Translate this page into:
Impact assessment of Karanja deoiled cake and sundried biogas slurry as a mixed substrate on the nematicidal potential of Purpureocillium lilacinum
⁎Corresponding author. asharma5@amity.edu (Abhishek Sharma),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Developing bio-alternatives to chemical pesticides is of global scientific interest. First scientific report on value addition of sundried biogas slurry as fungal substrate. Current findings helped in interpreting the relationship between the nature of the substrate and virulence of the fungus. Purpurocillium lilacinum grown on Karanja deoiled cake and sundried biogas slurry (60:40) that controlled 71.8% nematodes in soil. The study provides a holistic approach that could provide plant protection and waste management simultaneously.
Abstract
The present study explored the feasibility of waste biomass viz., Karanja (Pongamia pinnata) deoiled cake (KDC), and sundried biogas slurry (BGS) as a mixed substrate to develop Purpureocillium lilacinum 6029 based fungal formulation against plant-parasitic nematode, Meloidogyne incognita. The fungus cultured on KDC-BGS in a 60/40 ratio showed maximum egg mass inhibition (96.8%) and protease activity (376.65 U/g) along with superior colonization ability (100% colonization of egg mass on the third day). Incidentally, P. lilacinum spores obtained from the traditional substrate (wheat) inhibited only 68.72% egg mass hatching with a comparatively poor colonization rate construing the linkage between the nature of substrate and virulence of the fungus. Bioefficacy studies on tomato plants in the greenhouse revealed that the formulation with KDC-BGS as a substrate was 1.19 times more effective than the wheat-based formulation in controlling nematodes. Enhanced physical-biochemical parameters of tomato plants treated with the bioformulation further substantiated KDC-BGS as a reliable and economical substrate for P. lilacinum.
Keywords
Karanja deoiled cake
Sundried biogas slurry
Purpureocillium lilacinum
Root-knot nematodes
Tomato
1 Introduction
In the existing crop production scenario, biocontrol has become an assuring alternative to synthetic pesticides. Purpureocillium lilacinum (earlier Paecilomyces lilacinus) is well-known for controlling plant-parasitic root-knot nematodes (RKNs), particularly Meloidogyne incognita (Kofoid and White). The ability of P. lilacinum to produce virulent spores that can infect sedentary stages, particularly the eggs and females of RKNs (Nesha and Siddiqui, 2017), make them an ideal candidate for commercial application in the form of solid or liquid bio formulations. Bioformulation is a physical mixture of biocontrol agents and substrates and inert ingredients like carriers that provide effective and economical control of pests. Although various factors determine commercial success, nutrient medium composition always remains a limiting factor (Martínez-Álvarez et al., 2016). Therefore, attention is now on developing a simple medium in composition, cheap in price, and available in large quantities (Nguyen et al., 2017).
Non-edible deoiled cakes are now gaining momentum as competitive nutrient substrates for microbial agents' optimal growth (Singh and Satyanarayana, 2006; Ramachandran et al., 2007). In one of our previous investigations, we cultivated P. lilacinum 6029 utilizing Karanja (Milletia pinnata) deoiled cake (KDC) as a principal nitrogen source. We established the significance of the C/N ratio by achieving the optimal growth of the fungus using sucrose as a metabolizable carbon source (Sharma et al., 2014b). However, sucrose being an expensive excipient, we explored sundried biogas slurry (BGS) as a more affordable carbon source in the prevailing investigation to minimize the input cost of the product development. BGS, also known as digestate, is a by-product of anaerobic digestion in a biogas plant. Fresh BGS is a rich source of nitrogen. Hence, it recently earned heightened interest as a potential feedstock for microalgal cultivation to produce biofuel (Zhu et al., 2016) besides its customary use as organic manure. Unfortunately, slurry on drying loses a great amount of ammonia nitrogen by volatilization and is considered futile for the purposes mentioned above. Interestingly, the slurry remains rich in carbohydrates and hence, can be explored as a potential carbon source in solid-state fermentation of potential strains.
Nutrient medium compositions further influence the virulence parameters of biocontrol fungi. Various solid substrates like wheat grains, wheat flour, wheat bran, rice flour, etc., increased the virulence of Beauveria bassiana isolates against the brown-tail moth, Euproctis chrysorrhoea (Bena-Molaei et al., 2011). Still, there is a dearth of research on agro-industrial waste valorization for biopesticide development. The current study demonstrates the technical feasibility of utilizing low-cost KDC (biodiesel waste) and sundried BGS as a mixed substrate for P. lilacinum 6029 on both bioformulation development dimensions, i.e., high spore yield and virulence of spores. Additionally, we evaluated the developed product's shelf life and field-efficacy (greenhouse study) against nematode infestation in tomato plants.
2 Materials and methods
2.1 Microorganisms
Purpureocillium lilacinum 6029 was procured from the Indian Type Culture Collection (ITCC), Indian Agriculture Research Institute (IARI), New Delhi. Sterile distilled water with 0.01% Tween-80 was used to prepare the spore suspension from the fungal slant. The fermentation media was subsequently inoculated with the freshly prepared suspension.
Egg masses of M. incognita were received from the Division of Nematology, IARI, New Delhi. They were used to maintain the pure population on brinjal (Solanum melongena) plants growing in earthen pots with autoclaved soil at the micromodel complex, IIT Delhi. Inoculums (egg masses) of M. incognita were carefully handpicked from the roots by sterilizing forceps. Second-stage juveniles (J2) were collected following 3 days of incubation of egg masses in sterile distilled water.
2.2 Substrates
Based on our previous research (Sharma et al., 2014a, 2014b), KDC was chosen as a nitrogen substrate for P. lilacinum 6029 in the current study. The Karanja oil was extracted from the cold press, and the residual cake was further deoiled in the laboratory using the soxhlet apparatus. To overcome the low C/N ratio crisis of deoiled cake, sundried BGS was adopted as a carbon amendment during SSF. The fresh BGS was procured from Shri Krishna Gowshala, Ghaziabad (U.P.), India, and was sundried for two weeks at IIT Delhi. KDC and BGS were then milled and sieved to get particle size nearby 2.0 mm. The physicochemical properties of KDC and BGS were determined using standard methods (Table 1). Chemicals and reagents of analytical grade were purchased from Merck and Himedia (India). Data in the table were exhibited in the form of “Mean ± Standard deviation”.
Parameters
KDC
BGS
C
42.26 ± 2.4
41.6 ± 2.1
N
4.87 ± 0.87
0.72 ± 0.12
P
0.89 ± 0.03
0.59 ± 0.02
K
1.3 ± 0.02
0.91 ± 0.04
pH
5.5 ± 0.23
7. 61 ± 0.4
C/N
8.68 ± 0.11
57.78 ± 0.19
Moisture
4.6 ± 0.36
5.1 ± 0.23
2.3 Development of bionematicide formulation through mixed solid substrate fermentation
Mixed Solid Substrates Fermentation (SSF) was carried to know the efficiency of various combinations of KDC-BGS (80:20, 60:40, 40:60, and 20:80, respectively) to develop potent bioformulation of P. lilacinum 6029. The fermentation was carried out using 10 g of the substrate in a 250 ml Erlenmeyer flask. The substrate was inoculated with one ml of fungal spore suspension. The initial moisture content (65%) of the mixed substrate was managed by adding sterile distilled water. The moisture from the spore suspension water was also considered. Flasks were then incubated at 28 °C for ten days. SSF of P. lilacinum on wheat grains (traditional substrate) under similar conditions was taken as control. Each assay was done with three replicates. For the formulation development, P. lilacinum grown on optimal substrate composition was mixed with fly-ash (inert carrier) in the ratio of 1:2 and dried to 4% moisture under shade.
The selection of optimal substrate composition was performed based on spore count and virulence of spores. The spores in fermented matter were harvested by adding 10 ml of 0.01% solution of Tween 80 to 1 g of fermented product and vortexed for 2 min. Proper dilutions were made and spore were counted (spores/g) using Neubeaur's haemocytometer.
Egg mass hatching inhibition of M. incognita and protease production by P. lilacinum was deemed virulence for fungus (Huang et al., 2005). For assaying egg mass hatching inhibition, spores from the fermented substrates were harvested by the method mentioned above and adjusted to 107spores/ml through dilutions. 24-well plates, each containing 2 ml of the test spore concentration, were used to determine the percentage of egg mass inhibition. Four uniform-sized egg masses were added to each well containing fungal spores. Wells with egg masses in sterile distilled water were served as control. The counting of hatched juveniles was performed daily for seven days through a stereoscopic microscope.
The proteolytic activity of the fungus in the fermented medium was examined by the modified caseinolytic method (Braga et al., 2011). Briefly, a known quantity of the fermented matter was mixed with distilled water (1:5, w/w) and agitated on a rotary shaker (180 rpm, 30 min, 30 °C). The contents of the flasks were filtered through Whatman No. 1 filter paper and the filtrate was centrifuged at 10,000 × g for 10 min (4 °C). The supernatant collected was used as crude enzyme extract.
The colonization rate of fungal spores harvested from the formulation or wheat grains on egg masses was studied with ten egg masses per Petri plate, and five replicates per fungal spore treatment. The colonization was inspected daily unto the 7th day under the stereoscopic microscope at 40× magnification as a mark of parasitism.
2.4 Shelf life study
CFU count was analyzed to investigate the shelf life of the formulation. Briefly, formulation (100 g) was kept in a 500 ml dark bottle was stored at 4 and 26 °C separately for six months. The CFU count/g dried substrate was determined using the dilution plate method. A periodic assay of the virulence (egg mass hatching inhibition) was also conducted to check the efficacy of the spores harvested from the bionematicide formulation stored at different temperatures.
2.5 Dose-response relationship
A dose–response relationship was established by plotting a dose–response curve and assaying IC50 (Concentration required to inhibit 50% of egg mass hatching) for our nematicidal bioproduct. The spore suspension was adjusted to various doses, viz. 108, 107, 106, 105, 104, 103, and 102 spores/ml by diluting with SDW and were examined against egg masses of M. incognita. The hatching rate of the eggs was observed daily for seven days, and IT50 (Time required to inhibit 50% of egg mass hatching) was also calculated for each dose.
2.6 Scanning electron microscopy (SEM)
SEM study was executed to review the morphological response of P. lilacinum 6029 cultivated on selected KDC-BGS medium in 60:40 ratio. After being prefixed with a 2.5% glutaraldehyde solution at 4 °C overnight, the samples were harvested by centrifugation and washed three times with a 0.1 M sodium phosphate buffer solution (pH 7.2). The samples were dehydrated by a series of 25, 50, 75, 90, and 100% ethanol treatment and later on mounted on silver stubs, coated with gold by cathodic spraying (Polaron gold). Finally, the samples were examined using SEM EVO 50(ZEISS EVO series) fitted with a Bruker AXS EDX system, IIT Delhi, under the following analytical condition: EHT = 20.00 kV, WD = 9.5 mm, Signal A = SE1.
2.7 Bioefficacy evaluation of the formulation under greenhouse conditions
Based on the dose–response study, pot experiments with P. lilacinum formulation @ 107 spores/g soil were carried to study its efficacy on the tomato crop (Lycopersicum esculatum, var. GS-600) infested with nematodes. The experiments were conducted in a greenhouse at IARI, New Delhi, without any temperature or illumination controls and subjected to environmental conditions. The tomato seeds were sown in separate nurseries. Three-week-old seedlings were uprooted smoothly to protect tender roots and transplanted in earthen pots @ one seedling/pot. Each pot contained 2 kg of autoclaved soil. After a week, M. incognita inoculum (10 egg masses) were introduced per pot. The treatment details are as follows: T1- control (good check); T2- M. incognita (Inoculated control); T3- Wheat-based fungal formulation @ 107 spores/g of soil; T4- KDC-BGS (60/40 ratio) based fungal formulation @ 107 spores/g of soil. Each treatment had ten replicates.
2.7.1 Nematode control
Nematode infestation and its control were determined via rating root gall index on the 1–5 scale (Barker, 1985), control efficacy (Sun et al., 2006), eggs/egg mass, egg mass/gram roots, and nematode population/250 cc soil.
The galling index was rated on a scale of 1 = No galling, 2 = trace (<25% of total root system galled), 3 = slight (25–50% of total root system galled), 4 = moderate (51–75% of total root system galled) and 5 = severe (>75% of total root system galled). The following formula calculated control efficacy: -
The number of egg masses per gram roots was determined through visual observation. Egg masses were detached gently with a pair of forceps and extracted in NaOCl solution. The egg masses were broken, and eggs were released into the water. The final volume of egg suspension was made up to 100 ml. 10 ml of this was pipette out after thoroughly bubbling and poured in a counting dish to count the number of eggs per egg mass. Modified Cobb's decanting and sieving method, followed by Baermann's funnel technique, was employed to determine the initial and final population of M. incognita in pots (Southey, 1986). Nematode reduction (%) was calculated using the Henderson and Tilton (1955) formula i.e.
Where, IPT and FPT = Initial and final population in treatment.
IPC and FPC = Initial and final population in infested control
2.7.2 Plant growth parameters and biomass yield
After seven months, the plant biomass was harvested. Data about plant growth parameters viz. shoot and root length, fresh and dry shoot weight, fresh and dry root weight, and the number of fruits were recorded. Completely expanded leaves were used to determine chlorophyll a, chlorophyll b, and total chlorophyll content, according to Harborne (1998). Likewise, fully expanded leaves were selected for nutrients (N, P, K, and CP) analysis (Tandon, 1995).
2.8 Statistical analysis
Data were statistically analyzed by one-way ANOVA, followed by Duncan's multiple range tests at P < 0.05 using SPSS (version 10) software. The IC50 and IT50 values were determined by probit analysis (LdP Line).
3 Results and discussion
3.1 Development of bionematicide formulation of P. lilacinum 6029
3.1.1 Optimization of substrate composition
For the current investigation, we formulated a screening strategy based on in vitro assessments of antagonistic activity apart from the conventional parameter, i.e., spore production, to evaluate substrate compositions for P. lilacinum 6029 to control root-knot nematode. Wheat grains produced the highest spore count (1.5 × 1010 spores/g) (Table 2). However, among the four combinations of KDC-BGS, the 40/60 ratio produced maximum spores (9.3 × 109 spores/g) significantly (p < 0.05) at par with the 60/40 ratio (8.2 × 109 spores/g) followed by 20/80 and 80/20 (6.4x 108 spores/g and 4.7 × 108 spores/g) respectively. Evidently, the spore count was directly proportionate to the concentration of BGS in the mixed substrate up to a certain extent. It was possibly due to a high percentage of carbon in sundried BGS, which might have helped bring the optimum C/N ratio of the mixed substrate. Carbon sources and C/N ratios influence the viability, endurance, and virulence of fungal spores (Rangel et al., 2004; Shah et al., 2005; Hutwimmer et al., 2008). Besides, the BGS supplementation made the substrates more aerated and porous for microbial activity. P. lilacinum, being saprophytic, in all likelihood, utilized carbon from sundried BGS as an energy source. Hammer et al. (2014) suggested that fungi naturally incline towards minerals and nutrients accessible from organic substrates, thereby corroborating our findings. Data are means of four replicates. In each column, data followed by the same letter are not significantly different at P < 0.05 by DMRT.
Substrate(KDC-BGS)
Spore count(109 spores/g)
Egg mass hatching inhibition (%)
3rd day
5th day
7th day
80/20
4.7 ± 0.72 a
84.15 ± 1.23 a
85.95 ± 2.03 a
90.68 ± 2.18 a
60/40
8.2 ± 0.9b
82.67 ± 2.12 a
85.2 ± 1.77 a
89.1 ± 2.24 a
40/60
9.3 ± 0.78b
64.35 ± 2.74b
68.5 ± 1.38b
79.79 ± 1.83b
20/80
6.4 ± 0.65c
47.02 ± 1.85c
55.02 ± 2.11c
71.1 ± 1.73c
wheat grains (control)
15 ± 0.45 d
45.21 ± 1.24c
52.21 ± 1.82c
68.72 ± 1.48c
Spores from 80/20 and 60/40 ratio of the substrates showed at par virulency inhibiting egg mass hatching up to 90.68 and 89.1% respectively on the seventh day. Spores obtained from wheat grains were least potent as they inhibited only 68.72% of egg mass hatching of M. incognita after a week. Our findings accentuate the significance of biowastes as culture substrates for producing virulent fungal spores for biocontrol applications. Wu et al. (2009) reported varied conidial virulence harvested from different substrate compositions on diamondback moth larvae. Likewise, the conidia from white rice amended with PDB showed more virulence on Dactylaria higginsii than conidia from white rice alone (Wyss et al., 2001). The current study noticeably displays the impact of culture substrates (KDC-BGS) on the virulence of P. lilacinum spores, although a conclusive linkage between nutrition and virulence remains unclear.
The presence of KDC facilitates the production of protease as the activity was directly proportional to the amount of KDC (r2 = 0.9761) in KDC-BGS compositions (Fig. 1). Also, the positive correlation between protease activity and egg mass hatching inhibition, implying that that protease enzyme is a major virulent factor in causing infection to the nematode eggs. Maximum protease activity and egg mass hatching inhibition (382.02 U/g & 90.68% inhibition) were seen in 80/20, whereas minimum in 20/80 ratios (236.82 U/g & 71.1% inhibition) among the four combinations of KDC-BGS. Ultrastructural and histochemical studies have inferred the association of hydrolytic enzymes in penetrating the nematode cuticle and eggshell (Chen et al. 2015). We assume that the high nitrogen content in KDC encouraged hydrolytic enzymes in the quick degradation of eggs of M. incognita in the present investigation. Our findings are in corroboration with the study by Khan et al. (2004), who noticed morphological changes in the eggshell and reduction in egg hatching of M. javanica and related all these events with the secretion of serine proteases by P. lilacinus 251.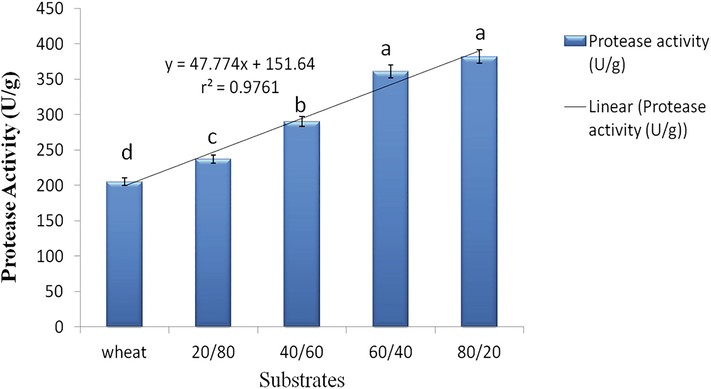
Effect of substrate compositions on protease activity of P. lilacinum 6029. Data are means of four replicates. In each bar, data followed by the same letter are not significantly different at P < 0.05 by DMRT.
Therefore, based on spore counts and virulence, the 60/40 ratio was chosen as the optimal combination of KDC-BGS for the SSF of P. lilacinum 6029. The fermented product was shade dried, grounded, and mixed with fly-ash (inert carrier) in a 1:1 ratio to develop bio-formulation to control root-knot nematode infection under lab and greenhouse conditions.
3.1.2 Structural characterization of the substrate after fermentation by scanning electron microscopy
Scanning electron microscopy (SEM) of the unfermented and fermented substrate (60/40 ratio) revealed luxuriant fungal growth on KDC-BGS based nutrient medium (Fig. 2 a, b). We found that the dense mycelia of P. lilacinum colonized a larger part of the optimized substrate. The fungal hyphae appeared to grow inside the substrate distorting the surface (Fig. 2c).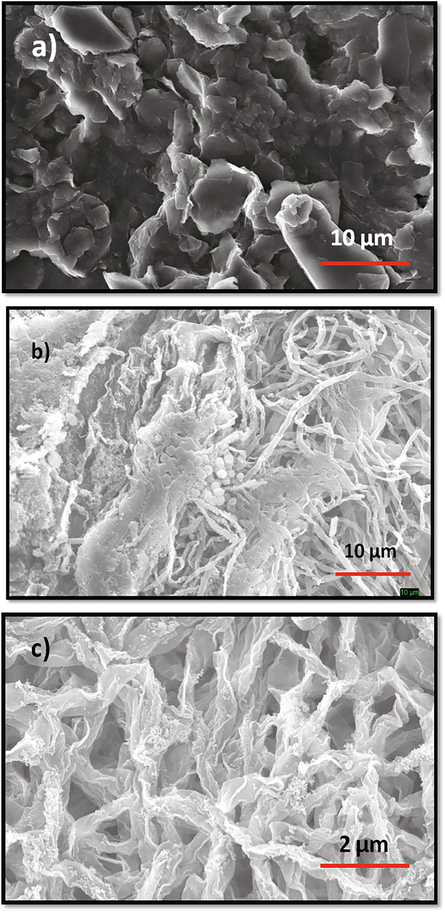
Scanning electron micrograph of unfermented (a- 10 µm) and P. lilacinum 6029 10-day fermented KDC-BGS (b) at 10 µm (c) 2 µm (closer view).
3.1.3 Colonization potential of fungal spores
The effect of fungal spores derived from KDC-BGS based formulation and wheat grains (control) on the colonization rate of egg masses is shown in Fig. 3. Colonization of egg masses by P. lilacinum 6029 spores was validated by inspecting the emerging hyphae from the egg mass surface under a stereomicroscope at 40X. On the 1st day, spores from formulation registered a 36.4% colonization on egg mass, but promptly increased to 89.2% on the 3rd day and completely colonized on the 5th day. On the contrary, fungal spores from the traditional substrate, wheat (control), showed a gradual increase in egg mass colonization, recording 100% colonization on the seventh day. An effective parasite should utilize a gelatinous matrix (GM) of nematodes as a source of nutrients and grow on it (Sharon et al., 2007). Since mycelia of P. lilacinum in the present study was detected on the GM, we understand that the fungus withstands the antimicrobial compound in the GM, which is in line with those of Zaki and Bhatti (1990); Eapen et al. (2005) and hence considerably colonized egg masses of nematodes.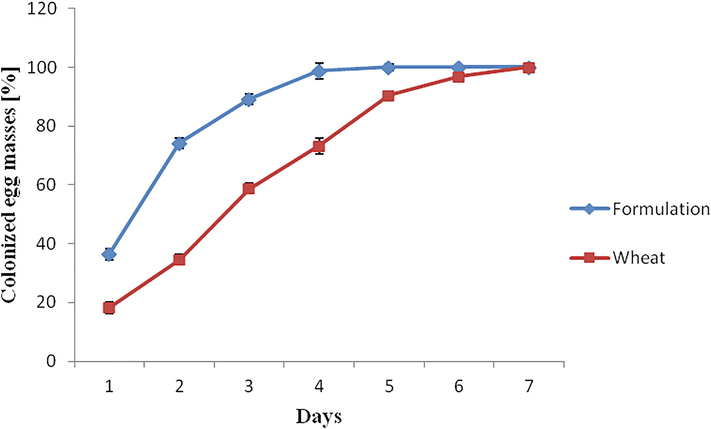
Percentage of egg masses of root-knot nematodes colonized by P. lilacinum 6029 spores obtained from the formulations (KDC-BGS and wheat grain-based) under in vitro condition. Data are means of four replicates.
3.1.4 Shelf-life
The shelf life study took place at two different temperature regimes, i.e., 4 °C (low temperature) and 26 °C (room temperature) for six months (Fig. 4). The viability of the sample stored at low temperatures remained constant throughout storage. At 26 °C, the biocontrol agent remained significantly (p < 0.05) viable during the first month of storage. The CFU count gradually declined from the first month onwards, but 53.21% of the initial count of formulation (4.32 × 109 CFU/g) was viable at optimum temperature even after six months of storage. Duan et al. (2008) had noted similar observations and reported that alginate pellets of P. lilacinus were much more stable when stored at low temperatures (4 or −20 °C) than at 25 or 40 °C. The initial increase in the CFU load during the first month and its maintenance till the third month at room temperature suggested sufficient nutrient support from KDC and BGS. After the third month, a decline in CFU indicates a scarcity of nutrients in the formulation. It is noteworthy that no other microorganism other than P. lilacinum 6029 was detected in all Petri plates during CFU count, implying nil contamination in the formulation during storage.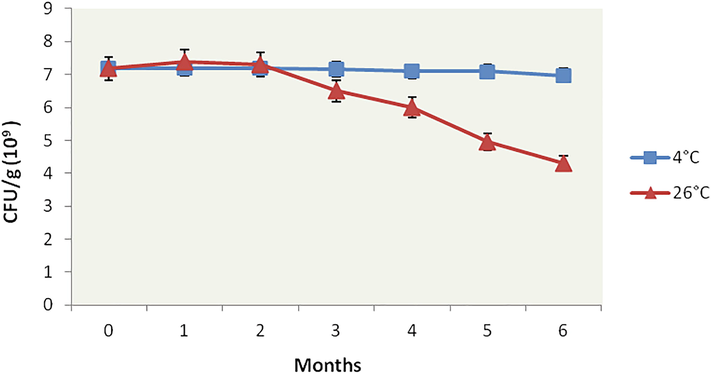
Shelf life study of P. lilacinum 6029 formulations stored at 4 and 26 °C for six months. Data are means of four replicates.
3.1.5 Dose-response relationship
Before the field expression of our bioformulation, a definitive determination of optimum fungal inoculum density was warranted. In this regard, a dose–response relationship was examined (Table 3). We found that the response intensity (egg mass hatching inhibition) was directly proportional to the dose of P. lilacinum spores. Maximum inhibition of egg mass hatching (95.6%) and minimum IT50 (1.41 days) was achieved by 108spores/ml. As the dose was reduced to 107, 106, and 105 spores/ml, percentage inhibition of egg mass hatching also declined to lower values (94.5, 88.7, and 73.7%, respectively). The fungal dose of 103 spores/ml was found to be the threshold value for P. lilacinum 6029 spores of formulation as a dose lower to it did not affect the egg mass hatching. Fernandez et al. (1989) reported a 97% reduction in the hatching of eggs when the GM was exposed to 109 spores/g of P. lilacinus while only 60% of eggs hatched at 105 spores/g concentration. A dose–response relationship curve suggested that the IC50 value of the formulation was 104 spores/g of the formulation. It is worth mentioning here that since dose efficacies viz. 108 and 107 spores/ ml toward egg mass hatching inhibition was at par (p < 0.05), we picked 107 spores/g for a greenhouse study. A lower dose minimizes the application cost, thereby increasing the net profit of the crop. Data are means of four replicates. In each column, data followed by the same letter are not significantly different at P < 0.05 by DMRT. IT: Inhibition time.
Dose(Spores/ml)
% egg mass hatching inhibition on 7th day
IT50(days)
108
95.6 ± 1.73 a
1.41
107
94.5 ± 1.51 a
1.52
106
88.7 ± 2.05b
2.05
105
73.7 ± 2.46c
2.93
104
45.9 ± 1.78 d
6.44
103
17.8 ± 2.24e
42.26
102
0.0f
Not Determined
3.2 Bioefficacy evaluation of the formulation under greenhouse conditions
3.2.1 Nematode control
The data about the effect of two different substrates based fungal formulation on M. incognita infestation in L. esculentum var. GS-600 is given in Table 4. Greenhouse study showed that T4, i.e., formulation with KDC-BGS as base substrate @107spores/ g of soil significantly (p < 0.05), managed the nematode infestation (62.5%) in tomato plants. Formulations developed on different nutrient sources also adversely affected M. incognita reproduction on tomato crops. The data revealed that the minimum egg masses/g root was recorded in T4 (9.1), followed by T3 (11.8). Although the GM serves as an agent protecting the eggs against microorganisms, we observed a better efficacy of our formulated product in damaging it. Hence, our findings are in contrast with the study proposing that the antimicrobial properties of GM prevent the egg parasitization (Orion and Kritzman, 2001), and in line with Anastasiadis et al. (2008) who demonstrated the potential of P. lilacinus 251 in colonizing the eggs of Meloidogyne spp. Data are means of ten replicates. All means are significantly different from each other at P < 0.05 by DMRT. Inoculum density of P. lilacinum 6029:107spores/g soil (T3 and T4).
Treatment
Gall Index at the scale of 1–5
Control Efficacy (%)
Egg masses /g root
Eggs/egg mass
Nematode population/250 cc of soil
% Nematode reduction over IC i.e. T2
Initial
Final
T1
1
–
nil
nil
nil
nil
–
T2
4.8
31.3 ± 1.60
193 ± 4.06
350 ± 4.35
280 ± 4.58
T3
2.3
52.08
11.8 ± 2.17
95 ± 5.29
355 ± 5.57
110 ± 2.64
62.5
T4
1.8
62.5
9.1 ± 1.22
76 ± 4.39
359 ± 6.92
81 ± 3.61
71.8
The data indicated that P. lilacinum 6029 greatly reduced the final nematode population per 250 cc of soil, with the lowest population (81) recorded in T4 followed by T3 (110 nematodes per 250 cc soil). The maximum percentage of nematode reduction over IC was observed in T4 (71.8%), implying the collective effect of fungus and its optimized the mixed substrate on nematode control. Our findings confirm the study where soybean cake defatted and coffee husks have acted as a powerful synergist with P. lilacinum against nematodes in tomato (Brand et al., 2010). Here, nitrogen in KDC may enhance protease activity and, subsequently, the fungus’ virulence.
3.2.2 Physiological parameters
The impact assessment of formulation on various plant growth parameters of L. esculentum var. GS-600 viz., root length, shoot length, and respective weight (fresh and dry) are revealed in Table 5. The tallest plant (102.5 cm) was recorded from the T4 treatment, while the shortest appeared in the inoculated control pots (58.62 cm). Employment of formulation improved both fresh and dry shoot weight over the IC. The formulation developed with wheat (T3) and KDC-BGS (T4) as a base substrate resulted in 23.95 and 21.75 cm mean root length. The smallest root length (12.01 cm) was recorded from the inoculated control plants. KDC-BGS based formulation significantly (p < 0.05) suppressed root galls on tomato, thereby showing an 86.11% increase of fresh root weight over IC in T4. Maximum fruit yield (622.32 g/plant) was obtained from T4, followed by T1 (588.67 g/plant) and T3 (465.21 g/plant). The improved plant growth in KDC-BGS based fungal formulation treated soil could be due to the release of nutrients from the organic substrates. The indirect contribution of the formulation to plant growth by limiting the soil's nematode population cannot be ruled out. Data are means of ten replicates. All means are significantly different from each other at P < 0.05 by DMRT.
Treatments
Length (cm)
Fresh weight (g)
Dry weight (g)
Fruit yield/plant (g)
Root
Shoot
Root
Shoot
Root
Shoot
T1
22.98
97.17
12.8
221.7
2.30
26.9
588.67
T2
12.01
58.62
7.2
125.2
1.23
15.2
137.97
T3
21.75
90.09
10.9
194.9
1.98
23.4
465.21
T4
23.95
102.5
13.4
230.5
2.32
29.7
622.32
SEm
± 0.35
± 0.95
± 0.11
± 1.26
± 0.07
± 0.35
± 2.56
3.2.3 Biochemical parameters
Table 6 provides the effect of formulation on the nutrient content in leaves of L. esculentum var. GS-600. A perusal of the data reveals an increase in nutrient uptake by the plants treated with the formulation. N, P, K, and crude protein in T4 were 2.41, 0.65, 1.64, and 15.06%, respectively. The results demonstrated the beneficial use of KDC and BGS in our formulation. Digested slurry, being organic, release fast-acting nutrients upon decomposition. These nutrients easily enter the soil solution, thus becoming straight away available to the plants besides serving as primary nutrients to develop saprophytes (Warman and Termeer, 2005). Data are means of ten replicates. All means are significantly different from each other at P < 0.05 by DMRT. N: Nitrogen, P: Phosphorous, K: Potassium, CP: Crude Protein.
Treatments
N (%)
P (%)
K (%)
CP (%)
T1
2.32 ± 0.03
0.61 ± 0.008
1.53 ± 0.04
14.50 ± 0.03
T2
1.82 ± 0.05
0.53 ± 0.007
1.09 ± 0.03
11.36 ± 0.05
T3
2.21 ± 0.02
0.59 ± 0.001
1.46 ± 0.02
13.81 ± 0.02
T4
2.41 ± 0.01
0.65 ± 0.009
1.64 ± 0.03
15.06 ± 0.01
Photosynthesis is an important plant growth parameter related to the chlorophyll content (Fig. 5). The total chlorophyll content in leaves markedly increased in the nematode inoculated plants treated with T4 (1.91 mg/g FW) followed by T1 (1.89 mg/g FW) and T3 (1.43 mg/g FW). Inoculated control, T2 had registered least chlorophyll a (0.829 mg/g FW), chlorophyll b (0.305 mg/g FW) and total chlorophyll content (1.134 mg/g FW) in leaves. P. lilacinum does not have residual toxicity and is known to promote plant growth (Nesha and Siddiqui, 2017).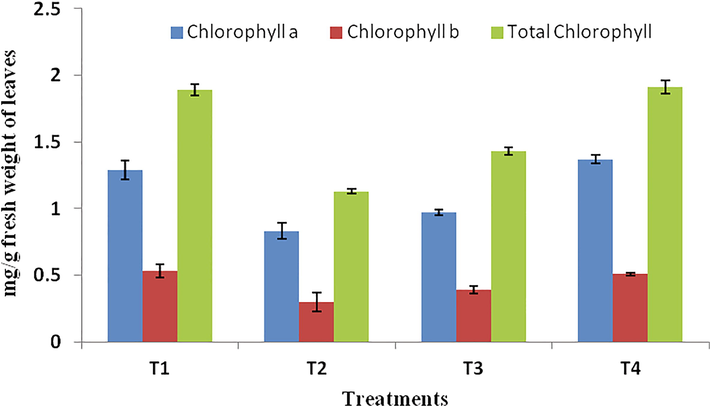
Effect on chlorophyll a, chlorophyll b, and total chlorophyll in leaves of tomato plant inoculated with the P. lilacinum based formulations. Data are means of ten replicates. All means are significantly different from each other at P < 0.05 by DMRT.
4 Conclusion
In summary, we designed original and optimized nutrient substrates that enabled the mass production of P. lilacinum spores with enhanced virulence properties. Additionally, cultivating the fungus on KDC-BGS symptomizes the waste management of biodiesel and biogas by-products that ultimately lead to the development of bio formulation for root-knot nematode control in an economically and environmentally favorable approach.
Disclosure of funding
This research was funded by National Oilseed and Vegetable Oil Development Board, India, Grant No. 32-140/NOVOD/2008/1427. and Researchers Supporting Project Number (RSP‐2020/182), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments:
The authors acknowledge the financial support of the National Oilseed and Vegetable Oil Development Board, Gurgaon, India (Grant No. 32-140/NOVOD/2008/1427). The authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP‐2020/182), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Prot.. 2008;27:352-361.
- [CrossRef] [Google Scholar]
- The application of microplot techniques in nematological research. In: BakerC K.R., Carter C., Sasser J.N., eds. An Advanced Treatise on Meloidogyne: Methodology. Raleigh: North Carolina State University Graphics; 1985. p. :127-134.
- [Google Scholar]
- Effect of culture substrates on virulence of Beauveria bassiana(Ascomycota: Cordycipitaceae) conidia against the browntail moth, Euproctis chrysorrhoea(Lepidoptera: Lymantriidae) Biocontrol Sci. Tech.. 2011;21:619-624.
- [CrossRef] [Google Scholar]
- Production and partial characterization of Duddingtonia flagrans(AC001) crude extract and its in vitro larvicidal action against tricho strongy lid infective larvae. Biocontrol Sci. Tech.. 2011;21:1313-1320.
- [CrossRef] [Google Scholar]
- Production of fungal biological control agents through solid state fermentation: a case study on Paecilomyces lilacinus against root-knot nematodes. Micologia Aplicada Int.. 2010;22:31-48.
- [Google Scholar]
- Effect of storage conditions on the survival of two potential biocontrol agents of nematodes, the fungi Paecilomyces lilacinus and Pochonia chlamydosporia. Biocontrol Sci. Tech.. 2008;18:605-612.
- [CrossRef] [Google Scholar]
- Tropical soil microflora of spice-based cropping systems as potential antagonists of root-knot nematodes. J. Invertebr. Pathol.. 2005;88:218-225.
- [CrossRef] [Google Scholar]
- In vitro effectivity of Paecilomyces lilacinus as biological control agent for Meloidogyne incognita. Revista Protect. Vegetal. 1989;4:66-70.
- [Google Scholar]
- A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem.. 2014;77:252-260.
- [CrossRef] [Google Scholar]
- Phytochemical Methods a guide to Modern Techniques of Plant Analysis. Springer Science & Business Media; 1998.
- Tests with Acaricides against the Brown Wheat Mite12. J. Econ. Entomol.. 1955;48:157-161.
- [CrossRef] [Google Scholar]
- An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Res. Microbiol.. 2005;156:719-727.
- [CrossRef] [Google Scholar]
- Algorithm-based design of synthetic growth media stimulating virulence properties of Metarhizium anisopliaeconidia. J. Appl. Microbiol.. 2008;105:2026-2034.
- [CrossRef] [Google Scholar]
- Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol. Control. 2004;31:346-352.
- [CrossRef] [Google Scholar]
- Development of a powder formulation based on Bacillus cereus sensu lato strain B25 spores for biological control of Fusarium verticillioides in maize plants. World J. Microbiol. Biotechnol.. 2016;32
- [CrossRef] [Google Scholar]
- Effects of Paecilomyces lilacinus and Aspergillus niger alone and in combination on the growth, chlorophyll contents and soft rot disease complex of carrot. Sci. Hortic.. 2017;218:258-264.
- [CrossRef] [Google Scholar]
- Newly Isolated Paecilomyces lilacinus and Paecilomyces javanicus as novel biocontrol agents for Plutella xylostella and Spodoptera litura. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2017;45:280-286.
- [CrossRef] [Google Scholar]
- A role of the gelatinous matrix in the resistance of root-knot nematode (Meloidogyne spp.) eggs to microorganisms. J. Nematol.. 2001;33:203.
- [Google Scholar]
- Oil cakes and their biotechnological applications – A review. Bioresour. Technol.. 2007;98:2000-2009.
- [CrossRef] [Google Scholar]
- Variations in UV-B tolerance and germination speed of Metarhizium anisopliae conidia produced on insects and artificial substrates. J. Invertebr. Pathol.. 2004;87:77-83.
- [CrossRef] [Google Scholar]
- Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett.. 2005;251:259-266.
- [CrossRef] [Google Scholar]
- Nematicidal activity of Paecilomyces lilacinus 6029 cultured on Karanja cake medium. Microb. Pathog.. 2014;75:16-20.
- [CrossRef] [Google Scholar]
- Statistical optimization of growth media for Paecilomyces lilacinus 6029 using non-edible oil cakes. Ann. Microbiol.. 2014;64:515-520.
- [CrossRef] [Google Scholar]
- Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur. J. Plant Pathol.. 2007;118:247-258.
- [CrossRef] [Google Scholar]
- Phytase production by thermophilic mold Sporotrichum thermophile in solid-state fermentation and its application in Dephytinization of sesame oil cake. Appl. Biochem. Biotechnol.. 2006;133:239-250.
- [CrossRef] [Google Scholar]
- Laboratory Methods for Work with Plant and Soil Nematodes. HMSO; 1986.
- Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. J. Invertebr. Pathol.. 2006;93:22-28.
- [CrossRef] [Google Scholar]
- Tandon, H. 1995. Methods of analysis of soil, plants, waters and fertilizer. In: Fertilizers Development and Consultation Organization, New Delhi, India, p. 144.
- Evaluation of sewage sludge, septic waste and sludge compost applications to corn and forage: yields and N, P and K content of crops and soils. Bioresour. Technol.. 2005;96:955-961.
- [CrossRef] [Google Scholar]
- Media composition influences on growth, enzyme activity, and virulence of the entomopathogen hyphomyceteIsaria fumosoroseus. Entomol. Exp. Appl.. 2009;131:30-38.
- [CrossRef] [Google Scholar]
- Evaluation of agar and grain media for mass production of conidia of Dactylaria higginsii. Plant Dis.. 2001;85:1165-1170.
- [CrossRef] [Google Scholar]
- In vivo parasitism of Meloidogyne javanica by an oviparasitic fungus, Paecilomyces lilacinus. Nematol Mediterr. 1990;18:141-143.
- [Google Scholar]
- Microalgal cultivation with biogas slurry for biofuel production. Bioresour. Technol.. 2016;220:629-636.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101399.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







