Translate this page into:
In vitro impact of the long term medication for blood pressure, diabetes type II and cholesterol medicines on the gut microbiota
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The human gastrointestinal (GI) tract contains a variety of microbial species that constitute the gut microbiota. The gut microbiota has a role in host immune health and defence against pathogenic organisms. The composition of gut microbiota is affected by several factors that impact the role of the GI tract, one of these factors influences medications, in particular antibiotics, however, recent findings revealed that non-antibiotic medications significantly impact the diversity of gut microbiota as well. Therefore, this study was conducted to investigate the effect of three commonly prescribed medicines in vitro.
Methods
Amlodipine, metformin and atorlip (atorvastatin calcium) tablets, which are frequently administered for lowering blood pressure, diabetes type II and cholesterol respectively, were included in this study. To study the antibacterial activity against potential pathogens, agar-well diffusion assay and minimum inhibitory concentration (MIC) was used.
Results
The outcomes indicated that metformin and atorlip showed less activity against both Gram-positive and Gram-negative bacteria. The drug of lowering cholesterol amlodipine significantly inhibited the growth of all tested bacterial strains. The zone of inhibition ranged between 12 and 30 mm. B. casei was highly susceptible and the zone of inhibition was 30 mm, conversely the opportunistic pathogen P. aeruginosa showed resistance against amlodipine and the zone of inhibition was only 12 mm. The MIC values ranged from 0.039 to 0.612 mg/L. Conclusion: The present findings revealed that two of the used medicines (metformin and atorlip) showed no antimicrobial properties, in contrast, amlodipine reflected a potent impact against all examined microbes either probiotics or potential pathogens at very low concentrations.
Keywords
Gut microbiota
Probiotics
Minimum inhibitory concentration
Hypertension
Diabetes
Cholesterol
1 Introduction
Microorganisms commence colonizing the human body by the first moments of birth (Milani et al., 2017). The community of such collection of microbes is called the microbiome. This term includes these microorganisms and all related genetic materials and the secreted substances of these microbes that occupy the human body (Mousa et al., 2022). The microbiota of humans is a very complex community and dynamic changes are occurring in these microorganisms during the different human age levels, nevertheless, the stage after adulthood and before aging represents the most stable period for gut microbiota which contribute to host growth (Thursby and Juge, 2017; Herzog et al., 2021). Consequently, human health is regulated positively or negatively by the microbiota that is inhabited in or on the body organs (Vos et al., 2022). A direct relationship between gut microbiota and the host health was reported. These microorganisms, particularly probiotics, contribute in metabolizing nutrients, digesting polysaccharides that are complex and indigestible and providing the essential molecules that the host-body lack its pathways to synthesize substances such as certain vitamins as well as protecting against pathogens and evolving the immune system (Lazar et al., 2019). For instance, the short-chain fatty acids (SCFAs) as gut microbiota secondary metabolites (Silva et al., 2020). SCFAs are short-chain organic molecules composed of less than 6 carbon atoms, such compounds are produced as a result of fermentation of complex polysaccharides by obligate anaerobic bacteria in the large intestine (Den Besten et al., 2013). Acetate, butyrate and propionate are the notable SCFAs that demonstrated to play a significant role in human health through protecting from inflammatory response, inducing mucus production, preserving intestinal barrier integrity, enhancing insulin secretion and minimizing the risk of colon cancer as well as inhibiting the activity of histone deacetylase (HDAC) that effect on brain functions (Markowiak-Kopeć et al., 2020; Silva et al., 2020). Alteration in the SCFAs production levels due to disruption in gut microbiota, i.e. losing the bacterial taxa that produce such molecules, were correlated to several human diseases such as autism (Liu et al., 2019), multiple sclerosis (Melbye et al., 2019) and Inflammatory Bowel Diseases (IBD).
Throughout the host life the diversity of microbiota is affected by several factors including, environmental factors, host genetics, antibiotics, nutrition and health state either during wellness or diseases (Laursen et al., 2021; Senn et al., 2020). Among the all factors, antibiotics possess the inhibitory affect on the distribution of gut microbiota, several studies demonstrated the potent impact of medications on gut microbiota (Ramirez et al., 2020; Konstantinidis et al., 2020). However, the impact of long-term use of medicines on the diversity of gut microbiota is rarely studied. Therefore, the aim of this study is to investigate the impact of drugs on the gut bacterial population.
2 Materials and methods
2.1 Materials
Bacterial culture media including nutrient broth (NB), nutrient agar (NA), Mueller Hinton agar, De Man Rogosa and Sharpe (MRS) agar medium and the standard antibiotics were obtained from Himedia (Mumbai, India). Medications for diabetic type II, cholesterol, and hyper tension were purchased from local medicine suppliers.
2.2 Preparation of bacterial inoculum
Gut microbiota was used in this study. These include probiotics bacterial strains such as Brevibacterium casei, Lactobacillu splantarum KACC 15,357 and potential pathogens Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27584, Acinetobacter sp.ATCC 49139, Klebsiella pneumonia ATCC 13,883 and Salmonella typhimurium ATCC 14028. All strains were inoculated into NB and NA media except for L. plantarum which was cultivated by MRS medium. The plates were incubated at 35 ± 2 °C for 18–24 h in a shaker incubator at 120 rpm. Then it was stored at 4 °C until further use.
2.3 Effect of medicines on the growth of probiotic strains
A stock solution of three drugs for cholesterol (20 mg), diabetes (500 mg) and blood pressure (5 mg) (amlodipine, metformin and atorlip) was prepared by dissolving tablets in 1:1 (v/v) DMSO/distilled water (DW). The bacterial inoculum was prepared for agar-well diffusion assay by adding bacterial culture into test tubes containing 5 ml sterile DW to obtain bacterial suspension equal to 0.6 optical density (OD 600 nm). The optical density was measured with a spectrophotometer and the cell density was approximately 1 × 108 CFU/ml. The bacterial strain suspension was swabbed onto Mueller-Hinton agar plates and MRS plates were used for probiotic L. plantarum. The diluted medicine with a final concentration of 10 mg was added into the well, and streptomycin (10 µg) was used as a positive control. Plates were kept at refrigerator 4 °C for 20 mins and further incubated at 35 ± 2 °C for 18–24 h. The inhibition zone was measured in mm.
2.4 Statistical analysis
Analysis of variance (ANOVA) was used to determine the variation of the medication's effect on the tested bacteria. The Microsoft Excel software was used to test the significance and the “p” value less than 0.05 was considered to be statistically significant.
3 Results
The influence of three commonly prescribed long-term medications for chronic diseases, Amlodipine, Metformin, and Atorlip (atorvastatin calcium) were screened for their antimicrobial activity against several human gut probiotics and potential pathogens. The chemical structure of the medicines was illustrated in Fig. 1.
Chemical structure of amlodipine, metformin and atorlip.
The findings of susceptibility to these medications revealed that metformin and atorlip showed no antibacterial activity at 10 mg concentration against the selected bacteria. Moreover, significant results were reported with amlodipine which possesses potent antibacterial activity against all the tested bacteria as indicated in Fig. 2, the gut probiotic B.casei and E. coli ATCC 25,922 both significantly influenced by this drug with a zone of inhibition of 30 mm and 24 mm, respectively (Fig. 3). The activity of amlodipine against remaining strains including L. plantarum KACC 15357, Acinetobacter sp.ATCC 49139, K. pneumonia ATCC 13,883 and S. typhimurium ATCC 14,028 was moderate range between 16 and 20 mm zone of inhibition. In contrast, P. aeruginosaATCC 27,584 was less susceptible by amlodipine with and the zone of inhibition was 12 mm. In comparison to the control antibiotic, the effect of pressure medication towards bacterial strains including B. casei, Acinetobacter sp., L. plantarum was higher in contrast to other microbes which were more susceptible to streptomycin than amlodipine (Fig. 2) (p > 0.05).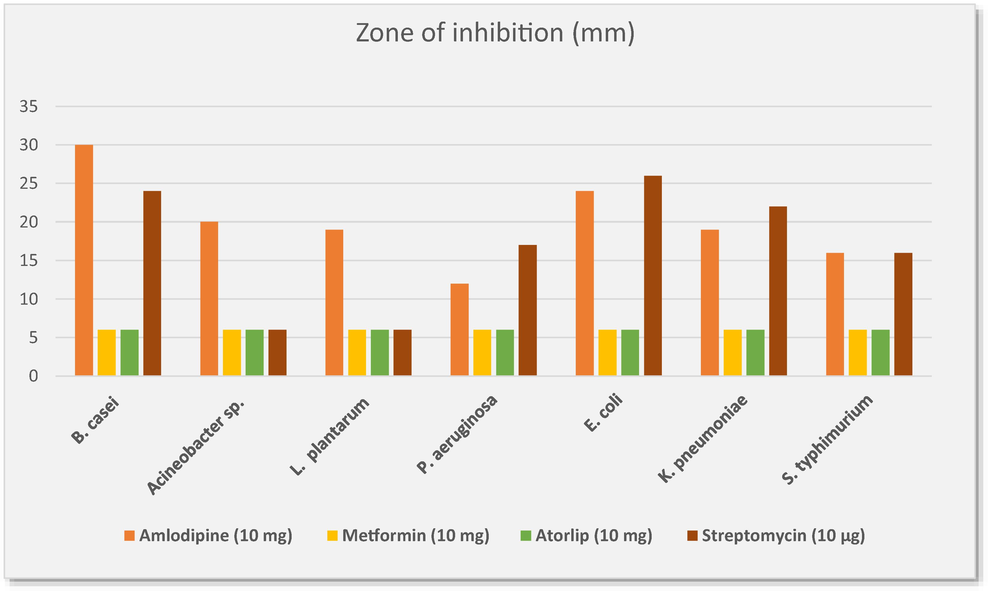
Antimicrobial activity of amlodipine, metformin and atorlip on probiotic and pathogenic bacterial strains.
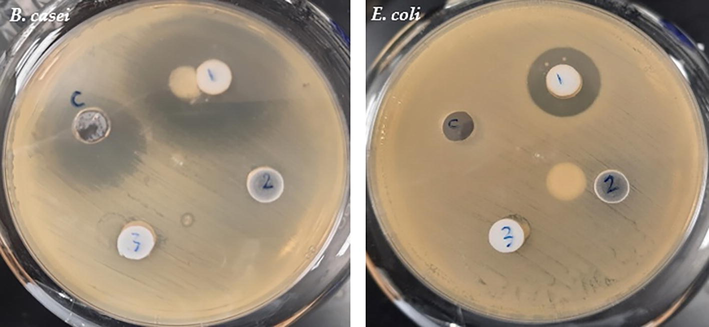
The impact of three medications against B. casei and E. coli. C; standard antibiotic control (streptomycin, 10 µg), 1: amlodipine (10 mg), 2: metformin (10 mg) and 3: atorlip (10 mg).
To determine the minimum inhibitory concentration (MIC) of amlodipine, micro-broth plate (96 well) method was used at various concentrations; 5, 2.5, 1.25, 0.625, 0.312, 0.156, 0.078 and 0.039 mg. The findings of the MIC test showed high consistency with the agar-well diffusion assay. The MIC values for B. casei and Acinetobacter sp. were the lowest by 0.078 and 0.039 mg, however, approximately the half of tested bacteria showed MIC value of 0.156 mg including L. plantarum, E. coli and S. typhimurium while the growth of K. pneumonia mostly inhibited at a concentration of 0.312 mg. Similarly, among all bacterial strains as in well diffusion results, P. aeruginosa showed the lowest susceptibility to pressure medications and the MIC value was 0.612 mg.
4 Discussion
The human body contains>100 trillion symbiotic microbes, which have a significant impact on human health (Young, 2017; Wang et al., 2017). Recently research reported that gut microbiota interaction with several medications by which the microbiome may interfere with the pharmacologic treatments leading to impact the drug's efficacy (Zhang et al., 2020; Walsh et al., 2018), in contrast, these medications may show antimicrobial properties against gut microbiota leading to imbalance or disruption the gut microbiota structure (Vich Vila et al., 2020), a growing number of studies have demonstrated that intestinal dysfunctions and pathological states may be caused by dysbiosis in the gut microbiota (Zha et al., 2020). Several studies reported that antibiotics play a significant role in the imbalance the gut microbiome composition leading to rise the antibiotic resistance (Francino, 2016; Langdon et al., 2016; Ramire et al., 2020), however, other studies attributed the prevalence of antimicrobial resistance to components other than antibiotics, such components including non-antibiotic molecules, metals and biocides have antimicrobial activity. Among them, it is believed that prescribed medicines significantly affect the composition of the gut microbiota (Ko et al., 2017). Statins are well-known medications used as first-line therapy for lowering cholesterol (Berne et al., 2005). Statin drugs found to have negative impact on gut microbiota structure in multiple microbiome studies (Dias et al., 2020; Vieira-Silva et al., 2020). Kim et al. (2019) have demonstrated that statin consumption is implicated in increasing the abundance of bacterial genera including Bacteroides, Butyricimonas, and Mucispirillum that are associated with intestinal inflammation in mice due to the release of inflammatory factors such as Interleukin 1 beta (IL-1β) and Transforming growth factor β (TGFβ1). Although, the present finding did not show any antibacterial properties for cholesterol-lowering drug atorlip tablets (Atorvastatin calcium) at the concentration of 10 mg/L against tested the tested bacteria. In a study conducted by Ko et al. (2017) found that MIC for list of statins drugs are atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin and simvastatin, which exhibited an antibacterial properties against both gut and pathogenic bacteria at concentrations between 32 and > 1,024 µg/mL. Despite that metformin showed no activity towards any of bacterial strains included in this study at concentration of 10 mg/well, nevertheless, microbiome studies showed that such medicine possess a negative impact on gut microbiota. Studies investigated the effect of metformin on diversity of gut microbes in patients with obesity and type 2 diabetes mellitus showed a significant alteration in gut microbiota composition, members of Bacteroidetes and Verrucomicrobiaphyla were significantly shifted while the most affected genera were Escherichia, Akkermansia and Bacteroides (Zhang and Hu, 2020; Silamiķele et al., 2021). Chen et al. (2022) reported that administration of metformin resulted in elevating the abundance of gut probiotic SCFAs-producer Akkermansiamuciniphila which have a positive impact on the host body health. Amlodipine is a drug that lowers blood pressure and treats coronary artery disease (Cussotto et al., 2019), the interaction studies between gut microbiota and amlodipine mostly focused on the influence of microbiota on metabolized of amlodipine . Yoo et al. (2016) showed that the systemic amlodipine bioavailability was elevated when this drug was administered alongside antibiotics, co-administered, which indicates the involvement of gut microbiota in the biotransformation of amlodipine leading to a decrease its bioavailability and eventually resulted in resistance to amlodipine. Conversely, the effect of this medication on the diversity of gut microbes was not investigated yet according to our knowledge,the in vitro findings of this study on the impact of amlodipine on the list of gut bacterial strains predicted that such medication potentially possess significant effect on composition of gut microbiota.
5 Conclusion
In conclusion, non-antibiotic therapies proved to remarkable induce a notable shift in gut microbiome diversity as demonstrated in many studies. The present findings revealed that two of the used medicines (metformin and atorlip) showed no antimicrobial properties, in contrast, amlodipine reflected a potent impact against all examined microbes either probiotics or potential pathogens at very low concentrations. Further works are required to study the impact of these drugs on probiotic or pathogenic strains.
Acknowledgment
The author extend their appreciation to the Researchers supporting project number (RSP-2022R479) King Saud University, Riyadh, Saudi Arabia.
Ethical approval
Not applied
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol.. 2005;4(1):1-11.
- [CrossRef] [Google Scholar]
- Psychotropics and the microbiome: a chamber of secrets…. Psychopharmacology. 2019;236(5):1411-1432.
- [CrossRef] [Google Scholar]
- Gut microbiome and health: Mechanistic insights. Gut. 2022;71(5):1020-1032.
- [CrossRef] [Google Scholar]
- The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res.. 2013;54(9):2325-2340.
- [CrossRef] [Google Scholar]
- Gut bacterial microbiome composition and statin intake—A systematic review. Pharmacol. Res. Perspect.. 2020;8(3):e00601.
- [CrossRef] [Google Scholar]
- Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front. Microbiol.. 2016;1543
- [CrossRef] [Google Scholar]
- The importance of age in compositional and functional profiling of the human intestinal microbiome. Plos one. 2021;16(10):e0258505.
- [Google Scholar]
- Alterations in gut microbiota by statin therapy and possible intermediate effects on hyperglycemia and hyperlipidemia. Front. Microbiol.. 2019;10:1947.
- [CrossRef] [Google Scholar]
- Statins: antimicrobial resistance breakers or makers? PeerJ. 2017;5:3952.
- [CrossRef] [Google Scholar]
- Effects of antibiotics upon the gut microbiome: a review of the literature. Biomedicines. 2020;8(11):502.
- [CrossRef] [Google Scholar]
- The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med.. 2016;8(1):1-16.
- [CrossRef] [Google Scholar]
- Gut microbiota development: influence of diet from infancy to toddlerhood. Rev. Artic. Ann. Nutr. Metab.. 2021;77(3):21-34.
- [CrossRef] [Google Scholar]
- Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front. Nutr.. 2019;6:21.
- [CrossRef] [Google Scholar]
- Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep.. 2019;9(1):1-9.
- [CrossRef] [Google Scholar]
- The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107.
- [CrossRef] [Google Scholar]
- Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand.. 2019;139(3):208-219.
- [CrossRef] [Google Scholar]
- The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev.. 2017;81(4)
- [Google Scholar]
- Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front. Microbiol.. 2022;13:825338
- [Google Scholar]
- Antibiotics as major disruptors of gut microbiota. Front. Cell Infect. Microbiol.. 2020;10:572912
- [CrossRef] [Google Scholar]
- Microbial colonization from the fetus to early childhood—a comprehensive review. Front. Cell Infect. Microbiol.. 2020;637
- [CrossRef] [Google Scholar]
- Metformin strongly affects gut microbiome composition in high-fat diet-induced type 2 diabetes mouse model of both sexes. Front. Endocrinol. (Lausanne). 2021;12:626359
- [CrossRef] [Google Scholar]
- The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol.. 2020;11:25.
- [CrossRef] [Google Scholar]
- Introduction fo the human gut flora. Biochem. J.. 2017;474(11):1823-1836.
- [CrossRef] [Google Scholar]
- Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun.. 2020;11(1)
- [CrossRef] [Google Scholar]
- Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581(7808):310-315.
- [Google Scholar]
- Drug–gut microbiota interactions: implications for neuropharmacology. Br. J. Pharmacol.. 2018;175(24):4415-4429.
- [CrossRef] [Google Scholar]
- The human microbiota in health and disease. Engineering. 2017;3(1):71-82.
- [CrossRef] [Google Scholar]
- Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. J. Hypertens.. 2016;34(1):156-162.
- [CrossRef] [Google Scholar]
- The role of the microbiome in human health and disease: an introduction for clinicians. BMJ.. 2017;356
- [CrossRef] [Google Scholar]
- Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes, Metab. Syndr. Obes. Targets Ther.. 2020;13:5003.
- [CrossRef] [Google Scholar]
- Zhao, C., Hu, Y., Chen, H., Li, B., Cao, L., Xia, J., Yin, Y., 2020. An in vitro evaluation of the effects of different statins on the structure and function of human gut bacterial community. PloS one, 15(3), e0230200. https://doi.org/10.1371/JOURNAL.PONE.0230200.







