Translate this page into:
In vitro bactericidal and imipenem synergistic effect of nano-silver against multiple drug-resistant Pseudomonas aeruginosa
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Pseudomonas aeruginosa (P. aeruginosa) is an aerobic gram-negative pathogen. It induces decline of lung functions and increase mortality rate. It has a high antimicrobial resistance rate limiting number of antibiotics which can be used.
Objectives
Evaluation of bactericidal and antibiotic synergistic effect of Nano-silver (Ag-NP) against Multiple drug-resistant P.aeruginosa (MDR P. aeruginosa).
Material and methods
P.aeruginosa were isolated from clinical specimens. MicroScan WalkAway-96SI System was used for laboratory Identification, antimicrobial susceptibility. Minimal inhibitory concentration (MIC) was determined by a microdilution method. Time kill assay determined by incubation MRSA with different concentrations of Ag-NP (0, 50, 100, and 200 μg/ml) in a shaking incubator at 37 °C for 24 h. Growth curves of bacterial cell cultures were attained through repeated measures of the optical density (O.D.) at 600 nm. The disc diffusion method was used to evaluate synergistic of Ag-NP with antibiotics.
Results
The MIC value of Ag-NPs against MDR P. aeruginosa was in at of 5 μg/ml. The bacterial growths of cells treated with 1.25, 2.5, 5 and 10 µg/ml Ag-NPs were inhibited. After 4 h, almost all treated bacterial cells were dead. All of the combinations showed significant synergistic effect (P-value 0.003), and the result showed the highest synergism at concentrations of Imipenem at MIC (32 µg/ml) and 16 µg/ml.
Conclusion
Nano-Silver has high therapeutic activity against MDR P. aeruginosa, it can be suggested as an alternative or adjuvant with antibiotics for MDR P. aeruginosa treatment. Further studies are required for understand synergistic effect of Ag-NP combining, and assessment of its safety.
Keywords
Nano-silver
Pseudomonas aeruginosa
Imipenem
Drug resistant
1 Introduction
Pseudomonas aeruginosa is a widespread nosocomial pathogen (Fowler and Nancy, 2014; Breidenstein et al., 2011), can cause to cause dangerous opportunistic infections among immunocompromised patients, for example, chronic lung infections in cystic fibrosis patients (Winstanley et al., 2016). P. aeruginosa has been classified as a superbug because of its extraordinary adaptability, intrinsic resistance to antibiotics and ease of acquiring microbial resistance, which also contribute to its multidrug resistance and pan-drug resistance to existing antibiotics (Livermore, 2002; Oliver et al., 2015).
The rapid incidence and spread of MDR P. aeruginosa strains leads it a high morbidity and mortality of infections caused by MDR P. aeruginosa (Fowler and Nancy, 2014). Clinically isolated strain, specially, have witnessed a serious situation. Therefore, there is an urgent need to develop novel antimicrobial compounds or combinations with potent antibacterial activity against clinically isolated MDR P. aeruginosa.
Among the range of materials whose antimicrobial property is being investigated, AgNP appear as a promising new antibacterial agent that could be helpful to confront this and other drug-resistant bacteria. Different studies have established the bactericidal effect of Ag-NP in Gram negative and Gram-positive bacteria, but the bactericidal mechanism of this compound has not been clearly elucidated. Morones et al. (Yoon et al., 2008) defined the antibacterial activity of silver nanoparticles in four types of Gram-negative bacteria: Escherichia coli, Vibrio cholera, P.aeruginosa, and Salmonella tiphy and suggested that Ag-Np attach to the surface of the cell membrane and disturb its function, penetrate bacteria, and release silver ions (Morones et al., 2005).
Silver nanoparticles showed antibacterial activity against some drug-resistant bacteria (Inoue et al., 2010; Birla et al., 2009). In addition, several studies regarding the synergistic activity of Ag-Np in combination with other compounds have been reported: a combination of amoxicillin and Ag-NP showed greater bactericidal efficiency towards E.coli than when they were applied separately (Li et al., 2005), and interactions between Ag-NP and polymyxin B showed synergistic effects for Gram negative bacteria (Ruden et al., 2009).
Carbapenem such as imipenem and meropenem play key role treatment of P. aeruginosa infections. Lately, a problem of carbapenem resistance has loomed with emergence of Carbapenem resistant P. aeruginosa (Maltezou, 2009; Lister et al., 2009). Treatment of imipenem-resistant infection is very difficult, due to imipenem resistance genes are located on transferable genetic elements such as plasmid. These infections are associated with high mortality and morbidity rates (Bebrone, 2007). In the present study, we investigated the antimicrobial activities of Ag-NP alone and in combination with Imipenem against MDR P. aeruginosa.
2 Material methods
2.1 Bacterial isolate
Ninety P. aeruginosa isolate from clinical specimens at Microbiology laboratory, Department of clinical analysis at Gynecology and Children hospital, Hafr Elbatin, KSA. Mid-stream urine, suction tip, pus and blood specimens were collected aseptically for bacteriological examination. Handling, transporting, and storing of collected samples were made at refrigeration temperature. All samples were inoculated on Blood agar, incubated at 37 °C for overnight, and colonies were processed.
2.2 Antimicrobial testing
The MicroScan WalkAway-96SI System was used in laboratory Identification, antimicrobial susceptibility testing and P. aeruginosa detection was performed with Neg Combo Panel Type 32 (Dade Behring®, USA). All procedures were performed according to the manufacturer’s instructions.
2.3 Antimicrobial activity of NANO-silver
Nano-silver were obtained from SIGMA ALDRICH, SAINT LOUIS, USA. MIC defined as the lowest concentration of AgNP preparation that prevented bacterial growth. MIC The minimal inhibitory concentration (MIC) was determined by a microdilution method, using LB broth (Sigma–Aldrich) and final inoculum of 105 CFU/ml. Bacteria were incubated with serial twofold dilutions of Ag-NP, and the effect on cell viability was measured after a 24 h period of incubation. Bacterial cell viability was measured by using a colony-forming capacity assay in nutrient agar (Kohanski et al., 2007). All the assays were run in parallel with negative and positive control.
2.4 Time-kill assay
To examine the growth curves of bacterial cells exposed to Ag-NPs, Mueller-Hinton broth with different concentrations of Ag-NPs powder (0, 1.25, 2.5, 5, and 10 μg/ml) was used, and the bacterial cell concentration was adjusted to 105 CFU/ml (Salomoni et al., 2017). Each culture was incubated in a shaking incubator at 37 °C for 24 h. Growth curves of bacterial cell cultures were attained through repeated measures of the optical density (O.D.) at 600 nm.
2.5 Synergistic effect of nano-silver with antibiotics
The disc diffusion method was used to evaluate the synergistic of silver nanoparticles with antibiotics. Based on the CLSI standards (Wayne, 2011). By using the spread plate method, the Mueller-Hinton agar plates were inoculated with the turbidity adjusted bacterial suspension, and antibiotic discs (Hi Media Chemicals Pvt. Ltd., Mumbai, India), were placed on plates containing MDR P. aeruginosa and was incubated at 37 °C for 24 h after which the inhibition zone diameter of plates containing colloidal silver, MDR P. aeruginosa, and antibiotic disc and those containing only the antibiotic disc and P. aeruginosa organisms were measured, and zone of inhibition (ZOI) was measured by subtracting the disc diameter from the total inhibition zone diameter. The synergistic effect was quantified by the equation (B − A)/A × 100, where A and B are the ZOI for antibiotic and antibiotic + silver nanoparticles, respectively.
2.6 Statistical analysis
All experiments were conducted in triplicate to validate the producibility of the experiments. Statistical analysis was carried out by one-way ANOVA at a P-value of 0.05 by Microsoft Excel software (Microsoft, USA).
3 Results
3.1 Bacterial strains
Of ninety P. aeruginosa isolates, 15 were MDR P. aeruginosa. Multiple drug-resistant P. aeruginosa was defined as isolate showed resistance to three or more of the following eight sentinel antimicrobial agents: amikacin; aztreonam; cefepime; ceftazidime; ciprofloxacin; colistin; imipenem; and piperacillin/tazobactam.
3.2 Antimicrobial activity of nano-silver
Cell viability assay was used to assess the bactericidal effect of Ag-NP, multiple-drug resistant P. aeruginosa was subjected to twofold Ag-NP serial dilutions for 24 h. Nano-Silver affected bacterial cellular viability in a dose-dependent manner. Bacterial cell viability was measured by using a colony-forming capacity assay. MDR P. aeruginosa was inhibited at concentrations over 5 µg/ml at 105 CFU where no visible bacterial growth in agar plate.
3.3 Time-kill assay
Growth curves of P. aeruginosa treated with Ag-NPs showed that Ag-NPs can inhibit the growth and reproduction of bacterial cells, Fig. 1. The bacterial growths of P. aeruginosa treated with 0, 1.25 2.5, 5 and 10 µg/ml Ag-NPs were inhibited. After 4 h, almost all treated bacterial cells were dead. The bacterial growth of the cells treated with 1.25 and 2.5 µg/ml Ag-NPs were also lower than that of cells in the control group.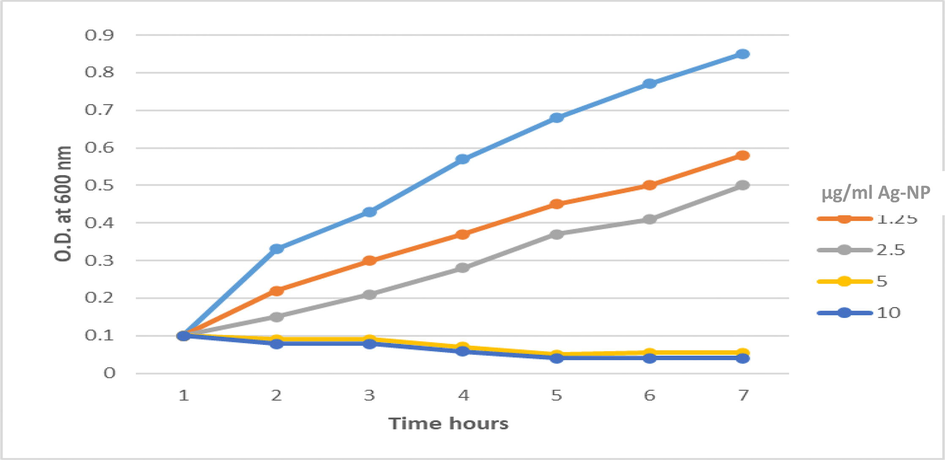
Growth curve of MDR P. aeruginosa with different concentrations of Nano-silver.
3.4 Synergistic effect of nano-silver with imipenem
Nano-Silver showed a significant synergistic effect when it combined with imipenem P-vale 0.003, Table 1. Also, the synergistic effects of Ag-NP were investigated with Imipenem against MDR P. aeruginosa using the disc diffusion method, and the effects evaluated by determination of the synergism percentage. The result showed the highest synergism at concentrations of Imipenem at (32 µg/ml) and 16 µg/ml, Fig. 2.
Source of Variation
SS
df
MS
F
P-value
F crit
Between Groups
1066.8
2
533.4
9.212435233
0.003764567
3.885294
Within Groups
694.8
12
57.9
Total
1761.6
14
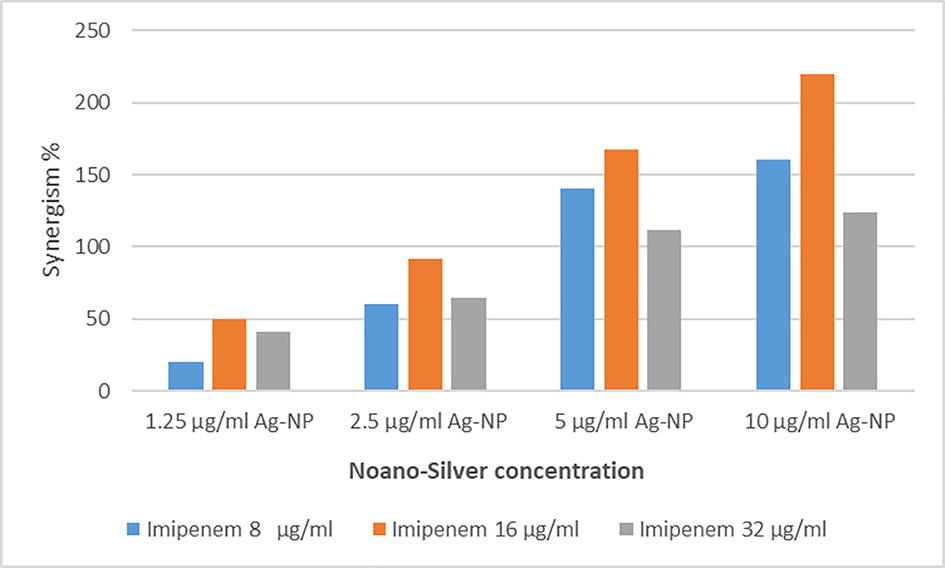
Synergistic effect of combination of Ag-NP with Imipenem at different concentrations for them.
4 Discussion
The increasing trend of antimicrobial resistance alarms about the dilemma that could be faced when treating these infections. The surveillance results showed a high incidence of MDR P. aeruginosa in our hospitals.
Many studies evaluated the antibacterial activity of Ag-NP (Chaloupka et al., 2010; Gade et al., 2010). Nano-Silver reported antibiofilm and antibacterial effect against multidrug-resistant bacteria, this helps in solving the most serious problem to worldwide public health (Ghotaslou et al., 2017; Ansari et al., 2011). Here, we evaluated the antibacterial activity of Ag-NP against MDR P. aeruginosa, the results showed increases in the antibacterial activity of Ag-NP directly with the increasing of Ag-NP, Fig. 1. The results obtained by Tiwari et al. showed that treating bacterial cells by Ag-NP caused protein leakage from the bacterial cell along with increasing of Ag-Np concentration and led to cell death (Tiwari et al., 2008). The current study reported complete susceptibility of MDR P. aeruginosa over MIC (5 µg/ml), Fig. 1. This proves the works of other authors that colloidal silver is a powerful antimicrobial agent that can destroy over 650 microorganisms at a very low concentration without having any deleterious effect on the body tissues (Caufield et al., 2000). These findings indicate that the antibacterial activity of 2.5 μg/mL of Ag-NPs could slightly inhibit bacterial growth yet not enough to outpace the speed of reproduction of the bacterial cells. Many researches have been performed studying the effect of Ag-NPs on pathogenic microorganisms.
Our results showed that the combination of Ag-NP with Imipenem significantly increased its activity (P-value 0.003), Table 1, and indicate the synergistic effect of Ag-NP. The result showed that Ag-NP influenced Imipenem activity at different concentrations with the highest effect at combinations of Imipenem/AgNP (32/10 µg/ml) and (16/5 µg/ml), Fig. 2. A similar result was obtained by Shaimaa et al., the combination of chemical and biological Ag-NP with Imipenem significantly increased the antimicrobial activity of Imipenem (Hasson et al., 2019). The results reported by Fayaz et al. (2010) and Rai et al. (2012) on P. aeruginosa were somewhat similar to our findings.
The probable mechanism involved in the synergistic effect of antibiotics with Ag-NPs could be the formation of complexes between the antibiotics and Ag-NPs; through the bonding of the antibiotic’s active and functional groups like hydroxyl and amino groups to the large surface area of Ag-NPs by chelating (Fayaz et al., 2010; Oni et al., 2002), Silver Nanoparticles antibacterial mechanism works by inhibiting oxygen metabolism, which finally kills the microbes in a very short time (Shahverdi et al., 2007).
In the current study combinations of Ag-NPs and Imipenem showed enhanced antimicrobial activity of Imipenem against MDR P. aeruginosa and overcome resistance problem. These are encouraging results, as it may be possible to achieve an effective antimicrobial effect at lower antibiotic concentrations against multiple-drug resistant bacteria. Further studies are required to understand the synergistic effect of nanosilver combinations and assess the safety and efficacy of new antibiotics Ag-NPs combinations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ansari, M.A., Khan, H.M., Khan, A.A., 2011. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MSRA on isolates from skin infections.
- Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. J. Biochem. Pharmacol.. 2007;74:1686-1701.
- [Google Scholar]
- Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. J. Lett. Appl. Microbiol.. 2009;48:173-179.
- [Google Scholar]
- Breidenstein, Elena BM, César de la Fuente-Núñez, and Robert EW %J Trends in microbiology Hancock. 2011. 'Pseudomonas aeruginosa: all roads lead to resistance', 19: 419-26.
- Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. J. Michael. J. Infect. Hardin Immun.. 2000;68:4018-4023.
- [Google Scholar]
- Nanosilver as a new generation of nanoproduct in biomedical applications. J. Trends Biotechnol.. 2010;28:580-588.
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. J. Nanomed. Nanotechnol. Biol. Med.. 2010;6:103-109.
- [Google Scholar]
- Emergence of carbapenem resistance due to the novel insertion sequence IS Pa 8 in Pseudomonas aeruginosa. J. PLoS One. 2014;9 e91299
- [Google Scholar]
- Mycogenic metal nanoparticles: progress and applications. Biotechnol. Lett.. 2010;32:593-600.
- [Google Scholar]
- The in vitro effects of silver nanoparticles on bacterial biofilms. J. Microbiol. Biotechnol. Food Sci.. 2017;6:1077-1080.
- [Google Scholar]
- Boosting antimicrobial activity of imipenem in combination with silver nanoparticles towards S. fonticola and Pantoea sp. J Nano Biomed. Eng.. 2019;11:200-214.
- [Google Scholar]
- The Japanese Society for Biomaterials, The Australian Society for Biomaterials, and the Korean Society for Biomaterials, 2010. Antibacterial properties of nanostructured silver titanate thin films formed on a titanium plate. J. Biomed. Mater. Res. A J. Soc. Biomater.. 2010;92:1171-1180.
- [Google Scholar]
- A common mechanism of cellular death induced by bactericidal antibiotics. J. Cell Collins. 2007;130:797-810.
- [Google Scholar]
- Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. J. Nanotechnol.. 2005;16:1912-1917.
- [Google Scholar]
- Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. J. Clin. Microbiol. Rev.. 2009;22:582-610.
- [Google Scholar]
- Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Diseases. 2002;34:634-640.
- [Google Scholar]
- Metallo-β-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? J. Int. J. Antimicrob. Agents. 2009;33(405) e1-05. e7
- [Google Scholar]
- The increasing threat of Pseudomonas aeruginosa high-risk clones. J. Drug Resistance Updates. 2015;21:41-59.
- [Google Scholar]
- The discharging ears in Adults in Ibadan, Nigeria causative agents and antimicrobial sensitivity pattern. J Afr. J. Clin. Exp. Microbiol.. 2002;3:3-5.
- [Google Scholar]
- Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol.. 2012;112:841-852.
- [Google Scholar]
- Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. J. Antimicrob. Agents Ulrich Chemother.. 2009;53:3538-3540.
- [Google Scholar]
- Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. J. Nanomed. Nanotechnol. Biol. Med.. 2007;3:168-171.
- [Google Scholar]
- Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. J. Nanotechnol. Sci. Appl.. 2017;10:115.
- [Google Scholar]
- Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci. 2008:647-655.
- [Google Scholar]
- Wayne, P.A., 2011. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing.
- Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. J. Trends Microbiol.. 2016;24:327-337.
- [Google Scholar]
- Antimicrobial characteristics of silver aerosol nanoparticles against Bacillus subtilis bioaerosols. J. Environ. Eng. Sci.. 2008;25:289-294.
- [Google Scholar]







