Translate this page into:
Immunogenic protein profiling of pathogenic Escherichia coli strains isolated from infants with diarrhea in Quetta Balochistan
⁎Corresponding author. ali.akbar@um.uob.edu.pk (Ali Akbar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Escherichia coli (E. coli) is a widespread bacterial species that comprise a broad variety of strains and can be highly pathogenic often. Diarrhea caused by E. coli pathotypes is a leading public health problem in underdeveloped countries such as Pakistan. Five different diarrheagenic E. coli pathotypes (Enterohaemorrhagic, Enteropathogenic, Enteroaggregative, Enteroinvasive and Enterotoxigenic E. coli) were isolated from 200 samples of diarrheal children in Quetta, Pakistan. Total-cell protein profiles of all the strains isolated were resolved via SDS-Polyacrylamide Gel Electrophoresis (PAGE). More than 15 protein bands were found ranging from 15 kDa to 153 kDa in size. A dendrogram plot explained the relatedness of the pathotypes amongst them. The protein bands of 70 and 15 kDa, analyzed through GelAnalyzer 2010a© were found common among all the isolated pathotypes. Purified bands of these two proteins (70 and 15 kDa) from the gel were injected in experimental mice groups for immunization purposes. During immunogenicity tests, the mice that were immunized with 70 and 15 kDa proteins stayed alive for seven days despite subsequently being challenged with the E. coli pathotypes, while higher mortality 80% were observed in the non-immunized control group. Upon the postmortem it was revealed that all the vital organs were covered by the E. coli strains injected for challenge studies. The immunized mice and control mice sera subjected to the Agar Gel Immunodiffusion assay (AGID) test for antibody/antigen reaction were found positive for antibodies presence. The exploration of immunogenic proteins among all the prevalent pathotypes can be a breakthrough in the development of a combined, effective, and safe vaccine. Further investigation of these immunogenic proteins would be of great importance for future immunization endeavors.
Keywords
Infections
Bacteria
Antibodies
Antigen
Immunity
1 Introduction
In global health problems, diarrhea is one of the major issues which can cause an estimated 1.3 million deaths every year in children worldwide (Fiedoruk et al., 2015). Age group of 1–5 years in children are considered to be the most susceptible age with diarrhea (Regassa and Lemma, 2016). It has been reported that diarrhea are the second most common cause of death in children in the age 1–5 years (Lamberti et al., 2012; Ugboko et al., 2020). Acute or chronic child diarrhea may also be connected with poor mental development and stunted growth in this age group (Brander et al., 2019). The problem gets aggravated in developing countries as compared to the developed countries, where morbidity and mortality of children gets associated with diarrhea (Lamberti et al., 2012; Al Ahadeb et al., 2021). Multiple agents are usually responsible for the cause of diarrhea i.e. viruses, bacteria, and parasites. However, the emerging and re-emerging bacterial diarrhea is one core global health issue (Uppal et al., 2015; Baker et al., 2021). In Pakistan, 118,000 deaths per year have been reported due to recurrent diarrheal diseases in children under the age of five years (Shaikh and Haran, 2011).
Escherichia coli (E. coli) are well-known and most common bacterial species. Whereas Diarrheagenic E. coli (DEC) are classified as extra-intestinal pathogens or enteric pathogens strains that cause diarrhea in human hosts and clinically manifest a collection of virulence genes. It also has several other pathotypes that can cause animal and human infections of different clinical manifestations and severity (Ramos et al., 2020).
Drug resistant E. coli infections extend the length of stay in the hospitals, which pose economic pressure directly and indirectly over the population and health care systems (MacKinnon et al., 2020). To reduce child mortality, the key public health priority should be the proper control and management of such widespread infectious diseases. The current study aims to understand and identify the diarrheal cases in an appropriate and timely method by the differential protein profiling of these common pathogens. Future investigations in these lines, may help in decreasing the disease rate caused by diarrhea and will reduce the financial liability of public health.
In this scenario, five different isolates of E. coli pathotypes were characterized for differential protein profile from the children of age under five years affected with gastroenteritis. In vivo immunogenization as well as In vitro antibody detection were performed. To conceive suitable approaches to control this disease, the present research may pave the way for understanding the antigenicity of diarrhea-causing E. coli pathotypes in children under the age of five years in Quetta, Pakistan. The identification of immunogenic proteins from isolated pathotypes and their proteomic studies may help in better vaccine development and future research probabilities in this domain.
2 Material and methods
2.1 Bacterial isolates
Five different diarrheagenic E. coli isolates, Enterohaemorrhagic E. coli (EHEC), Enteropathogenic E. coli (EPEC), Enteroaggregative E. coli (EAEC), Enteroinvasive E. coli (EIEC), and Enterotoxigenic E. coli (ETEC) were analyzed for total cellular protein analysis in this study. These are locally circulating pathotypes, characterized earlier (Zil-e-Huma et al. 2019). The isolates were sub-cultured in the laboratory and used further for protein profiling in this study.
2.2 SDS-Polyacrylamide Gel Electrophoresis (PAGE)
The standard procedure was followed for the whole-cell protein preparation (Silva et al., 2018). Commercially available kit bicinchoninic acid (BCA) (Bio world®) was used for extraction and estimation of protein in prepared samples of E. coli against bovine serum albumin (Sigma®) as standard. The procedure for SDS PAGE was performed on protein extracts from E. coli pathotypes in 12% polyacrylamide. The separating gel was 12% by weight polyacrylamide, containing 6 mL polyacrylamide, 3.7 mL 0.5 M Tris-HCl buffer, 8.8 pH, and 75 µL of 10% SDS with 5.25 mL double distilled water. The gel was chemically polymerized with the addition of 150 μL ammonium persulphate and 15 μL of tetramethylene-diamine was used as a catalyst. The comb was removed after polymerization to load the prepared protein samples in the designated wells. A known protein ladder was used against dissociated proteins to determine the size. The gel was then stained for 4 h in Coomassie brilliant blue and subsequently de-stained and stored at 4 °C in fridge.
GelAnalyzer 2010a© determined molecular weights of proteins in the gel based on the molecular weight markers. For protein purification, bands were excised using a reference and passively eluted from the gel matrix. The solutions were centrifuged, and the supernatant was confirmed for the presence of proteins by subjecting it to SDS-PAGE.
2.3 Experimental animals
In this study, female Bagg Albino (BALB/c) inbred research mice (four to eight weeks old) were used for experimental purposes. Formal approval was obtained from the Ethical Committee of the Faculty of Life Sciences, CASVAB, UOB, Quetta for all animal-based experiments conducted following the institutional regulations. The eluted proteins of 15 kDa and 70 kDa were injected subcutaneously (100 µL/mouse) into the 4–8 weeks old female BALB/c mice (n = 5 mice/group) on every third day for a total period of 14 days. Control mice received the same volume (100 µL) of normal saline.
2.4 In vivo immunogenization study
Over a period of fourteen days, the eluted proteins (100 µL/mouse) were injected subcutaneously every third day to the test mice while the control group with 100 µL of normal saline. Blood (200–500 µL) was collected using the lancet from the submandibular vein of the mice. Collected sera from blood samples were stored at −20 °C. Both the immunized and control groups of mice were challenged with the pathogenic strains of E. coli isolates. The five E. coli strains as a mixture at the dose of 5 × 105 CFU/mouse were intraperitoneally injected to the controlled and previously immunized mice. Observations were made every seventh day and sera was collected from both the immunized and control groups of mice, which were subjected to AGID test for the detection of antibodies presence. Organs of the test and control mice were observed for the presence of pathogenic E. coli strains used in the challenge study.
2.5 Agar Gel Immunodiffusion assay test
Intracellular proteins were disrupted through sonication and a semi-solid gel of 0.8% agar was prepared by adding 0.8 g of Agarose (Oxide, England) in 0.85% NaCl (100 mL) solution and the pH of the solution was adjusted to 9.0. Wells of 5 mm diameter were punched in the hexagonal pattern once the gel was set while the diameter between the adjacent wells was maintained at 3 mm. Six peripheral wells were assigned for serum samples and a central well for antigen. To prepare the antigen, a freshly incubated broth sample of mixed pathotypes was centrifuged overnight. The next morning, the pellet was washed with Phosphate-buffered saline (PBS) and then 25 μL of sample buffer (without dye) was added. This mixture was incubated in a water bath at 90 °C and observations were made after 24 and 48 h of incubation. The positive test was the appearance of one or more precipitation lines before or at 48 h, whereas no lines were negative results (Vallat and Allen, 2004).
3 Results and discussion
The Diarrheagenic E. coli (DEC) can be categorized into five major categories depending on the expression of virulence genes (Zhou et al., 2018). In the study we have tested EAEC, EPEC, EHEC and mixed strain samples for protein.
3.1 Whole-cell protein profile
The protein profiles analysis can be helpful for the rapid identification of different microbial species. The SDS PAGE can probe the chemical characteristics by creating concise and stable protein banding patterns which in turn helps in the identification and comparison of different isolates (Ghazi et al., 2009).
According to the molecular weight markers, a few more than 15 different protein bands were resolved which were between 15 and 153 kDa. GelAnalyzer 2010a© revealed that the control E. coli (ATCC 25922) was significantly different from the tested isolates of pathogenic E. coli from this analysis (Fig. 1). However, when human and cattle fecal samples were analyzed by Naidu et al. (2011) variations were still seen in bands from different isolates ranging from < 20 kDa to >97.4 kDa. Therefore, these variations may be customary whereas the presence of pathotypes was confirmed through Rf value of the banding pattern. In this study two prominent protein bands 70 and 15 kDa were observed common amongst the total appeared bands of different pathotypes. These proteins band were found noticeable among this analyzed isolates of pathogenic E. coli strains.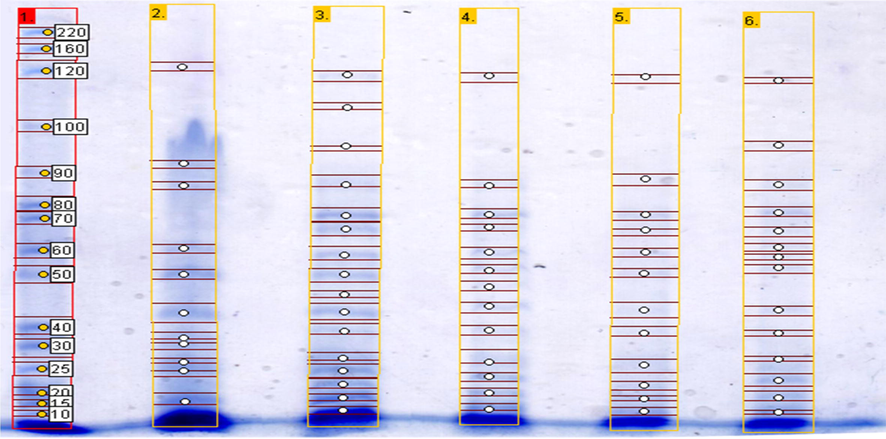
Representative graph of proteins expressed by E. coli pathotypes, resolved by SDS-PAGE protein profiling at 12.5% polyacrylamide gel and stained with Coomassie brilliant blue. Lane 1 represents molecular marker (standard), Lane 2 represent E. coli control, Lane 3 represent EPEC, Lane 4 represent EHEC, Lane 5 represent EAEC, Lane 6 represent the mixed pathotypes.
3.2 SDS-PAGE based cluster analysis
Molecular weights and relative mobility were calculated through GelAnalyzer 2010a©. Multivariate Statistical Package (MVSP) helped analyze the genetic variations through the Eucleadian distance procedure with Unweighted Pair Group Method with Arithmetic Averages (UPGMA) (Sneath and Sokal, 1973). According to the Cluster analysis and dendrogram, the E. coli pathotypes were sorted into two lineages. In the numerical analysis, four different pathotypes and one ATCC control strain were separated into three clusters and joined at 61 values of similarity of entire cellular protein profiles (Fig. 2). With Euclidean distance of 20, cluster one included EAEC and EHEC strains whereas, cluster two included EPEC and mix pathotypes with Euclidean distance of 33. ATCC reference strain was an outlier and distant from other pathotypes with Euclidean distance 50. The degree of divergence and genetic similarity between the different serotypes of DEC and the clusters were also shown by Campos et al. (2004). While EHEC and EAEC showed similarity among each other in cluster B, clusters A and C were found to be different serotypes of EPEC.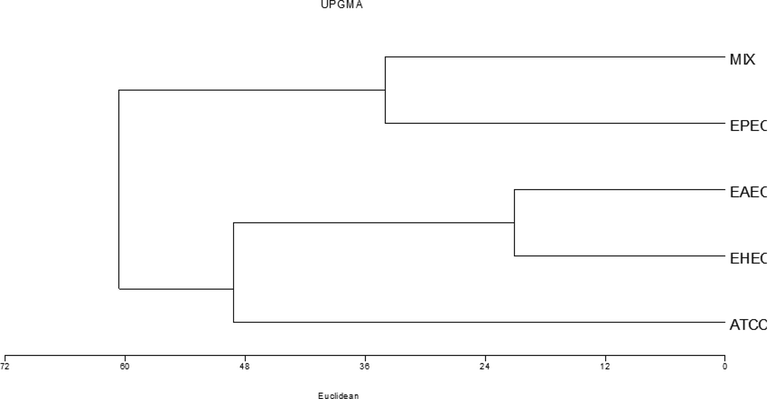
SDS–PAGE profiles Dendrogram of whole-cell proteins of pathotypes of E. coli by the unweighted pair group method with an arithmetic average algorithm (UPGMA).
3.3 Immunogenicity test
The common bands of 70 kDa and 15 kDa were extracted and purified to be used in the animal trials. These were used for the immunization of the mice groups and mice were challenged with infection. The immunized mice when challenged with the common 70 kDa and 15 kDa proteins were proved to be effective against the introduced pathogen in the test mice group. These test mice live relatively longer (7 days) compared to the control group. Four out five (80%) of the control mice died immediately after the introduction of pathogen. When these control mice were examined in a postmortem the infection was found spread to all the vital organs which were confirmed by isolating the infectious agent E. coli from all these organs. These results conforming that the protein used for the protection of the test group mice were effective and made the mice able to fight the infection for long compared to the control group in the study. This shows that the protein can be effectively used for the prophylaxis and vaccination purpose against the pathogenic E. coli serotypes.
Vaccine development for the infamous infectious disease is one of the major objectives of this area of research (Zhang and Sack, 2012). Infections around the world such as enterotoxigenic E. coli (ETEC) diarrheal infection is becoming one of the leading causes of global morbidity and mortality with 1.3 million deaths every year (Walker et al., 2007). These increasing global numbers are forcing the resources to be invested in vaccine development against such health hazards (Black et al., 2010). The studies suggest that a vaccine against ETEC is feasible however, it is still a dream for the suffering patients and their families (Boedeker, 2005). Since the occurrence of ETEC infections is skyrocketing in young children and infants even in the non-endemic areas (Qadri et al., 2007) it actually increases the need for vaccine development. Studies do support the fact that humans as well as animals (Steinsland et al., 2003), and even the test animals from the laboratories (Roy et al., 2008) may develop immunity against recurrent infection if they have been exposed to ETEC pathotypes. Some of the studies even support the idea during investigational human trial models of ETEC of the same pathotypes, that both active, as well as passive immunization, was strong enough to provide protection against further infection (Tacket et al., 1988). When the whole-genome sequencing was not available, ETEC antigens such as factors of fimbrial colonization and toxins were the main focus of the vaccine development. However, these antigenic factors have proven to be insufficient. The other studies which included the virulence factors to develop the immune responses are still under consideration and have proven to be still ineffective. Therefore, there is a dire need for the recognition of new pathogenic proteins involved in the infectious process (Svennerholm and Tobias, 2008). With the increasing rate of emerging infections in this era, the need of new, safe and highly effective vaccines based on the agents antigenic protein is increasing (Rauch et al., 2018).
There are some existing virulence proteins that may have proven competent novel vaccine development options. Two of such immunogenic proteins are EtpA and flagellin and have shown in vitro protection (Roy et al., 2009). The molecular epidemiology of EtpA in infection is unknown while the flagellin is part of the flagellated ETEC strains. Nonetheless, the experimental expression of both of these proteins is confirmed during human experimental infections (Fratamico et al., 2016).
3.4 Agar Gel Immunodiffusion test
The AGID test helped to test the sera of both the immunized as well as non-immunized mice from the study. The antigen–antibody reaction was easily visualized during this test while both the 70 and 15 kDa proteins were tested. The appearance of white crescent-shaped bands between the lysates of E. coli pathotypes and the tested proteins was seen at the time of reaction. However, these bands were absent in the sera of the non-immunized mice Fig. 3. The absence of active antigen proteins was conformed through the test as well as the reactive nature of the test proteins with the infectious sera was also confirmed. Hakim et al. (2017) used the AGID test for the conformation of three E. coli serotypes with the previously prepared rabbit antisera of the serotypes.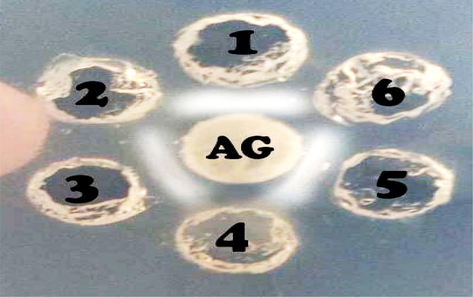
Agar Gel Immunodiffusion (AGID) test showed different sera behaving differently. Bands showing precipitation lines with E. coli pathotypes lysate as antigen (AG). Well 1 was positive control, well 2, 4 and 6 were negative control whereas, well 3 and well 5 were 15 kDa and 70 kDa serum respectively.
4 Conclusion
Diarrheagenic E. coli is one of the leading causes of diarrhea in children especially in third world countries like Pakistan, but with a lack of sufficient information. There is a need to establish easy and quick methods for the identification of DEC virulence genes. Detailed investigations and a more dynamic description of the E. coli strains are necessary to identify these gastroenteritis agents in infants, as well as appropriate therapeutic or prophylactic options are also needed to be established. Two common proteins identified in the present study are found to be immunogenetically competent for further detailed investigations. The suggested proteins may lead the way towards future vaccine candidates against these four DECs in a single dose. Therefore, extended immunological analysis and clinical studies in humans would be valuable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impact of Cinnamomum verum against different Escherichia coli strains isolated from drinking water sources of rural areas in Riyadh, Saudi Arabia. J. King Saud Uni.-Sci. 2021:101742.
- [CrossRef] [Google Scholar]
- Infectious disease in an era of global change. Nat. Rev. Microbiol.. 2021;1–13
- [CrossRef] [Google Scholar]
- Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969-1987.
- [Google Scholar]
- Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol.. 2005;21(1):15-19.
- [Google Scholar]
- Determinants of linear growth faltering among children with moderate-to-severe diarrhea in the Global Enteric Multicenter Study. BMC Med.. 2019;17(1)
- [CrossRef] [Google Scholar]
- Diarrheagenic Escherichia coli categories among the traditional enteropathogenic E. coli O serogroups: a review. Memorias do Instituto Oswaldo Cruz. 2004;99(6):545-552.
- [CrossRef] [Google Scholar]
- Conventional and molecular methods in the diagnosis of community-acquired diarrhoea in children under 5 years of age from the north-eastern region of Poland. Int. J. Infect. Dis.. 2015;37:145-151.
- [CrossRef] [Google Scholar]
- Advances in molecular serotyping and subtyping of Escherichia coli. Front Microbiol.. 2016;7:644.
- [CrossRef] [Google Scholar]
- Phenotypic and whole cell protein analysis by SDS-PAGE for identification of dominants lactic acid bacteria isolated from Algerian raw milk. World J. Dairy Food Sci.. 2009;4(1):78-87.
- [Google Scholar]
- Evaluation of some Escherichia coli antigenic components as a discriminatory and diagnostic tool. J. Chem. Pharm. Sci.. 2017;10(2):989-994.
- [Google Scholar]
- Systematic review of diarrhea duration and severity in children and adults in low-and middle-income countries. BMC Pub. Health.. 2012;12(1):276.
- [Google Scholar]
- Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: a systematic review and meta-analysis. Antimicrob. Resist. Inf. Cont.. 2020;9(1):1-22.
- [CrossRef] [Google Scholar]
- Detection of shiga toxin genes (stx1 & Stx2) and molecular characterization of shiga-toxigenic Escherichia coli isolated from diverse sources in Gulbarga Region, India. Pharmacophore. 2011;2(5):253-265.
- [Google Scholar]
- Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun.. 2007;75(8):3961-3968.
- [CrossRef] [Google Scholar]
- New vaccine technologies to combat outbreak situations. Front. Immunol.. 2018;9:1963.
- [CrossRef] [Google Scholar]
- Assessment of diarrheal disease prevalence and associated risk factors in children of 6–59 months old at Adama District Rural Kebeles, Eastern Ethiopia, January/2015. Ethiopian J. Health Sci.. 2016;26(6):581-588.
- [CrossRef] [Google Scholar]
- Escherichia coli as commensal and pathogenic bacteria among food-producing animals: health implications of extended spectrum β-lactamase (ESBL) production. Animals. 2020;10(12):2239.
- [CrossRef] [Google Scholar]
- The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect. Immun.. 2008;76(5):2106-2112.
- [CrossRef] [Google Scholar]
- Vaccination with EtpA glycoprotein or flagellin protects against colonization with enterotoxigenic Escherichia coli in a murine model. Vaccine. 2009;27(34):4601-4608.
- [CrossRef] [Google Scholar]
- Treating common illnesses among children under five years: a portrayal of health-seeking behaviours and practices in the northern areas of Pakistan. World Health Populat.. 2011;12(4):24-34.
- [CrossRef] [Google Scholar]
- A Rapid Extraction Method for mammalian cell cultures, suitable for quantitative immunoblotting analysis of proteins, including phosphorylated GCN2 and eIF2a. Meth. X. 2018;5:75-82.
- [CrossRef] [Google Scholar]
- Numerical Taxonomy. The Principles and Practice of Numerical Classification. W H Freeman & Co; 1973.
- Protection from natural infections with enterotoxigenic Escherichia coli: longitudinal study. Lancet. 2003;362(9380):286-291.
- [CrossRef] [Google Scholar]
- Vaccines against enterotoxigenic Escherichia coli. Expert Rev. Vacc.. 2008;7(6):795-804.
- [CrossRef] [Google Scholar]
- Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. New England J. Med.. 1988;318(19):1240-1243.
- [CrossRef] [Google Scholar]
- Childhood diarrhoeal diseases in developing countries. Heliyon. 2020;6(4):e03690
- [CrossRef] [Google Scholar]
- A comparative study of bacterial and parasitic intestinal infections in India. J. Clin. Diagn. Res.. 2015;9(3):DC01-DC04.
- [CrossRef] [Google Scholar]
- Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees). Paris: Office International Des Epizooties; 2004.
- Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vacc.. 2007;25(14):2545-2566.
- [CrossRef] [Google Scholar]
- Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vacc.. 2012;11(6):677-694.
- [CrossRef] [Google Scholar]
- Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: a hospital based study. BMC Infect Dis.. 2018;18(1):1-10.
- [CrossRef] [Google Scholar]
- Incidence of diarrheagenic Escherichia coli pathotypes in children suffering from diarrhea in Tertiary Care Hospitals, Quetta, Pakistan. Pak. J. Zool.. 2019;51(6):2015-2021.
- [CrossRef] [Google Scholar]







