
–
ASH4
12 ± 0.0
11 ± 0.0
7.1 ± 0.1
–
–
–
–
–
ASH5
13.1 ± 0.1
12.2 ± 0.2
10.1 ± 0.1
10 ± 0.0
8.0 ± 0.0
–
–
–
ASH6
12.05 ± 0.05
11.1 ± 0.1
10.3 ± 0.1
10.2 ± 0.2
8.15 ± 0.5
–
–
–
ASH7
13 ± 0.0
12 ± 0.0
11.2 ± 0.1
8.1 ± 0.1
7.05 ± 0.05
–
–
–
ASH8
12.2 ± 0.2
11.5 ± 0.5
10 ± 0.0
8 ± 0.0
7 ± 0.0
–
–
–
ASH2
12.2 ± 0.0
11.5 ± 0.0
10 ± 0.1
9.10.1
7 ± 0.1
–
–
–
ASH1
12.05 ± 0.05
11.1 ± 0.1
10 ± 0.0
8 ± 0.0
–
–
–
–
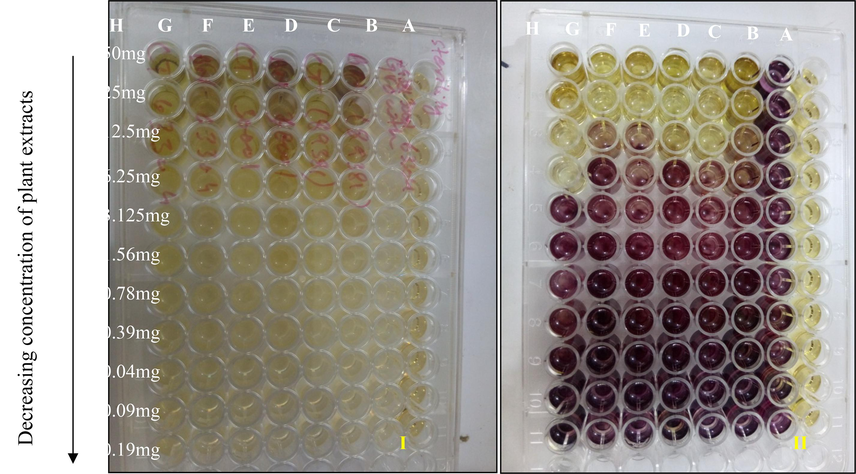
MIC of different concentrations of B. nobilis (BN) and C. ternata (CT) plant extracts determined by MTT reduction assay. Where rows A = sterility control, B = growth control, C = BN plant extract and ASH6 isolate, D = CT plant and ASH6 isolate, E = BN plant extract and ASH3 isolate, F = CT plant extract and ASH3 isolate, G = BN plant extract and ASH8 isolate and H = CT plant extract and ASH8 isolate. Figure I shows the color of the plate before adding MTT, while figure II shows the development of purple coloration after the addition of MTT.
3.3.2 Microbroth dilution assay
After 24 h of incubation, 96 well plates containing different concentrations of plant extracts and S.aureus were absorbed in liquid media at 600 nm. MIC was considered as that concentration of plant extract whose absorbance was less than that of growth control and closely related to sterility control. Microbroth dilution assay was more sensitive in measuring MIC of plant extracts. The MIC of C. ternata plant was 0.78–1.56 mg/ml, and the MIC of B. nobilis plant was 1.56–6.25 mg/ml (Table 3a).
Bacterial strains
MIC mg/ml
CT
BN
ASH3
1.56
3.125
ASH4
0.78
1.56
ASH5
1.56
3.125
ASH6
1.56
3.125
ASH7
0.78
1.56
ASH8
1.56
6.25
ASH2
0.78
1.56
ASH1
1.56
3.125
3.3.3 MTT reduction assay
96 well plate was used for growing S.aureus in the presence of different concentrations of plant extracts. After 24 h of incubation, cell viability was checked with the help of an MTT solution. 22 µl MTT was added to all test wells. The appearance of purple color indicated the presence of viable bacterial cells. The purple color started fading away with decreasing the concentration of plant extracts until it became light purple or colorless, indicating the absence of viable bacterial cells. The MIC of the C. ternata plant determined by the MTT reduction assay was 1.56–3.125 mg/ml, whereas the MIC of B. nobilis plant was 1.56–6.25 mg/ml (Table 3b). MIC = Minimum inhibitory concentration MTT = 3-(4,5-Dimethylthiazol-2-yl)-2.5-Diphenyltetrazolium Bromide. CT = Choysia ternate. BN = Bismarckia nobilis.
Bacterial strains
MIC mg/ml
CT
BN
ASH3
1.56
3.125
ASH4
3.125
6.25
ASH5
1.56
3.125
ASH6
1.56
3.125
ASH7
1.56
3.125
ASH8
1.56
3.125
ASH2
3.125
3.125
ASH1
3.125
3.125
3.4 Study of Bactericidal/ bacteriostatic activity of plant extracts
After the determination of MIC by microbroth dilution assay, 50 µl aliquotes of MIC and above concentrations of BN and CT plants were taken from 96 well plates, serially diluted in normal saline, and spread onto MHA plates. The number of colonies was counted after 24 h of incubation to calculate CFU/ml. MBC was calculated by comparing the results with growth control. MICindex was calculated from MIC and MBC to determine the mode of action of plant extracts. Tables 4a and 4b outline the antibiosis mechanism of CT and BN plant extracts against two S. aureus isolates, ASH4 and ASH6, respectively. MIC = Minimum inhibitory concentration MBC = Minimum bactericidal concentration
Plant extracts
MIC
MBC
MICindex
Mechanism
C. ternata
0.78
6.25
0.1248
Bactericidal
B. nobilis
1.56
12.5
0.1248
Bectericidal
Plant extracts
MIC
MBC
MICindex
Mechanism
C. ternata
1.56
25
0.0624
Bactericidal
B. nobilis
3.125
25
0.125
Bactericidal
3.5 Determination of antifungal activity of plant extracts
3.5.1 Isolation and identification of C.neoformans
C. neoformans was identified based on colony morphology, microscopic appearance, and urease test (Fig. 6a, b, and c, respectively). It formed mucoid, creamy, and smooth colonies on the SDA medium after one week of incubation at room temperature. Furthermore, C. neoformans appeared as encapsulated, spherical cells lacking hyphae or pseudohyphae. C. neoformans changed the color of the media from yellow to pink due to ammonia production.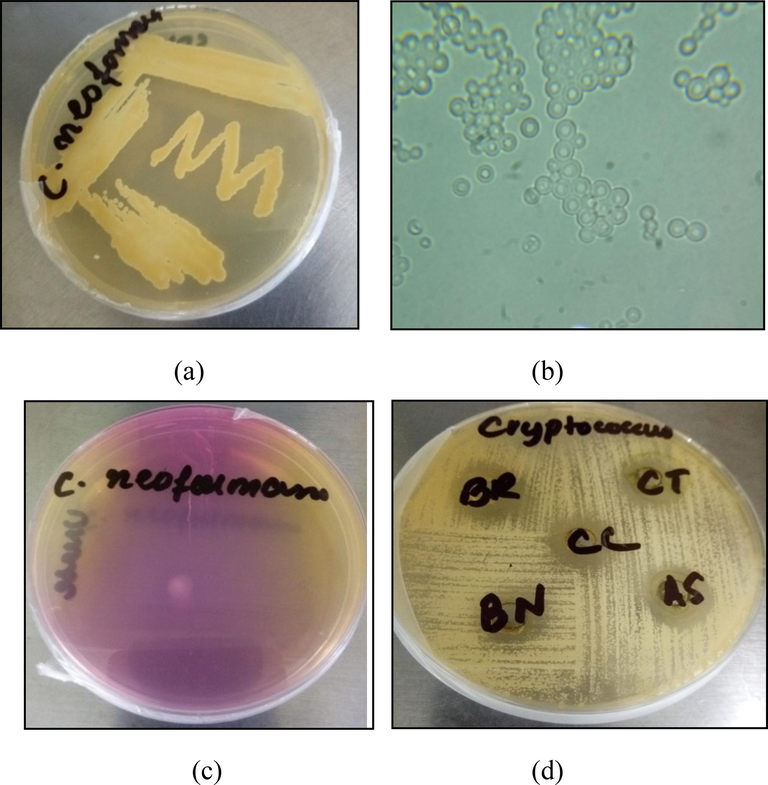
Morphological Identification of C.neoformans and antifungal activity against it. (a) Colony morphology – formation of mucoid, creamy, and smooth colonies on Sabouraud Dextrose Agar (SDA) medium; (b) Microscopic Appearance – encapsulated, spherical cells lacking hyphae or pseudohyphae; (c) Urease positive indicated by the change in media's color from yellow to pink owing to production of ammonia. (d) Antifungal activity of 70 % methanolic extracts of B. nobilis (BN), C. ternata (CT), B. recurvata (BR) and C. cataractarum(CC) against Cryptococcus neoformans. BN, CT, BR, and CC inhibited fungal growth and formed zones of inhibition measuring 14.25 ± 0.25 mm, 13.25 ± 0.05 mm, 17.35 ± 0.15 mm, and 16.25 ± 0.05 mm, each at a concentration of 100 mg/ml.
3.5.2 Antifungal activity of plant extracts
70 % methanolic extracts of all plants showed significant antifungal activity against C.neoformans by agar well diffusion assay (Fig. 6d). 100 µl of each plant extract (100 mg/ml) was added to the SDA plate inoculated with 0.5McFarland fungal suspension. After one week of incubation at room temperature, the zone of inhibition was calculated (Table 5).
70 % Methanolic Plant Extracts
Zone of Inhibition (mm)
B. nobilis
14.25 ± 0.25
C. ternata
13.25 ± 0.05
B. recurvata
17.35 ± 0.15
C. cataractarum
16.25 ± 0.05
4 Discussion
Over time, the uncontrolled use of antibiotics has led to the emergence of drug-resistant staphylococcal infections, making their treatment increasingly challenging. The prevalence of multidrug-resistant S. aureus strains is a global issue, with high levels of resistance to multiple antibiotics, including penicillin, ampicillin, oxacillin, and ceftriaxone. Methicillin-resistant Staphylococcus aureus (MRSA) poses a significant global health threat. The estimated global prevalence of MRSA is 14.69 %. The 30-day all-cause mortality rate for bloodstream infections with MRSA is estimated to be 20 % to 40 %. Moreover, the overall mortality rate attributed to Staphylococcus aureus infections was over 1 million deaths in 2019. It is associated with a wide range of infections, including skin and soft tissue infections, endocarditis, osteomyelitis, bacteremia, and lethal pneumonia. These figures underscore the urgent need for continued research and development of effective alternative strategies to combat drug-resistant staphylococcal infections.
The limited effectiveness of certain antibiotics has heightened the importance of alternative treatment approaches, such as phytomedicine, in addressing this critical public health concern. Plant extracts have been traditionally used to treat infectious diseases due to their abundance of effective phytochemicals, which offer an alternative strategy for combating infections. The current study used Bismarckia nobilis and Choysia ternata plants to study their antimicrobial effect. Plant extracts were prepared in 70 % methanol and n-hexane. N-hexane extracts of Bismarckia nobilis and Choysia ternata plants showed antibacterial activity against eight S.aureus isolates. Alam et al. (2020) also studied the antibacterial activity of the silver nanoparticles derived from the seeds of B. nobilis plant by the green method. The anti-inflammatory activity of essential oils, ternanthranin, and two synthetic analogs isolated from C.ternata were also studied (Pinheiro et al., 2015). However, the current study is the first report of the antibacterial activity of B. nobilis and C. ternata leaf extract against any bacterial species. The zone of inhibition of B.nobilis plant extract was 12.1–13.1 mm, and the zone of inhibition of C.ternata plant extract was 13.1–15.1 mm for different S.aureus isolates (Table 1).
After determining antibacterial activity, MIC of B. nobilis and C. ternata plants was determined by agar well diffusion assay, microbroth dilution assay, and MTT reduction assay. Although the MIC determined by all three methods was concordant with each other, microbroth dilution assay was the most sensitive method. So the MIC of the plant determined by microbroth dilution assay was in the range of 0.78–3.125 mg/ml and 1.56–12.5 mg/ml for CT and BN plant extracts, respectively (Table 3a). In order to find the mode of action of these plant extracts, MBC was also calculated to find out the MICindex value. The MICindex of BN and CT plants was 0.1248 against S.aureus isolate ASH4 (Table 4a). When the MIC/MBC ratio is ≤ 4, the plant extract is regarded as bactericidal, but if the ratio is ˃ 4, then the plant extract is considered bacteriostatic (Krishnan et al., 2010). Since the value is less than 4, the mode of action of B. nobilis and C. ternata plants is bactericidal. The current investigation is first time reporting the antibacterial activity of these plants against S.aureus. On the other hand, 70 % methanolic extract of all four plants showed antifungal activity against Cryptococcus neoformans fungus, the leading cause of meningitis in AIDS patients. The zone of inhibition of Bismarckia nobilis, Choysia ternata, Chamaedora cataractarum, and Beaucarnea recurvata was 14.25 mm, 13.25 mm, 16.25 mm, and 17.35 mm, respectively (Table 11). The antifungal activity of Lauris nobilis species was reported by Sırıken et al. (2018).
These plants remain largely unexplored for their antimicrobial potential, with limited studies around them. Bismarckia nobilis has been reported as a smooth muscle relaxant. Methanolic extract of B.nobilis is effective for treating diarrhea, hypertension, and asthma because of its spasmodic, antidiarrheal, and vasodilator activities (Saqib et al., 2019). B.nobilis has also been used by the people of Mahajanga, Madagascar, as a mouthwash to treat oral diseases (Ranjarisoa et al., 2016).
GC–MS analysis of C. ternata revealed the presence of the natural compound isopropyl N-methylanthranilate named ternanthranin. Essential oil and ethanolic extract of the C. ternate showed pain-killing activity (Radulović et al., 2011). Antispasmodic and simulative properties of the two compounds isopropyl N‐methylanthranilate and methyl N‐methylanthranilate isolated from C. ternata were also reported (Radulović et al., 2013b). Pinheiro et al. (2015) studied the anti-inflammatory properties of essential oils of C. ternata.
Members of the Arecaceae Family have, however, shown antimicrobial potential. For instance, Farahmandfar et al. (2019) found that the ultrasonic extracts of Arum maculatum (particularly in the ethanol: water (50:50) solvent) had higher extraction yield and antioxidant potential than the maceration extracts. The extracts (water, ethanol, and 50:50 ethanol: water) were active against the tested food-borne pathogens, gram-positive and gram-negative bacterial strains, including S.aureus. Results of the microdilution assay showed that the ultrasonic-assisted ethanol extract (EE-US) was the most effective against S. aureus and had the lowest MIC against it at 12.5 mg/ml. Plant extracts are more effective against Gram-positive bacteria compared to Gram-negative bacteria. This may be due to the differences in the cell wall structure of these two types of bacteria. Studies have shown that plant extracts can cause cell wall disruption and decrease cytoplasmic pH in Gram-positive bacteria, leading to their inhibition. The number and position of phenolic hydroxyl groups, double bonds, and delocalized electrons in plant compounds can also affect their antibacterial activity.
Based on these results, it can be theoretically expected that C.ternata and B.nobilis plant extracts can be used for the treatment of S.aureus associated infections. Similarly, all four plants (Bismarckia nobilis, Choysia ternata, Beaucarnea recurvata, and Chamaedora cataractarum) can be potent antifungal agents for treating Cryptococcal infections. So, we can use these plant extracts for the development of antibiotics. These antibiotics would be economical and safe to use and can serve as potent antifungal and antibacterial agents.
The current study assessed the antibacterial potential of the selected plants since it used eight strains of S.aureus and three different techniques to determine the minimum inhibitory concentration (MIC). However, it presents several limitations. Above all, the selected bacterial strains originate from the same region. To further validate the efficacy of the extracts, they should be tested against drug-resistant strains from different geographical areas (at the global or domestic level). Furthermore, the study does not explore the phytochemical composition of the extracts nor identify the active metabolites. Additionally, the safety or toxicity of the plants used has not been addressed. Moreover, the yield and composition of phytochemicals can be influenced by and, thus, vary due to several factors like growth environment, plant parts, harvest season, and extraction method, potentially influencing its activity.
As mentioned above, the limitations provide a broad path for future prospects. The active plants can be assessed for their efficacy in combatting a wide range of bacterial and fungal pathogens. Bacterial biofilms often pose greater resistance to antibiotics. Plant extracts can be studied for their potential to inhibit biofilm formation. The extracts can also be paired with conventional antibiotics to evaluate their synergistic potential for more effective treatment.
Phytochemical profiles of plant extracts can be analyzed, and the active plant metabolites can be identified through GC–MS or HPLC-MS analysis. Further research can explore the mechanisms of action adopted by the antimicrobial components, paving the way for targeted therapies. The plants can additionally be checked for other biological properties such as anti-viral, anti-cancer, or anti-inflammatory effects. Experimenting with cancer cells and mouse models can assist in identifying additional properties and determining their safety and toxicity status. Moreover, these plant extracts can also be utilized as bio-preservatives in processed food items by preventing the growth of microorganisms and increasing the shelf life of food.
5 Conclusion
In conclusion, the current study highlights the potent antimicrobial attributes of indigenous plants of Bismarckia nobilis, Choysia ternata, Chamaedora cataractarum, and Beaucarnea recurvata. The efficacy of n-hexane and methanolic extracts against S. aureus and C. neoformans, respectively, implicates their therapeutic relevance. The n-hexane extracts of Bismarckia nobilis and Choysia ternate exhibited potent antibacterial activity against S. aureus. Moreover, the methanolic extracts of all four plants displayed potent antifungal activity. The study remains confined to in vitro experiments and sidesteps phytochemical analysis. Analyzing the bioactive constituents of these plant extracts and bridging the gap between in vitro assays and in vivo investigations would enhance the clinical translatability of these findings. In a broader context, our findings contribute to the growing body of knowledge aimed at harnessing the potential of nature's arsenal to develop plant-based therapeutics to mitigate microbial infections.
6 Consent to participate
All authors consent to participate in the manuscript publication
7 Consent for publication
All authors approved the manuscript to be published.
8 Declaration of funding
Researchers Supporting Project Number (RSPD2024R1083), King Saud University, Riyadh, Saudi Arabia.
Author contributions
KM AU and TN conceptualized the research. KM, AL and AR conducted experiment. HY, MA, KAMB, ZA contributed in data acquisition and data analysis, and written the first draft of paper. HMA helped in software and language editing. KM, HY, AU and TN critically revised and improved the manuscript. All authors red and approved the final draft for submission.
CRediT authorship contribution statement
Kausar Malik: Conceptualization, Supervision. Ayesha Liaqat: Data curation, Writing – original draft. Ammara Riaz: Validation, Formal analysis. Humaira Yasmin: Project administration, Resources. Muhammad Asad: Investigation, Formal analysis. Amin Ullah: Conceptualization, Formal analysis, Resources. Khadija Abdul Majid Butt: Methodology, Validation. Zainab Akram: Writing – original draft. Hossam M. Aljawdah: Software, Visualization, Investigation. Tariq Nadeem: Conceptualization, Project administration, Writing – review & editing.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSPD2024R1083), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and biological activity of silver nanoparticles from medicinal palm tree bismarckia nobilis seeds. Asian J. Chem. 2020
- [Google Scholar]
- Virulence-associated enzymes of Cryptococcus neoformans. Eukaryot. Cell. 2015;14(12):1173-1185.
- [Google Scholar]
- Evaluation of antinociceptive and/or anti-inflammatory activity of Choisya Aztec Pearl. Planta Med.. 2014;80(16):P2B23.
- [Google Scholar]
- Steroidal saponins from the leaves of Beaucarnea recurvata. Phytochemistry. 2011;72(9):946-951.
- [Google Scholar]
- Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci. Nutr.. 2019;7(2):465-475.
- [Google Scholar]
- Determination of MICs for Mycobacterium avium-M. intracellulare complex in liquid medium by a colorimetric method. J. Clin. Microbiol.. 1995;33(7):1842-1846.
- [Google Scholar]
- Antimicrobial activity evaluation of Cassia spectabilis Leaf Extracts. Int. J. Pharmacol.. 2010;6(4):510-514.
- [Google Scholar]
- Isolation of quinoline alkaloids from three Choisya species by high-speed countercurrent chromatography and the determination of their antioxidant capacity. Rev. Bras. 2017;27(3):297-301.
- [Google Scholar]
- Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med.. 2005;202(2):209-215.
- [Google Scholar]
- A cytotoxicity and comparative antibacterial study on the effect of Zataria multiflora Boiss, Trachyspermum copticum essential oils, and Enrofloxacin on Aeromonas hydrophila. Avicenna Journal of Phytomedicine. 2012;2(4):188.
- [Google Scholar]
- Bismarck palms failing in Southwest Florida. In: Paper Presented at the Proceedings of the Florida State Horticultural Society. 2012.
- [Google Scholar]
- Preparation of plant extracts from indigenous medicinal plants. International Journal of Science and Technology. 2012;1(12):688-692.
- [Google Scholar]
- Anti-inflammatory activity of Choisya ternata Kunth essential oil, ternanthranin, and its two synthetic analogs (methyl and propyl N-methylanthranilates) PLoS One. 2015;10(3)
- [Google Scholar]
- Identification of a new antinociceptive alkaloid isopropyl N-methylanthranilate from the essential oil of Choisya ternata Kunth. J. Ethnopharmacol.. 2011;135(3):610-619.
- [Google Scholar]
- Effects of methyl and isopropyl N-methylanthranilates from choisya ternata kunth (rutaceae) on experimental anxiety and depression in mice. Phytother. Res.. 2013;27(9):1334-1338.
- [Google Scholar]
- Influence of methyl and isopropyl n-methyl antranilates on carbon tetrachloride-induced changes in rat liver morphology and function. Facta Universitatis-Series: Physics, Chemistry and Technology. 2013;11(1):67-73.
- [Google Scholar]
- Use of plants in oral health care by the population of Mahajanga, Madagascar. J. Ethnopharmacol.. 2016;193:179-194.
- [Google Scholar]
- Evaluation of smooth muscle relaxant potential of Bismarckia nobilis (Hildebr. & Wendl.) in diarrhea, hypertension and asthma by ex-vivo and in-vivo method. Bol. Latinoam. Caribe Plant. Med. Aromat.. 2019;18(2):204-221.
- [Google Scholar]
- Palm fiber-based dietary supplements. Google Patents; 2006.
- Antibacterial activity of laurus nobilis: a review of literature. Medical Science and Discovery. 2018;5(11):374-379.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103122.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







