Translate this page into:
In silico analysis of LPMO inhibition by ethylene precursor ACCA to combat potato late blight
⁎Corresponding author. kperveen@ksu.edu.sa (Kahkashan Perveen),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
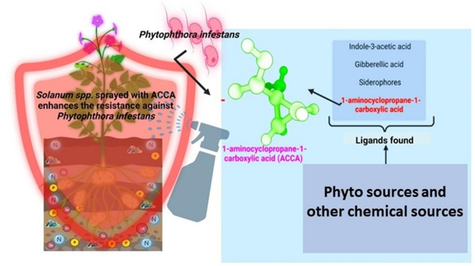
Potato late blight (PLB), caused by the pathogen Phytophthora infestans, severely threatens potato production worldwide. This study investigates the potential of the ethylene precursor 1-amino-cyclopropane-1-carboxylic acid (ACCA) to inhibit Lytic Polysaccharide Monooxygenases (LPMOs) in P. infestans, a key protein involved in the disease’s pathogenesis. Our findings demonstrate that ACCA significantly enhances the immune response in potato plants against P. infestans, with a binding energy of −8.85 kcal/mol. Integrating ACCA treatment into existing PLB management strategies could offer a novel and sustainable approach to combat this devastating disease. This research provides valuable insights into reducing the global impact of PLB and improving food security through innovative control measures.
Keywords
1-amino-cyclopropane-1-carboxylic acid
Molecular docking
Molecular dynamic simulation
Sustainable potato production
1 Introduction
Phytophthora infestans, commonly known to cause late blight or potato blight, is a highly destructive disease affecting potato plants. Thriving in humid environments with temperatures ranging from 4 to 29 °C, the pathogen can lead to extensive rotting of the plant leaves and tubers within two weeks under optimal conditions (Cooke et al. 2011). Notably, this disease was responsible for the catastrophic Irish Potato Famine in the mid-nineteenth century and continues to pose a significant threat to global crop production (Montarry et al., 2010). Despite various management strategies, PLB remains challenging due to the pathogen's adaptability. This study explores the novel use of ACCA to inhibit a key pathogen enzyme. Educational institutions and governmental organizations worldwide have established various forecasting programs to manage the disease (Montarry et al., 2010; Fry et al., 2015). Late blight is characterized by circular or irregularly shaped lesions on leaves, petioles, and stems, ranging from dark green to purplish black (Fry et al., 2013). Spore-producing structures may develop on the under-leaf surfaces beneath the lesions' margins. The rot can infiltrate potato tubers as deep as 15 cm, facilitating further infection by secondary fungi and bacteria such as those in the genus Erwinia, resulting in considerable losses during storage, transit, and sale (Cooke et al. 2011; Arora et al. 2014).
The development of P. infestans in potato plants is a multifaceted and intricately controlled process encompassing numerous phases of invasion, colonization, and propagation. As an oomycete pathogen, P. infestans begins its infection cycle by generating sporangia, which release mobile zoospores under optimal environmental conditions such as high humidity and mild temperatures. Upon contact with a vulnerable host plant, these zoospores form cysts and subsequently develop germ tubes that infiltrate the plant's epidermal cells, either directly or via natural openings like stomata. Inside the host plant, P. infestans expands and multiplies through the formation of specialized structures known as haustoria, which aid in the extraction of nutrients from plant cells (Fry et al. 2013; Whisson et al. 2016; Naumann et al. 2020). To suppress host defenses, manipulate plant signaling pathways, and facilitate its colonization and proliferation, the pathogen utilizes a range of secreted effector proteins. Host resistance (R) proteins can recognize these effectors, inducing a localized hypersensitive response (HR) that triggers programmed cell death and effectively limits the growth and spread of the pathogen. Nevertheless, P. infestans has evolved strategies to bypass host resistance by diversifying its effector repertoire, allowing it to avoid detection and sustain its virulence. As the infection advances, P. infestans generates sporangia on the plant surface, which are then dispersed by wind or rain, leading to new infection cycles in nearby plants. This destructive pathogenesis leads to the rapid development of water-soaked lesions and necrosis on leaves and stems, thereby causing significant crop losses and posing a threat to worldwide food security, emphasizing the importance of devising effective and sustainable management approaches to combat P. infestans in potato production (Nowicki et al., 2012; Fry et al., 2015; Whisson et al., 2016).
Phytophthora can survive in various environments, including improperly stored tubers, rubbish piles, field plants, and greenhouses. The pathogen produces both sexual oospores and asexual sporangia, which can be windborne, infecting nearby plants within hours (Fry et al., 2013; Arora et al., 2014). Zoospores, a type of asexual spore with flagella, germinate when temperatures drop below 15 °C, encysting and forming germ tubes as temperatures rise. Foliage blighting occurs within four to six days post-infection, with a new crop of sporangia forming as long as the weather remains cool and moist (Cooke et al., 2011; Arora et al., 2014).
This study centers on the copper-dependent LPMOs and their involvement in plant-pathogen interactions. We selected this protein for putative inhibition in P. infestans by a range of relevant effectors from plant hosts to predict mechanistic pathogen-host interplay and identify potential biocontrol strategies for this devastating pathogen. The LPMOs are crucial to P. infestans plant infection, warranting further investigation (Vandhana et al. 2022). We conducted a virtual screening of several plant metabolites and growth regulators known for their housekeeping roles in plant physiology, particularly under biotic and abiotic stress. The initial screening of potent ligands was followed by a molecular re-docking approach (Xuan-Yu et al., 2011), revealing 1-amino-cyclopropane-1-carboxylic acid (ACCA) as the most potent compound against P. infestans with a probable mechanism involving LPMO-binding target (Sabbadin et al. 2021; Kaur et al. 2022). ACCA is involved in the induction of the virulence system against P. infestans attack. Given the multitude of microbes that cause damage to Solanum spp., this study aims to identify strategies to enhance the virulence via the exogenous application of ACCA.
2 Materials and methods
2.1 Target protein & ligand preparation
LPMO were retrieved from RCSB PDB (https://www.rcsb.org/structure/6Z5Y) and then minimized by Chimera (Fig. 1A). The Ramachandran plot is shown in Fig. 1B. To determine the function of LPMOs (PDB ID: 6Z5Y) when it binds with 1-amino-cyclopropane-1-carboxylic acid (ACCA) (Chem ID: 535), the 3D structures of both in.sdf format were retrieved from PubChem (NCBI 2023). The Chimera UCSF team employed a 900-step conjugate gradient energy minimization method followed by a 1000-step steepest-descent approach for further optimization. Subsequently, the ligands were converted to.pdb format using Open Babel (version 3.1.1) and minimized again for 1000 iterations using the steepest descent algorithm. The AMBER ffSB14 force field was then utilized after assigning Gasteiger charges to establish the partial charges. Finally, the ligand underwent geometric optimization with DMol3 (Fig. 2). It is well-documented that crystallographic structures often depict LPMOs as dimers, a phenomenon recognized as a crystallographic artifact. This dimerization has been observed across various LPMO families and does not necessarily represent their physiological monomeric state. Our study utilized the dimeric form observed in the crystallographic structure (PDB 6Z5Y) to explore potential intermolecular interactions. However, the focus remained on understanding the functional aspects of the LPMO monomers, which are essential for the enzyme's activity on polymeric substrates. The copper site of the monomeric LPMO is crucial for its interaction with glycosidic bonds, facilitating the cleavage process (Sabbadin et al., 2021; Askarian et al., 2021).
(A) Structure representation and active sites of the selected target protein, Lytic Polysaccharide Monooxygenases (LPMOs), with PDB ID: 6Z5Y from Phytophthora infestans. (B) The Ramachandran plot showing the quality of the protein structure.
Geometrically optimized structure of 1-amino-cyclopropane-1-carboxylic acid (ACCA) using DMol3, showing the minimized energy configuration.
2.2 Virtual screening
BIOVIA Discovery Studio Visualizer version 2022 (Leonardo et al. 2015) connected the chemical targets to the protein's active site. This method allowed for producing a final product with strong binding affinity. AutoDock Vina was utilized to determine the binding site of the protein complex and create the receptor grid. The ligand conformation with the highest binding energy was selected for re-docking and further analysis.
2.3 Molecular re-docking studies
Following the virtual screening, 1-amino-cyclopropane-1-carboxylic acid (ACCA), the most potent ligand, was used to construct the receptor grid using AutoDock MGL version 1.5.6. Both ligand and receptor were saved in.pdbqt format. The grid point spacing was set to 0.57 Å with an exhaustiveness value of 8. Output files were examined using PyMol alongside Discovery Studio Visualizer 2021 in.pdbqt format. Validation and enhancement of ligand binding were achieved by examining co-crystallized ligands. The target protein molecules facilitated the binding of 1-amino-cyclopropane-1-carboxylic acid (ACCA). The inhibitory concentration of every candidate molecule was assessed by leveraging virtual screening outcomes to determine the candidate demonstrating the most robust interaction with copper-dependent lytic polysaccharide monooxygenases (LPMOs). The PDB 6Z5Y structure was simplified using the steepest descent method (1000 steps) before applying the AMBER ff4 force field. Additionally, prior to initiating the experiment, the protonation states of the copper-dependent lytic polysaccharide monooxygenases (LPMOs) were checked for neutralization. Polar hydrogen bonds, Kollman and Gastieger charges, and electrostatic forces produced the receptor and ligands. After merging nonpolar hydrogens, receptor, and ligand molecules were saved in.pdbqt format. A grid box with dimensions X=32, Y=31, and Z=36 with 2.40 Å spacing was generated. The Lamarckian Genetic Algorithm was used to dock protein–ligand complexes, identifying those with the lowest binding free energy (ΔG). AutoDock 4.2.6 was used for molecular docking experiments (Toukmaji et al., 1996).
2.4 Molecular dynamics simulation
To emulate molecular dynamics, Desmond software (Schrödinger LLC) was used on a 100 ns time scale (Bowers et al., 2006). To begin molecular dynamics simulations, initial docking research was conducted to predict ligand binding states in a static environment accurately. MD simulations then used the classical equation of motion to monitor atom movements over time (Jorgensen et al., 1983; Jorgensen et al., 1996; Debnath et al., 2023). Simulations were conducted to examine the physiological state of ligand binding using the System Builder tool. The systems utilized an orthorhombic box model of the solvent (TIP3P) and applied a force field derived from OPLS 2005 (Shaw et al. 2010; Shivakumar et al., 2010). NaCl at 0.15 M was used to simulate physiological conditions, and the simulation was conducted at 300 K and atmospheric pressure. Models were relaxed before starting, and stability was assessed by tracking protein and ligand RMSD with trajectories recorded every 100 ps (Perveen et al., 2023; Shaw et al., 2010).
3 Results
3.1 Virtual screening of ligands
The ligand with the lowest binding energy score of −8.85 kcal/mol, indicating the highest binding affinity for LPMO, was 1-amino-cyclopropane-1-carboxylic acid (ACCA). The low binding energy of −8.85 kcal/mol suggests a strong interaction between ACCA and LPMO, indicating potential efficacy in inhibiting the enzyme. This ligand underwent further refinement within the 6Z5Y binding cavity and was identified as the most potent among seven ligands tested for the receptor transcription protein LPMO (Table 1).
Active compounds
Gibbs free energy [ΔG] (in kcal/mol)
Gibberellic acid
−5.39
Indole-3-Acetic acid
−4.58
Citric acid (as Siderophore)
−4.23
Hydroxamate
−5.54
1-amino-cyclopropane-1-carboxylic acid (ACCA)
−8.85
Dextran
−5.87
Xanthan
−6.98
3.2 Molecular re-docking
Molecular docking identified the most effective intermolecular configuration between LPMO and seven ligands, revealing binding affinities listed in Table 1. During re-docking tests, 1-amino-cyclopropane-1-carboxylic acid (ACCA) exhibited a distinct binding pocket with LPMO, binding tightly to the core with a free energy of −8.85 kcal/mol (Fig. 3). During the initial docking studies, we identified interactions between ACCA and residues away from the substrate binding and catalytic sites in the dimeric context. Recognizing that these interactions might not fully represent the inhibitory potential, we conducted re-docking and molecular dynamics simulations focusing on the monomeric form of LPMOs. These simulations were crucial to observing ACCA's migration and binding stability closer to the monomeric catalytic site.
Molecular docking results of copper-dependent Lytic Polysaccharide Monooxygenases (LPMOs) bound to 1-amino-cyclopropane-1-carboxylic acid (ACCA), with a 2-D interaction diagram on the right panel illustrating key binding interactions.
3.3 Molecular dynamics simulation (MDS) & MMGBSA analysis
MD simulations were conducted on the most potent ligand ACCA and the pathogenic protein lytic polysaccharide monooxygenases to assess complex stability and quality until convergence. The results from our 100 ns molecular dynamics simulations provided significant insights. The stability of the ACCA-LPMO complex over this period suggested that ACCA can indeed migrate closer to the catalytic copper site, indicating its potential as an effective inhibitor. The observed stability and interactions in the monomeric context support the hypothesis that ACCA can impact the enzyme’s activity despite initial distal interactions observed in the dimeric form. The root mean square deviation (RMSD) of the Cα-backbone of LPMO bound to ACCA showed a deviation of 1.5 Å, indicating that the protein–ligand complex remained stable throughout the simulation (Fig. 4A, Table 2). This stability suggests that ACCA effectively binds to the LPMO without causing significant structural perturbations. Following 100 ns, notable variations were observed in the protein compared to the reference structure, especially within residues 17–24 of the LPMO bound to ACCA, as illustrated in Fig. 4B and Table 2. These variations indicate localized flexibility, which may be crucial for the protein's function. The Radius of Gyration (Rg) plot of the C-alpha backbone depicted in Fig. 4C demonstrated that the LPMOs bound to ACCA exhibited a deviation of 0.24 Å throughout the 100 ns simulation, according to Table 2. This minor deviation in the Rg plot confirms the compactness and stability of the protein–ligand complex. Additionally, LPMO bound to ACCA maintained stability throughout the simulation, evidenced by the presence of three consistent hydrogen bonds, which play a vital role in the stability and specificity of the protein–ligand interaction (Fig. 5).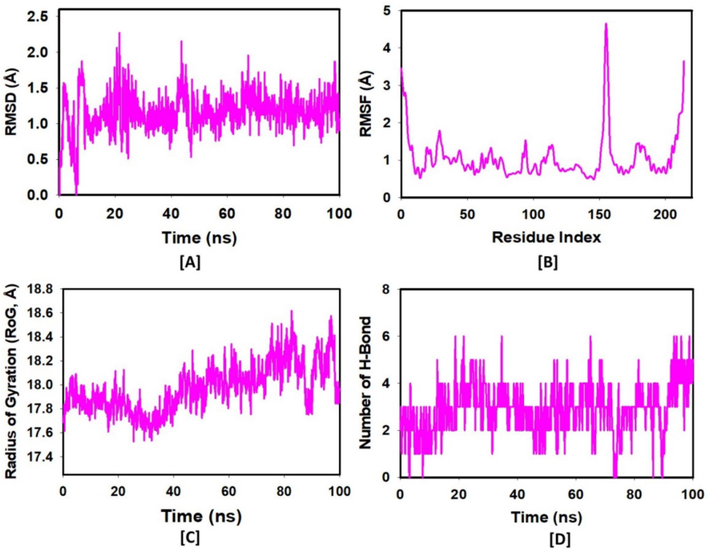
(A) RMSD plot showing the stability of the Cα-backbone of LPMOs bound to ACCA over a 100 ns simulation. (B) RMSF plot highlighting the flexibility of residues 17–24 in the LPMOs-ACCA complex. (C) Radius of Gyration plot indicating the compactness of the LPMOs-ACCA complex during the 100 ns simulation. (D) Hydrogen bonding interactions between LPMOs and ACCA, demonstrating consistent hydrogen bonds over the simulation period.
S. No.
Molecular dynamics simulations
Values
1
RMSD
1.5 Å
2
RMSF
17–24 Fluctuation at residues
3
Radius of Gyration (Rg)
0.24 Å
4
Number of Hydrogen Bonding
3
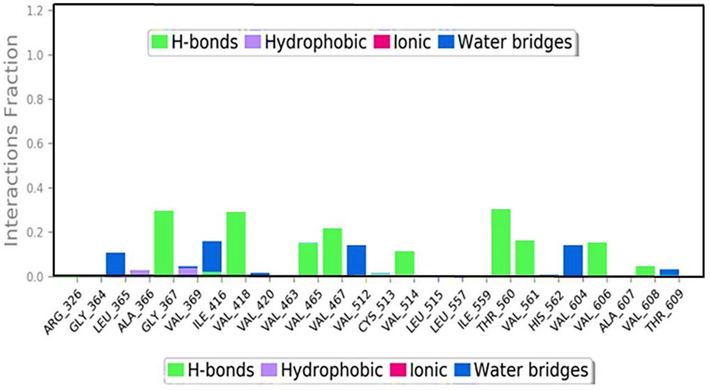
Various types of bonds formed during the 100 ns simulation run, including hydrogen bonds, hydrophobic interactions, ionic interactions, and water bridges, illustrating the stability and specificity of the LPMOs-ACCA complex.
ACCA exhibited pronounced hydrogen bonding interactions with the anticipated binding residues, forming a robust network of intermolecular connections. Additionally, the interaction between ACCA and the target protein involved diverse non-bonded interactions, as visually represented in Fig. 5. Moreover, Fig. 6 provides a visual depiction of the tightly bound conformation of ACCA with the LPMO protein throughout the entirety of the 100 ns simulation, indicating the sustained stability of the complex over time.
Stepwise interaction analysis of the LPMOs-ACCA complex over the 100 ns simulation, showing the evolution and stability of interactions throughout the simulation period.
The MMGBSA method, widely employed for assessing complex binding energy, was utilized to calculate the binding energy of each protein-ACCA complex in this study, considering various non-bonded interactions. The binding energies and their components for the interaction between LPMO and ACCA, calculated using the MMGBSA method, provide detailed insights into the strength and nature of the binding interactions. The overall binding free energy (ΔGbind) of −31.67 ± 4.60 kcal/mol indicates that the binding process between ACCA and LPMO is energetically favorable. This substantial negative value suggests a strong interaction between ACCA and LPMO, which is crucial for the efficacy of ACCA as an inhibitor. The lipophilic contribution (ΔGbindLipo) to the binding energy is −09.44 ± 0.72 kcal/mol, highlighting the importance of hydrophobic interactions in stabilizing the protein–ligand complex. These interactions are significant in ensuring that the ligand fits snugly within the protein's binding pocket. Additionally, the Van der Waals contribution (ΔGbindvdW) of −21.11 ± 3.21 kcal/mol emphasizes the role of these weak, non-covalent forces in maintaining the proper alignment and binding of ACCA to LPMO. Electrostatic interactions also play a critical role, as evidenced by the Coulombic contribution (ΔGbindCoulomb) of −15.66 ± 5.01 kcal/mol. This term accounts for the attractive forces between charged groups in ACCA and LPMO, which are essential for the specificity and strength of the binding. The hydrogen bonding contribution (ΔGbindHbond) of −4.54 ± 1.11 kcal/mol further supports the stability of the complex, as hydrogen bonds are key to the interaction between the ligand and the protein's active site. The solvation energy (ΔGbindSolvGB) of −30.14 ± 2.97 kcal/mol indicates favorable desolvation effects upon binding. This term reflects the free energy change associated with removing solvent molecules from the binding interface, which contributes significantly to the binding free energy. Lastly, the covalent contribution (ΔGbindCovalent) of −5.14 ± 0.24 kcal/mol, though typically less emphasized in non-covalent docking studies, adds to the overall binding stability by considering covalent interactions within the ligand or between the ligand and the protein. As a result, the complexes' binding energy and overall stability were elevated, as outlined in Table 3.
Energies (kcal/mol)
LPMOs with ACCA
ΔGbind
−31.67 ± 4.60
ΔGbindLipo
−09.44 ± 0.72
ΔGbindvdW
−21.11 ± 3.21
ΔGbindCoulomb
−15.66 ± 5.01
ΔGbindHbond
−4.54 ± 1.11
ΔGbindSolvGB
−30.14 ± 2.97
ΔGbindCovalent
−5.14 ± 0.24
4 Discussion
Potato late blight (PLB) represents a significant threat to global potato production, resulting in up to $10 billion in losses and management costs annually (Montarry et al., 2010; Arora et al., 2014; Fry et al., 2013). This issue remains prominent despite being 170 years since the devastating Irish Potato Famine. Substantial progress has been made by growers, agronomists, and laboratory scientists in comprehending the molecular pathogenesis of this crucial pathosystem and in developing effective management strategies to mitigate PLB (Cooke et al. 2011; Fry et al. 2013). P. infestans, a hemi-biotrophic oomycete, primarily infects potato plants, targeting stems, leaves, tubers, and fruits (Cooke et al. 2011). The pathogen secretes various signaling molecules and effectors that facilitate the initial stages of host infection. Key examples include cellulases, lipases, pectinases, and proteases, as well as secondary metabolites such as elicitins, glycoalkaloids, pyranones, and phytotoxins (Wang et al. 2019; Boevink et al. 2020).
Molecular dynamics (MD) simulations provided significant insights into the stability and interactions of the ACCA-LPMO complex. The significant contributions from lipophilic and Van der Waals interactions highlight the importance of hydrophobic and weak non-covalent forces in the stability of the ACCA-LPMO complex. These interactions ensure ACCA is properly aligned within the binding pocket, maximizing its inhibitory potential. The strong electrostatic interactions, as indicated by the Coulombic contribution, further enhance the binding specificity and strength, which are critical for the effective inhibition of LPMO. Hydrogen bonds help maintain the structural integrity of the protein–ligand complex, ensuring that ACCA remains firmly bound to LPMO. The favorable solvation energy contribution suggests that the desolvation process, which occurs when ACCA binds to LPMO, is energetically beneficial, further supporting the stability of the complex. The presence of non-bonded interactions, including hydrophobic contacts, ionic interactions, and water bridges, further enhances the stability of the complex (Bowers et al. 2006; Jorgensen et al. 1996). These interactions indicate that ACCA can effectively bind and stabilize LPMOs, suggesting its potential as a promising inhibitor for managing potato late blight.
LPMOs are pectin-degrading copper-dependent enzymes that play a pivotal role in P. infestans' ability to breach the plant cell wall, facilitating infection (Sabbadin et al. 2021; Jagadeeswaran et al. 2021). The enzymatic activity of LPMOs, driven by the reduction of the active-site Cu ion and subsequent re-oxidation by molecular oxygen or H2O2, is crucial for this process. Identifying ACCA as a potential inhibitor highlights its role in binding and stabilizing LPMOs, thereby inhibiting their activity (Quinlan et al. 2011; Bissaro et al. 2017, Shahid et al. 2019). The overexpression of LPMO-encoding genes during oomycete infection further underscores the significance of targeting these enzymes to disrupt the pathogen's life cycle (Sabbadin et al. 2021). Ethylene, a plant hormone known for enhancing stress resilience, is synthesized from ACCA, which serves as its precursor. This study demonstrates that ACCA can inhibit LPMO activity, thus potentially blocking the pathogenesis of potato late blight. However, to fully validate these findings, further experimental research is required to confirm ACCA's inhibitory effects on LPMO both in vitro and in planta. Recent studies have highlighted ACCA's efficacy in conferring resistance to various abiotic and biotic stresses, reinforcing its potential as a versatile and effective treatment in agricultural practices (Debnath et al., 2023; Debnath et al., 2024). ACCA's role in enhancing potato plant defense could be a cornerstone in developing sustainable agricultural practices, reducing reliance on chemical fungicides (Tokin et al., 2021). By integrating ACCA into crop management strategies, farmers can achieve more resilient crops, contributing to sustainable agriculture and food security.
This research underscores the importance of understanding the intricate molecular interactions between P. infestans and its host and the necessity for continued research and development of effective management strategies to mitigate the global impact of potato late blight on agricultural systems (Arora et al., 2014). Combining both dimeric and monomeric analyses, this integrative approach ensured a comprehensive understanding of ACCA's interaction with LPMOs. While the dimeric form provided a broader perspective on potential interaction sites, it primarily served as a benchmark for initial studies. The focus on monomeric functionality ensured physiological relevance, thereby validating ACCA's inhibitory potential in a realistic enzymatic context.
Future studies should further prioritize monomeric analyses to reflect the physiological state of LPMOs accurately. Experimental validation, such as enzyme inhibition assays and structural characterization of ACCA-LPMO complexes in their monomeric form, will be essential. Additionally, employing the dimeric form as a comparative benchmark can help identify and confirm critical interaction sites that are consistent across both forms. This approach will provide deeper insights into the dynamics of ACCA binding and its inhibitory mechanisms, enhancing our understanding of its potential applications in pest control and therapeutic interventions.
5 Conclusions
The discovery of the tremendous potential of 1-amino-cyclopropane-1-carboxylic acid (ACCA), an ethylene precursor, in enhancing Solanum spp. immunological response against P. infestans highlights the importance of exploring novel treatments to combat this disease. The promising binding value of −8.85 kcal/mol for ACCA suggests that exogenous application of this compound could bolster potato plant defenses against PLB. This study provides a promising foundation for developing ACCA-based treatments for PLB, potentially transforming sustainable potato farming practices. This research contributes to our understanding of effective PLB management strategies and underscores the need for continued investigation and innovation to overcome the disease's limitations on both local and continental levels. By leveraging cutting-edge research technology and interdisciplinary collaboration, we can develop effective, sustainable solutions to mitigate the impact of PLB on global potato production, ensuring food security for future generations.
CRediT authorship contribution statement
Najla A. Alshaikh: Writing – review & editing, Resources, Funding acquisition, Data curation. Kahkashan Perveen: . Sandip Debnath: Writing – original draft, Methodology, Formal analysis, Data curation. Amitava Paul: Writing – original draft, Validation, Formal analysis. Ong Ghim Hock: Writing – review & editing, Software, Investigation. R.Z. Sayyed: Writing – review & editing, Validation, Resources.
Acknowledgments
The authors would like to acknowledge the support provided by Researchers Supporting Project Number RSP2024R358, King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Late blight disease of potato and its management. Potato Journal. 2014;41(1):16-40.
- [Google Scholar]
- The lytic polysaccharide monooxygenase CbpD promotes Pseudomonas aeruginosa virulence in systemic infection. Nat. Commun.. 2021;12:1230.
- [CrossRef] [Google Scholar]
- Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol.. 2017;13:1123-1128.
- [Google Scholar]
- Devastating intimacy: the cell biology of plant-Phytophthora interactions. New Phytol.. 2020;228(2):445-458.
- [Google Scholar]
- Scalable algorithms for molecular dynamics simulations on commodity clusters. In: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing. 2006. p. :84-es.
- [Google Scholar]
- Epidemiology and integrated control of potato late blight in Europe. Potato Res.. 2011;54:183-222.
- [Google Scholar]
- Exploring the efficacy of 1-amino-cyclopropane-1-carboxylic acid (ACCA) as a natural compound in strengthening maize resistance against biotic and abiotic stressors: an empirical computational study. Front. Microbiol.. 2023;14:1232086.
- [Google Scholar]
- The enhanced affinity of WRKY reinforces drought tolerance in Solanum lycopersicum L.: An innovative bioinformatics study. Plants. 2023;12(4):762.
- [Google Scholar]
- Enhancing drought tolerance in cauliflower (Brassica oleracea var. botrytis) by targeting LFY transcription factor modulation via the ethylene precursor, ACCA: an innovative computational approach. Front. Plant Sci.. 2024;15:1255979.
- [Google Scholar]
- The 2009 late blight pandemic in the eastern United States–causes and results. Plant Dis.. 2013;97(3):296-306.
- [Google Scholar]
- Five reasons to consider phytophthora infestans a reemerging pathogen. Phytopathology. 2015;105(7):966-981.
- [Google Scholar]
- Do lytic polysaccharide monooxygenases aid in plant pathogenesis and herbivory? Trends Plant Sci.. 2021;26(2):142-155.
- [Google Scholar]
- Comparison of simple potential functions for simulating liquid water. J. Chem. Phys.. 1983;79(2):926-935.
- [Google Scholar]
- Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc.. 1996;118(45):11225-11236.
- [Google Scholar]
- An exopolysaccharide-producing novel Agrobacterium pusense strain JAS1 isolated from snake plant enhances plant growth and soil water retention. Sci. Rep.. 2022;12(1):21330.
- [Google Scholar]
- Molecular docking and structure-based drug design strategies. Molecules. 2015;20:13384-13421.
- [Google Scholar]
- Fitness costs associated with unnecessary virulence factors and life history traits: evolutionary insights from the potato late blight pathogen Phytophthora infestans. BMC Evol. Biol.. 2010;10(1):1-9.
- [Google Scholar]
- The importance of nutrient management for potato production part II: Plant nutrition and tuber quality. Potato Res.. 2020;63:121-137.
- [Google Scholar]
- National Center for Biotechnology Information (NCBI) (2023). PubChem Compound Summary for CID 535, 1-Aminocyclopropanecarboxylic acid. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/1-Aminocyclopropanecarboxylic-acid.
- Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis.. 2012;96(1):4-17.
- [Google Scholar]
- Enriching drought resistance in Solanum lycopersicum using Abscisic acid as drought enhancer derived from Lygodium japonicum: A new-fangled computational approach. Front. Plant Sci.. 2023;14:1106857.
- [Google Scholar]
- Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci.. 2011;108:15079-15084.
- [Google Scholar]
- Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes. Science. 2021;373(6556):774-779.
- [CrossRef] [Google Scholar]
- Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf.. 2019;180:588-599.
- [Google Scholar]
- Atomic-level characterization of the structural dynamics of proteins. Science. 2010;330(6002):341-346.
- [Google Scholar]
- Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput.. 2010;6(5):1509-1519.
- [Google Scholar]
- Inhibition of lytic polysaccharide monooxygenase by natural plant extracts. New Phytol.. 2021;232(3):1337-1349.
- [Google Scholar]
- Ewald summation techniques in perspective: a survey. Comput. Phys. Commun.. 1996;95:73-92.
- [Google Scholar]
- On the expansion of biological functions of lytic polysaccharide monooxygenases. New Phytol.. 2022;233(6):2380-2396.
- [Google Scholar]
- Phytophthora infestans RXLR effectors act in concert at diverse subcellular locations to enhance host colonization. J. Exp. Bot.. 2019;70(1):343-356.
- [Google Scholar]
- Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des.. 2011;7(2):146-157.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103436.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







