Translate this page into:
In vitro anticancer potential of dill seed extract against human hepatocellular carcinoma (Huh-7) cells
⁎Corresponding author. masiddiqui@ksu.edu.sa (Maqsood A. Siddiqui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background
Hepatocellular carcinoma is the most prevalent type of primary liver cancer and remains the foremost cause of cancer-related deaths globally. Dill (Anethum graveolens) seeds, rich in phytoconstituents, is renowned for their pharmacological properties.
Objectives
This study performed an in vitro evaluation to assess the cytotoxic effects of dill seed extract (DS-EE) on the Huh-7 hepatocellular carcinoma cell line. Moreover, the study investigated its effects on cell viability, cellular morphology, oxidative damage, levels of intracellular reactive oxygen species (ROS), mitochondrial membrane potential (MMP), and the expression of apoptosis-related genes in Huh-7 cells.
Methods
Huh-7 cells were treated with DS-EE at concentrations ranging from 5 to 100 μg/mL for a duration of 24 h.
Results
The cytotoxicity findings showed that DS-EE decreased cell viability and suppressed the growth of Huh-7 cells in a dose-dependent way, with an IC50 value of 60 μg/mL. Exposure to DS-EE extract for 24 h resulted in a significant elevation in lipid peroxidation (LPO) and a notable decrease in glutathione (GSH) content compared to the control. Furthermore, DS-EE significantly increased ROS production while notably decreasing the MMP level in Huh-7 cells. Moreover, DS-EE induces cell apoptosis by upregulating the expression of proapoptotic genes (p53, caspase-3, caspase-9, and Bax) and downregulating the expression of the antiapoptotic gene, Bcl-2. Conclusion: DS-EE exhibited a notable cytotoxic effect on Huh-7 cells by increasing oxidative damage and subsequently modulating the expression of apoptosis-related genes. The results of this study highlight the anticancer effectiveness of DS-EE, indicating its potential as a promising agent for hepatocellular carcinoma management.
Keywords
Dill seeds
Huh-7 cells
Cytotoxicity
Oxidative damage
ROS generation
Gene expression
1 Introduction
Cancer is undeniably a significant health concern worldwide, and its prevalence varies across different regions and countries. The global incidence of cancer escalated to almost 20 million new cases, accompanied by nearly 10 million fatalities (Al-Shamsi and Musallam, 2024). In developed countries, factors such as lifestyle, diets, environmental exposures, and access to healthcare may contribute to higher cancer rates (Minas et al., 2021). Liver cancer, specifically hepatocellular carcinoma, ranks among the six most commonly diagnosed and the third most common causes of cancer-related deaths globally (Rumgay et al., 2022). Early detection and intervention are crucial in improving outcomes for individuals with liver cancer (Guan et al., 2021). Additional data reveals that cancer remains a significant health challenge, with the burden continuing to rise annually (Ali et al., 2022). The field of cancer treatment has evolved into a dynamic area of research, encompassing both conventional and cutting-edge techniques. Various methods, including chemotherapy, radiation therapy, and surgery, are employed in combating cancer (Debela et al., 2021). Nonetheless, each of these approaches comes with its own set of drawbacks and limitations. Chemotherapy, though widely employed in cancer treatment, often induces severe side effects due to its non-selective action (Nurgali et al., 2018). Intrinsically, there is an urgent call to investigate alternative approaches for managing and preventing hepatocellular carcinoma. Natural products, with their diverse array of compounds and biological activities, present a compelling avenue for anticancer drug discovery (Chunarkar-Patil et al., 2024). Their potential to provide more targeted and tolerable therapies offers hope for improved outcomes in cancer treatment (Desai et al., 2008). Herbal medicines have emerged as a safe, non-toxic, and readily accessible reservoir of compounds with potential for treating cancer (Oyenihi et al., 2021). Herbs are thought to counteract the effects of diseases within the body due to their diverse array of characteristics (Isbill et al., 2020; Charneca et al., 2023). Research conducted this far has assessed the anticancer potential of numerous plants and plant-derived compounds (Chandra et al., 2023). Several of these botanicals and their constituents demonstrated notable efficacy against one or multiple types of cancer (Khan et al., 2019). Dill, more commonly referred to as Anethum graveolens, is an aromatic herb that belongs to the Apiaceae (Umbelliferae) family. Originating from the Mediterranean and Southwest Asia, this plant is known for its distinct fragrance (Singh et al., 2005). Dill seeds have been renowned for its medicinal and therapeutic uses in traditional medicine for an extended period. A. graveolens has been recognized for its biological and pharmacological properties, including antioxidant, anti-inflammatory, antidiabetic, antimicrobial, and anticancer effects (Afshari et al., 2019; Haidari et al., 2020; Al-Oqail 2021). Conventionally, in Saudi Arabia, native people utilize the Dill seeds as appetizers, carminatives, antispasmodics, and aphrodisiacs (Youssef, 2013). Phytochemical studies conducted on Dill seeds have identified a diverse array of phytoconstituents, including tannins, flavonoids, terpenoids, glycosides, phenolic acids, along with fatty acids such as capric, palmitic, stearic, and oleic acids (Jana and Shekhawat, 2010; Dahiya and Purkayastha, 2012). Hence, based on previous research on medicinal importance of dill seed, this study was aimed to assess the anticancer potential of dill seed extract against human hepatocellular carcinoma cells (Huh-7) as well as to elucidate the mechanism(s) involved in cell death.
2 Materials and methods
2.1 Reagents
All specified chemicals, reagents, and diagnostic kits were obtained from Sigma Chemical Company Pvt. Ltd., St. Louis, MO, USA, unless noted otherwise. The DMEM, antibiotic/antimycotic solution, and fetal bovine serum were sourced from Sigma company, USA. Culture vessels and other plastic consumables utilized in the study were acquired from Nunc, Denmark.
2.2 Plant material and extraction
Dill (Anethum graveolens) seeds were bought from a local market in Riyadh, Saudi Arabia. The dill seeds underwent manual screening before being ground into a coarse powder. Approximately 500 g of the grounded seeds were immersed in 1.5 L of ethanol for a duration of 3 days, with periodic agitation. Afterward, the extract underwent filtration using Whatman #1 filter paper. Then, the resulting filtrate was concentrated to dryness using a rotary evaporator operating at 40 °C under reduced pressure. The resulting ethanolic extract, designated as DS-EE, was then preserved at 4 °C for subsequent utilization.
2.3 MTT assay
Cell viability was assessed utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, following the protocol outlined by Siddiqui et al. (2008). To start, cells (1 × 104) were permitted to adhere within 96-well culture plates for 24 h in a CO2 incubator at 37 °C. After exposure, MTT solution (5 mg/ml in PBS) was added (10 μl per well in 100 μl of cell suspension), and the plates were then incubated for 4 h. Next, the supernatant was aspirated, and 200 µl of DMSO was carefully added to each well, followed by gentle mixing. The resulting color was measured at 550 nm. Control sets without treatment were run under identical conditions for comparison.
2.4 NRU assay
The neutral red uptake (NRU) assay was performed according to the methodology described by Siddiqui et al. (2008). After exposure, the cell culture medium was aspirated, and the cells were rinsed twice with PBS. Then, the cells were cultured for 3 h in medium containing neutral red (50 μg/ml). Following the incubation period, the medium was promptly aspirated, and the cells were rinsed with a solution containing 0.5 % formaldehyde and 1 % calcium chloride. Subsequently, the cells were further incubated for 20 min at 37 °C in a blend of acetic acid (1 %) and ethanol (50 %) to extract the dye. The absorbance of the extracted dye was recorded at a wavelength of 550 nm using a microplate reader. The acquired values were compared with those of the control group.
2.5 Morphological assay
Morphological changes induced by DS-EE in Huh-7 cells were assessed following exposure to increasing concentrations (5–100 μg/ml) of DS-EE for 24 h. Cell morphology was examined using an inverted phase contrast microscope (Olympus, CKX41, Japan) at 20× magnification to visualize alterations caused by the DS-EE treatment.
2.6 Intracellular glutathione (GSH) content
The GSH content was quantified following the method described by Chandra et al. (2002). Following exposure, cells were harvested, and cellular proteins were precipitated by treating 1 ml of sonicated cell suspension with 1 ml of 10 % trichloroacetic acid (TCA) on ice for 1 h, then centrifuged at 3000 rpm for 10 min. The supernatant was then mixed with 2 ml of 0.4 M Tris buffer (pH 8.9) containing 0.02 M EDTA, and 0.01 M 5,5′-dithionitrobenzoic acid (DTNB) to reach a final volume of 3 ml. The tubes were then placed in a water bath with shaking at 37 °C and incubated for 10 min, after which the absorbance of the resulting yellow color was measured at 412 nm.
2.7 Lipid peroxidation (LPO)
LPO was assessed using the thiobarbituric acid-reactive substances (TBARS) protocol described by Buege and Aust (1978). Following exposure, Huh-7 cells were collected by centrifugation, sonicated in ice-cold potassium chloride (1.15 %), and centrifuged at 3000 rpm for 10 min. The resulting supernatant (1 ml) was mixed with thiobarbituric acid (TBA) reagent (comprising 15 % trichloroacetic acid (TCA), 0.7 % TBA, and 0.25 N HCl) and heated at 100 °C for 15 min in a boiling bath. Subsequently, the samples were cooled, centrifuged at 1000 × rpm for 10 min, and the absorbance of the supernatant was measured at 550 nm.
2.8 ROS generation
For assessing reactive oxygen species (ROS) generation, we employed the fluorescent dye 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma Aldrich, USA) according to a previously outlined protocol (Farshori et al., 2022). Following a 24-hour exposure to DS-EE, cells were rinsed with PBS and then incubated at 37 °C for 60 min with 20 μM of DCFH-DA dye in an incomplete culture medium. Following this, intracellular fluorescence was examined using a fluorescence microscope.
2.9 Mitochondrial membrane potential (MMP)
MMP level was assessed following the protocol outlined by Zhao et al. (2022). In brief, both control and treated cells were washed with PBS. Following that, cells were subjected to a 1-hour incubation at 37 °C in darkness with 10 μg/mL of Rhodamine-123 fluorescent dye. After incubation, cells were again washed twice with PBS, and the fluorescence intensity of Rhodamine-123 was assessed using a fluorescence microscope (CKX41; Olympus, Japan), capturing images at a magnification of 20×.
2.10 RT-qPCR analysis
Huh-7 cells were exposed to 60 µg/ml of DS-EE for 24 h before RNA extraction. Total RNA was isolated from harvested cells using the RNeasy mini kit (Qiagen) in accordance with the guidelines provided by the manufacturer. The concentration of RNA was determined using the NanoDrop spectrophotometer (Thermo Scientific), and RNA purity was assessed by the OD260/OD280 absorbance ratio. Subsequently, 2 μg of total RNA was utilized for cDNA synthesis, performed using MLV reverse transcriptase kit (GE healthcare, UK) as per the method provided with kit. Amplification was conducted with the SYBR Green I (Roche) and the specific primers for p53, caspase-3, caspase-9, Bax, and Bcl-2 are reported in our previously study (Al-Oqail et al., 2017). The relative mRNA expression level for each gene, presented as fold change compared to GAPDH, serving as the housekeeping gene.
2.11 Statistical analysis
The results were expressed as the mean ± standard deviation derived from three separate experiments. Statistical evaluation involved employing one-way analysis of variance (ANOVA), followed by post-hoc Dunnett's test to compare values between control and treated groups. A significance threshold of p < 0.05 was considered as statistically significant.
3 Results
3.1 MTT assay
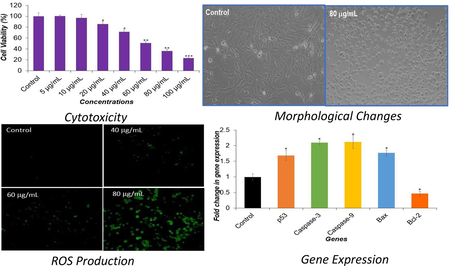
The cytotoxic effect of DS-EE in Huh-7 cells using the MTT assay is depicted in Fig. 1. A concentration-dependent decrease in percentage cell viability was observed following 24 h of DS-EE exposure, which was statistically significant (p < 0.001). Specifically, cell viability of 85 %, 71 %, 50 %, 36 %, and 23 % was recorded at DS-EE concentrations of 20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml, and 100 μg/ml, respectively, after 24 h of exposure in Huh-7 cells.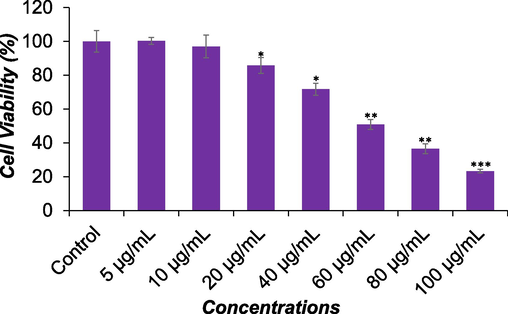
The cytotoxicity of DS-EE extract on Huh-7 cells was assessed through the MTT assay, wherein cells were subjected to varying concentrations of DS-EE for a period of 24 h. The data are expressed as mean ± S.D. *p < 0.05, **p < 0.01, and ***p < 0.001 vs control.
3.2 NRU assay
Fig. 2 depicts the cytotoxic impact of DS-EE on Huh-7 cells as determined by the NRU assay. A notable reduction in cell viability, correlated with increasing concentrations of DS-EE, was observed after 24 h of exposure, demonstrating a concentration-dependent effect (p < 0.001). Precisely, the cell viability of Huh-7 cells by NRU assay was recorded as 87 %, 73 %, 53 %, 39 %, and 29 % at 20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml, and 100 μg/ml of DS-EE, respectively, following the 24-hour exposure period.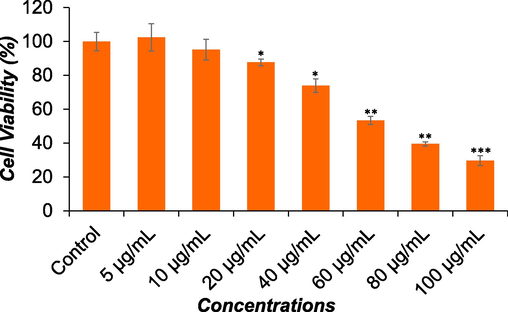
The cytotoxicity of DS-EE extract on Huh-7 cells was evaluated using the NRU assay. Cells were treated with different concentrations of DS-EE for a duration of 24 h. The data are expressed as mean ± S.D. *p < 0.05, **p < 0.01, and ***p < 0.001 vs control.
3.3 Morphological assay
Fig. 3 displays the morphological changes observed in Huh-7 cells following 24-hour exposure to DS-EE. Using a phase-contrast inverted microscope, alterations in the morphology of Huh-7 cells were visualized. The most pronounced effects were observed after exposure to DS-EE, with changes in cell morphology occurring in a concentration dependent manner. Huh-7 cells exposed to DS-EE concentrations of 40 μg/ml, 60 μg/ml, and 80 μg/ml reduced cell adhesion capacity, cells become rounded, and inhibited the growth of the cells compared to the control.
Changes in the morphology of Huh-7 cells induced by DS-EE extract. The cells were exposed to different concentrations of DS-EE extract for 24 h, and images were captured using a phase-contrast inverted microscope at 20× magnification.
3.4 Intracellular glutathione (GSH) content
Fig. 4A summarizes the depletion of glutathione (GSH) levels in cultured Huh-7 cells exposed to concentrations of 40 μg/ml, 60 μg/ml, and 80 μg/ml of DS-EE for 24 h. The results indicate that DS-EE decreased GSH level in a concentration-dependent manner. A significant decrease in GSH level was observed, with reductions of 12 %, 34 %, and 43 % at 40 μg/ml, 60 μg/ml, and 80 μg/ml of DS-EE exposure for 24 h, respectively, compared to the control.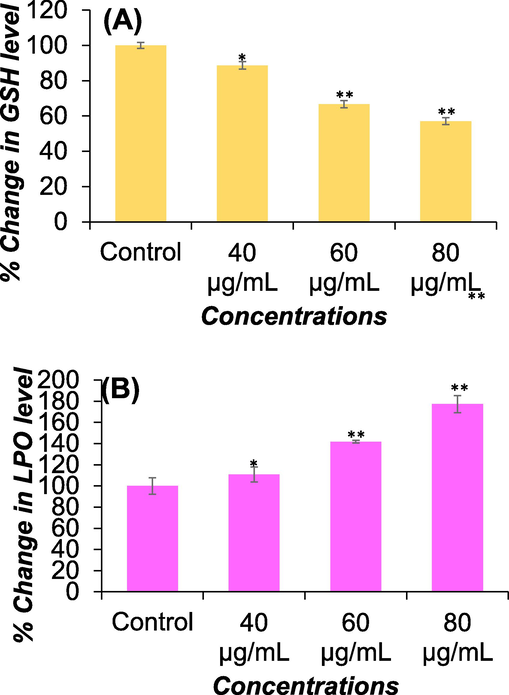
Oxidative stress assessments through (A) GSH and (B) LPO levels. GSH and LPO levels in Huh-7 cells was measured by treating the cells with DS-EE at concentrations ranging from 40 to 80 μg/ml. The data presented are the mean ± S.D. *p < 0.05 and **p < 0.01 vs control.
3.5 Lipid peroxidation (LPO)
The lipid peroxidation level induced by DS-EE in Huh-7 cells following a 24-hour exposure are presented in Fig. 4B. A concentration-dependent, statistically significant increase in lipid peroxidation was observed. Specifically, there was an increase of 110 %, 141 %, and 177 % at DS-EE concentrations of 40 μg/ml, 60 μg/ml, and 80 μg/ml, respectively, in Huh-7 cells exposed for 24 h.
3.6 ROS generation
Significant ROS generation (p < 0.01) was observed in Huh-7 cells exposed to DS-EE at concentrations of 40 μg/ml, 60 μg/ml, and 80 μg/ml for 24 h (Fig. 5A and 5B). The increase in ROS generation exhibited a concentration-dependent pattern, with levels reaching 128 %, 146 %, and 204 % compared to the untreated control following exposure to 40 μg/ml, 60 μg/ml, and 80 μg/ml of DS-EE, respectively.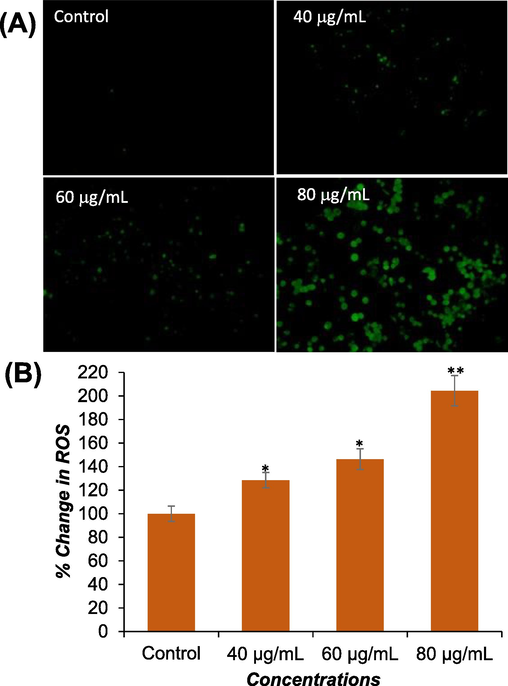
The induction of ROS generation by DS-EE in Huh-7 cells was investigated. (A) Fluorescence images were captured to visualize the intensity of DCF-DA dye after exposure to DS-EE at concentrations of 0, 40, 60, and 80 μg/ml for 24 h. (B) A graph was constructed to represent the percentage of ROS generation in Huh-7 cells. The data presented are the mean ± S.D. *p < 0.05 and **p < 0.01 vs control.
3.7 Mitochondrial membrane potential (MMP)
The effect of DS-EE exposure on mitochondrial membrane potential (MMP) was assessed in Huh-7 cells. A concentration-dependent, statistically significant (p < 0.01) decrease in MMP level was observed after 24 h of DS-EE exposure. The decrease in MMP level was measured as 13 %, 34 %, and 45 % at concentrations of 40 μg/ml, 60 μg/ml, and 80 μg/ml of DS-EE, respectively, compared to the untreated control (Fig. 6A and B).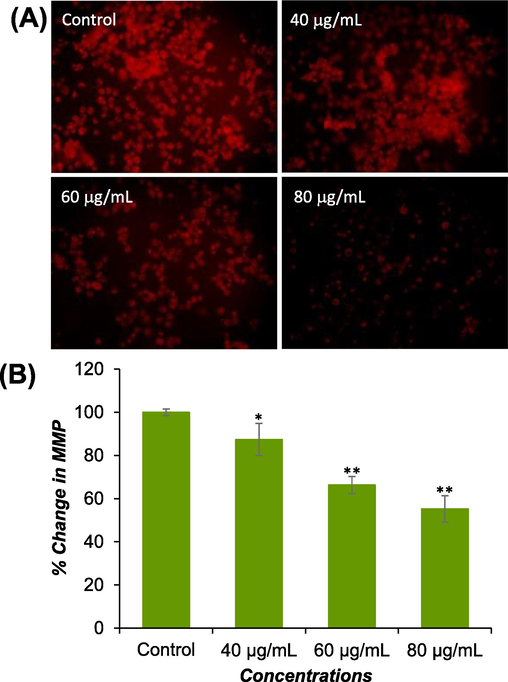
MMP levels were assessed using Rh-123 dye in both control and treated cells. (A) Images were captured to visualize intensity of Rh-123. (B) The percentage of MMP level relative to the control was determined at various concentrations of DS-EE. The data presented are the mean ± S.D. *p < 0.05 and **p < 0.01 vs control.
3.8 RT-qPCR analysis
The investigation focused on the impact of DS-EE on apoptosis-related genes in Huh-7 cells. Using real-time PCR, the mRNA levels of p53, caspase-3, caspase-9, Bax, and Bcl-2 were evaluated. As depicted in Fig. 7, the mRNA expression of p53, caspase-3, caspase-9, and Bax genes were increased by 1.6-, 2.0-, 2.1-, and 1.7-fold following 24 h of incubation with DS-EE, respectively. Conversely, Fig. 7 revealed a decrease in Bcl-2 gene expression by 0.46-fold after 24 h of incubation with DS-EE. These findings suggest that the DS-EE extract induced apoptosis in Huh-7 cells primarily through the modulation of apoptotic related genes.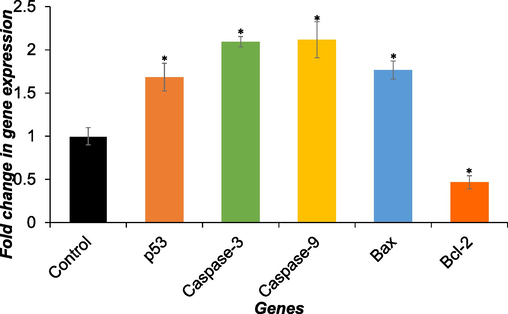
The expression of apoptosis markers genes (p53, Caspase-3, Caspase-9 Bax, and Bcl-2) was assessed in Huh-7 cells after treatment with 60 µg/ml DS-EE for 24 h. Quantification of gene expression was conducted via RT-qPCR. The data presented are the mean ± S.D. *p < 0.05 vs control.
4 Discussion
For centuries, medicinal plants have been integral to folk medicine, offering natural healing remedies with well-documented therapeutic benefits (Karunamoorthi et al., 2013). These encompass a wide range of areas, from cardiovascular disease prevention to anti-inflammatory, antimicrobial, antiaging, and anticancer activity (Anand et al., 2019). The crude extract and a vast array of natural compounds, such as tannins, flavonoids, terpenoids, glycosides, phenolic acids, from dill seeds have been discovered to possess anticancer properties (Jana and Shekhawat, 2010; Dahiya and Purkayastha, 2012). These researches highlight the significance of traditional dill seeds as invaluable reservoirs for the discovery of anticancer potential. Based on this premise, we initiated this study to primarily assess the cytotoxic activities of dill seed extract (DS-EE), and the mechanism(s) involved in cell death of Huh-7 cells. In this study, as indicated by MTT and NRU assays, DS-EE showed a potent cytotoxic activity with IC50 value of ∼60 μg/ml against human hepatocellular carcinoma cell line (Huh-7). Previous reports corroborated by our study, which highlighted that the essential oil extracted from dill seeds exhibits a dose-dependent anticancer and antiproliferative effect against human hepatocellular carcinoma cells (HepG2), with an IC50 value of 59.6 μg/ml (Al-Sheddi et al., 2019). These findings also align with other reports indicating that the A. graveolens demonstrated cytotoxic and inhibitory effects on other human cancer cell lines such as human breast cancer, human cervical cancer, and human colon cancer (Sharopov et al., 2013). These findings were further corroborated through morphological examination of Huh-7 cells treated with DS-EE. The light microscopic images depicted a loss of normal growth, cellular rounding, and inhibition of cell proliferation in Huh-7 cells exposed to DS-EE, indicative of apoptosis (Brady, 2004). Likewise, Al-Oqail and Farshori (2021) demonstrated that dill seed extract induces apoptosis in MCF-7, A-459, and HeLa cells, as evidenced by morphological alterations.
Further we explored the underlying mechanism of DS-EE-induced Huh-7 cell death. For this, Huh-7 cells were subjected to concentrations ranging from 40 to 80 μg/mL of DS-EE for 24 h, and analyses were conducted on oxidative stress markers (GSH and LPO), ROS generation, MMP, and expression of apoptotic marker genes. The increase in ROS leads to elevated LPO levels, which are known to reduce endogenous antioxidants like glutathione (GSH) (Gao et al., 2024). The results of the present investigation suggest that the administration of DS-EE at concentrations of 40, 60, and 80 μg/mL substantially increased lipid peroxidation (LPO) levels while simultaneously decreased glutathione (GSH) content in a dose dependent manner. Earlier research has documented that the exposure to plant extracts leads to heightened lipid peroxidation (LPO) and diminished levels of glutathione (GSH) in cancer cells (Al-Oqail 2021). Additionally, it has also been documented that the stimulation of plant extracts can trigger excessive generation of reactive oxygen species (ROS), resulting in oxidative stress and apoptotic cell death in cancer cells (Rostamabadi et al., 2023). As evidenced in this study, DS-EE treatment led to a dose-dependent increase in ROS production. These findings highlighted the involvement of oxidative stress and ROS generation in DS-EE-induced cell death in Huh-7 cells. The decreased mitochondrial membrane potential (MMP) has been linked to the initiation of apoptosis (Ly et al., 2003). As depicted in Fig. 6, there was a dose-dependent decline in mitochondrial membrane potential (MMP) subsequent to DS-EE treatment. This suggests that DS-EE induced mitochondrial dysfunction, consequently triggering the apoptotic pathway. Given that the apoptosis pathway is the most commonly induced type of cell death by plant extracts (Chaudhry et al., 2022), the study examined the ability of DS-EE to induce apoptosis in order to elucidate its potential mechanism of action. Apoptosis, a genetically regulated process, plays a crucial role in selectively eliminating cells, essential for cell turnover and normal development. It is tightly controlled through the expression of various genes, with the tumor suppressor gene p53 being paramount as the guardian of the genome. Triggered by either intrinsic or extrinsic pathways, apoptosis ultimately activates caspases, the enzyme responsible for executing key apoptotic features. This study utilized RT-qPCR gene expression profiling to investigate the cytotoxic impact of DS-EE on Huh-7 cells. The results indicated that DS-EE induce apoptosis, as evidenced by the notable increase in expression levels of proapoptotic marker genes, including p53, caspase-3, caspase-9, and Bax and a decrease in expression level of antiapoptotic gene Bcl-2. Consistent with our findings, prior investigations have also demonstrated that plant extracts induce apoptosis, exhibiting alterations in the apoptosis related genes in human hepatocellular carcinoma cell line (Pouraminaei et al., 2020).
5 Conclusion
In conclusion, this study demonstrated a concentration-dependent cytotoxic effect of dill seed extract (DS-EE) on human hepatocellular carcinoma (Huh-7) cells through oxidative stress. The research indicates a notable rise in lipid peroxidation (LPO) level and a decline in intracellular glutathione content (GSH), suggesting the involvement of DS-EE in triggering oxidative stress. The notable rise in intracellular ROS production and decline in mitochondrial membrane potential (MMP) suggest the contribution of DS-EE in cellular death mechanism. Furthermore, present findings indicated that DS-EE extract induces apoptosis in Huh-7 cells, accompanied by alterations in the expression of pro-apoptotic and anti-apoptotic genes. Additionally, this study has also contributed to a deeper understanding of how DS-EE induces apoptosis in Huh-7 cells, indicating its potential as a novel therapeutic option for hepatocellular carcinoma management.
CRediT authorship contribution statement
Mai M. Al-Oqail: Writing – original draft, Methodology, Data curation. Ebtesam S. Al-Sheddi: Methodology, Data curation. Nida N. Farshori: Writing – review & editing, Methodology, Data curation. Shaza M. Al-Massarani: Methodology, Data curation. Ebtesam N. Alsultan: Methodology, Data curation. Javed Ahmad: Methodology, Data curation. Abdulaziz A. Al-Khedhairy: Supervision, Resources. Maqsood A. Siddiqui: Writing – original draft, Methodology, Data curation, Conceptualization.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs; Chair for DNA Research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of antioxidant and anticancer effects of nanoemulsions prepared using dill essential oil. J. Arak Univ. Med. Sci.. 2019;22(4):40-51.
- [Google Scholar]
- The burden of cancer, government strategic policies, and challenges in Pakistan: A comprehensive review. Front. Nutr.. 2022;9:940514
- [Google Scholar]
- Anticancer efficacies of Krameria lappacea extracts against human breast cancer cell line (MCF-7): role of oxidative stress and ROS generation. Saudi Pharm. J.. 2021;29(3):244-251.
- [Google Scholar]
- Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. South Afr. J. Bot.. 2017;112:70-78.
- [Google Scholar]
- Antioxidant and anticancer efficacies of anethum graveolens against human breast carcinoma cells through oxidative stress and caspase dependency. BioMed. Res. Int.. 2021;2021:5535570.
- [Google Scholar]
- Not only a Western world issue: cancer incidence in younger individuals in the United Arab Emirates. CA Cancer J. Clin.. 2024;74(3):227-228.
- [Google Scholar]
- Evaluation of cytotoxicity, cell cycle arrest and apoptosis induced by Anethum graveolens L. essential oil in human hepatocellular carcinoma cell line. Saudi Pharm. J.. 2019;27(7):1053-1060.
- [Google Scholar]
- A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites.. 2019;9(11):258.
- [Google Scholar]
- Apoptosis methods and protocols. Totowa, NJ, USA: Humana Press; 2004.
- Scientific evidences of anticancer potential of medicinal plants. Food Chem. Adv.. 2023;2:100239
- [Google Scholar]
- Inhibition of fiber cell globulization and hyperglycemia-induced lens opacification by aminopeptidase inhibitor bestatin. Invest. Ophthalmol. vis. Sci.. 2002;43(7):2285-2292.
- [Google Scholar]
- Beyond seasoning—the role of herbs and spices in Rheumatic diseases. Nutrients.. 2023;15(12):2812.
- [Google Scholar]
- Cancer and apoptosis: the apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol.. 2022;13:842376
- [Google Scholar]
- Anticancer drug discovery based on natural products: from computational approaches to clinical studies. Biomedicines.. 2024;12(1):201.
- [Google Scholar]
- Phytochemical analysis and antibacterial efficacy of dill seed oil against multi-drug resistant clinical isolates. Asian J. Pharm. Clin. Res.. 2012;5(2):62-64.
- [Google Scholar]
- New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med.. 2021;9
- [Google Scholar]
- HPTLC estimation and anticancer potential of Aloe perryi petroleum ether extract (APPeE): A mechanistic study on human breast cancer cells (MDA-MB-231) J. King Saud Univ. Sci.. 2022;34(4):101968
- [Google Scholar]
- Elevated ROS levels caused by reductions in GSH and AsA contents lead to grain yield reduction in Qingke under continuous cropping. Plants.. 2024;13(7):1003.
- [Google Scholar]
- Early diagnosis and therapeutic strategies for hepatocellular carcinoma: from bench to bedside. World J. Gastrointest. Oncol.. 2021;13(4):197-215.
- [Google Scholar]
- The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials.. 2020;21:483.
- [Google Scholar]
- Opportunities for health promotion: Highlighting herbs and spices to improve immune support and well-being. Integr. Med.. 2020;19(5):30.
- [Google Scholar]
- Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn. Rev.. 2010;4(8):179-184.
- [Google Scholar]
- Traditional medicinal plants: a source of phytotherapeutic modality in resource-constrained health care settings. J. Evid. Based Complement. Altern. Med.. 2013;18(1):67-74.
- [Google Scholar]
- Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules.. 2019;10(1):47.
- [Google Scholar]
- The mitochondrial membrane potential (Δψ m) in apoptosis; an update. Apoptosis.. 2003;8:115-128.
- [Google Scholar]
- An overview of cancer health disparities: new approaches and insights and why they matter. Carcinogenesis.. 2021;42(1):2-13.
- [Google Scholar]
- Adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front. Pharmacol.. 2018;9:362658
- [Google Scholar]
- Unravelling the anticancer mechanisms of traditional herbal medicines with metabolomics. Molecules.. 2021;26(21):6541.
- [Google Scholar]
- The effect of Cressa Cretica hydroalcoholic extract on apoptosis and the expression of Bcl2, Bax and P53 genes in hepatoma cell line HepG2. Gene Rep.. 2020;20:100692
- [Google Scholar]
- Froriepia subpinnata leaf extract-induced apoptosis in the MCF-7 breast cancer cell line by increasing intracellular oxidative stress. Iran. J. Pharm. Res.. 2023;22(1)
- [Google Scholar]
- Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol.. 2022;77(6):1598-1606.
- [Google Scholar]
- Composition and bioactivity of the essential oil of Anethum graveolens L. from Tajikistan. Int. J. Med. Arom. Plants.. 2013;3(2):125-130.
- [Google Scholar]
- Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol in Vitro.. 2008;22(7):1681-1688.
- [Google Scholar]
- Chemical constituents, antimicrobial investigations, and antioxidative potentials of Anethum graveolens L. essential oil and acetone extract: Part 52. J. Food Sci.. 2005;70(4):M208-M215.
- [Google Scholar]
- Medicinal and non-medicinal uses of some plants found in the middle region of Saudi Arabia. J. Med. Plants Res.. 2013;7(34):2501-2513.
- [Google Scholar]
- Escin induces apoptosis in ovarian cancer cell line by triggering S-phase cell cycle arrest and p38 MAPK/ERK pathway inhibition. J. King Saud Univ. Sci.. 2022;34(1):101644
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103390.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







