Identification, evaluation the source of natural bioactive compounds from Calotropis gigantea l. flowers and their anticancer potential
⁎Corresponding authors at: Room No: 2A2, Research Chair in Laser Diagnosis of Cancers, Department of Physics and Astronomy, College of Science, King Saud University, P. Box: 2455, Riyadh, 11451, Saudi Arabia. dsandhana@ksu.edu.sa (Sandhanasamy Devanesan), malsalhi@ksu.edu.sa (Mohamad S. AlSalhi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
In the intensified search to derive biologically active compounds from natural products for various health care issues, attention is focused on non-edible plant sources. Among the wild plants, Calotropis [milk weeds] of the family Apocynaceae, are the repository of pharmaceutically active phytoconstituents. The flowers of C. gigantea are expected to be rich in bioactive compounds.
Methods
The bioactive compounds and in vitro anticancer activity of C. gigantea flower extract were investigated. The compounds in the extract were separated using GC–MS and confirmed their structures with NIST (National Institute of Standard and Technology) database. The cytotoxicity of flower extracts against A-549 lung cancer cell lines was evaluated by MTT assay. Among the six identified compounds, three biologically active compounds with short retention time and abundance were chosen for molecular docking for anticancer activities. Molecular docking using the software GLIDE version 6.5 was used to trace their interactions with the anti-lung cancer protein, IT69.
Results
The phytochemical screening indicated the rich presence of anticancer flavonoid and phenolic compounds in the flower extract. Inhibition of cell viability and growth and morphological alterations in cells were observed when A-549 cells were exposed to different concentrations of flower extract. The highest percentage of cell death (70 %) was observed in cells exposed to 100 µg/ml concentration. Molecular docking of three identified compounds with lung anticancer protein complex, IT69 proved that Butanoic acid, 3-[(phenyl methoxy) imino] trimethylsilyl ester is a good anti-lung cancer agent. Hence for pulmonary cancer, the identified compound can intensively be tested further.
Keywords
Calotropis gigantea
Milkweed
Bioactive compounds
Cytotoxicity
Anti-lung cancer activity
1 Introduction
Cancer is one of the most serious health burden disease results in major death worldwide. According to World Health Organization (WHO), about 30 % of the premature death of adults in the age between 30 and 70 from non-chronic diseases were due to cancers in 2020. In the year 2018, 18.1 million people has cancer and 9,6 million died worldwide (WHO, 2020). WHO predicts that the number may twice higher in the year of 2040. Lung cancer is the most diagnosed among all cancers, followed by breast cancer. Major cancer-related deaths are mainly due to lung cancer. Various rapid detection methods using nanomaterial with enhanced sensitivity have been developed for the diagnosis of cancer in the early stage (Chinnappan et al., 2019, Muthukumar, et al., 2023). Despite various modern diagnostic methods and the anti-tumor therapy (Chinnappan et al., 2020), bioactive compounds from medicinal plants play a major role in cancer therapy.
Natural products from medicinal plants have the potency to inhibit several diseases including cancer (Atanasov, et al., 2021; Qureshi, et al., 2006). According to World Health Organization, more than 80 % of the population in developing countries depends on traditional medicine (Oyebode, et al., 2016). Recently, phytochemicals isolated from plants and their potential therapeutic drugs and antimicrobial activities have been explored (Altemimi et al., 2017). Despite technical challenges in discovering drugs from natural products, new lead compounds are identified by several researchers from plant resources (AlSalhi, et al., 2019). In the pursuit to identify lead compounds for cancer therapy, many phytoconstituents and their related products are paid good attention (Dutta et al., 2019; Mushtaq et al., 2018).
Calotropis procera and C. gigantea in the family of Apocynaceae are a repository of medicinally active phytoconstituents (Hassan et al., 2023; Pathania et al., 2020). C gigantea L. is commonly known as giant milkweed or crown flower. It is an evergreen, lactiferous perennial shrub introduced globally from South Asia. This plant has been used in traditional medical systems such as homeopathy, ayurvedic and traditional medicine for many years (Damodaran, et al., 2019). The bioactive compounds such as cardenolides and steroids from this plant have been widely studied. The plant has many medicinal properties not limited to anti-inflammatory, anti-diarrheal, anti-diabetic and anti-tumor activity etc. (Gururaja and David, 2016). Various parts of this plant are being used in traditional medicine as it is a repository of pharmacologically active compounds (Gupta and Gupta, 2019). The apoptosis effect by the combination of extract and 5-FU was significantly improved by the mitochondria-dependent pathway mechanism. This pathway reduces the adenosine triphosphate production and enhances the reactive oxygen species production as the result of cell apoptosis (Winitchaikul et al., 2021). Alafnan et al. have demonstrated the antioxidant, enzyme inhibition and wound healing behavior of phytochemicals from C. gigantea as bioactive medical products (Alafnan, et al., 2021). The lignan glycoside from the latex of C. gigantea exhibited antitumor activity, especially in ancient medicine, flowers of C. gigantea L were used widely for the treatment of diabetes mellitus, bronchial asthma, rheumatoid arthritis, and nervous disorders. Extraction and characterization of bioactive compounds from C. gigantea flowers have been reported (Parhira et al., 2014). The flowers of C. gigantea have exhibited some analgesic, antimicrobial, and cytotoxic activity.
The present study was carried out (i) to identify and characterize the various bioactive phytochemical compounds present in the C. gigantea flower extract using both qualitative method and GC–MS technique, (ii) to evaluate the effect of flowers extract on cell viability and cytotoxicity using in vitro assay against human cancer cell line, namely A-549, and (iii) to predict the mechanism of anticancer action of the lead bioactive compounds by a molecular docking approach.
2 Materials and methods
2.1 Collection and preparation of flower samples
Healthy and fresh inflorescence of C. gigantea was collected from the King Saud University Campus area, Riyadh, Saudi Arabia. A voucher specimen of C. gigantea was deposited at the Herbarium of the Department of Botany, King Saud University. The collected flowers were washed thoroughly with normal tap water to remove the impurities, followed by distilled water. Thirty grams of clean and fresh C. gigantea flowers were dried under shaded conditions at room temperature for two days. Then, 10 g of the dehydrated flowers were dipped in 50 ml of almond oil. After 24 h of soaking, excess oil was drained and samples was separated into five class petri dish and placed into orbital incubator at 80 °C for 8 h. Then, sample was burnt using a furnace at 687 °C for 10 min (Devanesan al., 2018). The ash powder form was collected and used in further phytochemical analysis and in vitro cytotoxicity assay.
2.2 Qualitative phytochemical analysis of C. Gigantea flower
The qualitative phytochemical analysis of flower extract was conducted to identify the presence of tannins, terpenoids, alkaloids, flavonoids, steroids, carbohydrates, proteins, and saponins. The test was carried out according to the standard biochemical analyses given by Harborne, (Harborne, 1998; Harborne, 1973).
2.3 Gas Chromatography-Mass spectrometry analysis (GC–MS)
The bioactive components present in the C. gigantea flower extract were identified by using GC–MS technique. Ten grams of C. gigantea flower ash powder was dissolved in 100 ml of methanol, and the solvent was completely evaporator by using a vacuum evaporator. GC–MS analysis of methanol extract was carried out on a single quadrupole GCMS-QP2010 (Shimadzu). Interpretation of the mass spectrum was done using the database of the National Institute of Standards and Technology (NIST). The spectrum of unknown components was compared with the spectrum of known components stored in NIST library. The name, molecular structure, formula, and molecular weight of the components present in the flower extract were confirmed (Devanesan et al., 2018).
2.4 Cytotoxicity of flower extract by MTT assay
2.4.1 Effect on cell viability
MTT assay is a simple colorimetric analysis that is used to determine cell viability and cytotoxicity. The principle of the assay is the reduction of MTT dye (3-(4, 5-dimethylthiazole-2-yl)-2, 5- diphenyltetrazolium bromide) to purple formazan due to mitochondrial dehydrogenase enzyme present in the metabolically active cells. The insoluble formazan crystals in active cells are later solubilized and quantified using a UV–visible spectrophotometer [PerkinElmer LS-55, USA]. The darker the solution, the greater the viability of cells. Because only the viable cells can reduce the tetrazolium dye (Green, et al., 1984 23).
The MTT assay was done following the method prescribed by Mosmann et al with desired modifications (Mosmann, 1983 24). A-549 cell line was cultured in DMEM supplemented with fetal bovine serum (10 %), sodium bicarbonate (0.2 %), and antibiotic–antimycotic solution (1 ml/100 ml of medium). For the assay, A-549 (10,000 cells/ml) cell lines were seeded in 96-well tissue culture plates and incubated overnight in the CO2 incubator for attachment. Then, the medium was aspirated and replaced with the medium containing different concentrations (1–100 µg/ml) of flower extract. The cells were allowed to grow for 24 h. Then, MTT (10 µl /well containing 100 µl of cell suspension; 5 mg/ml of stock in PBS) was added in each well incubated for 4 h. After incubation, the reaction mixture was carefully taken out and 200 µl of DMSO was added to each well by pipetting up and down several times otherwise the content gets homogenized. After 10 min, the color developed was read at 550 nm. The untreated control was also run simultaneously under identical conditions.
2.4.2 Effect on cell morphology
To determine the effect C. gigantea flower methanol extract on changes in the morphology of cells, A-549 cell lines were exposed to different concentrations (5–100 µg/ml) of flower extract for 24 h. After the exposure, morphological changes in the cell induced by flower extract were observed under an inverted fluorescence microscope [OPTIKA, ITALY]. The cell images were obtained at 20 x magnification.
2.5 Molecular docking studies using schrodinger [GLIDE 6.4 version]
For molecular docking, three identified compounds in the extract of C. gigantea flower ash were chosen based on GC–MS interpretation. The compounds are Butanoic acid, 2[(phenylmethoxy)imino] trimethylsilyl ester; Acetic acid 2-[(phenyl methoxy) imino]trimethylsilyl ester and Butanoic acid, 3-[(phenylmethoxy)imino] trimethylsilyl ester. The available literature found that these compounds have anticancer activities, so in silico studies were made to find whether these compounds could execute anticancer activities. Docking with the anticancer protein complex (PDB code: 1 T69) was selected from the protein bank. It showed good binding to exhibit anticancer activities. So, it could be further researched to develop anticancer drugs. The compound's 2D structure was drawn and optimized using ChemDraw-12 software. The 3D structural optimization was executed by Argus lab 4.0 software. The optimized ligands were docked into anticancer protein complex 1 T69 using Glide (SP & XP) module. For the chosen active binding site with a 7 Armstrong distance docking glide grid was prepared. Glide 12 flexible docking was executed to confirm the active binding amino acid with biochemical bonding interaction. Data were expressed as mean ± standard deviation (SD).
3 Results
3.1 Phytochemical analysis of C. gigantea flowers
The methanol extract of flowers was subjected to phytochemical analyses. A preliminary qualitative phytochemical test of flower ash showed the presence of medically active constituents such as Coumarins (+++), flavonoids (+++), tannins (++), terpenoids (++), alkaloids (+++), steroids (++), carbohydrates (++), proteins (++), and saponins (+). Coumarins and flavonoid contents were high, and saponin content was less. Further, GC–MS was used for the separation and identification of compounds present in the flower extract. The results provide 6 major peaks determining the presence of bioactive compounds with their different therapeutic activities (Fig. 1). They include 1. Butanoic acid, 2[(phenyl methoxy)imino]trimethylsilyl ester, 2. Butanoic acid, 3-[(phenyl methoxy)imino] trimethylsilyl ester, 3. Acetic acid 2-[(phenyl methoxy) imino]trimethylsilyl ester, 4. Hexadecanoic acid methyl ester, 5. Pentadecanoic acid,14-methyl-, methyl ester, and 6. Benzenepropanoic acid, 3,5-bis(1,1-dimethyl ethyl)-4-hydroxy-, methyl ester. The molecular formula and structure of the compounds derived from the NIST library were presented in Table 1.
![GC–MS spectrum showing the abundance and peak retention time of the compounds present in the flower extracts of C. gigantea. Six compounds with short retention [RT] and abundance are seen in the graph.](/content/185/2024/36/2/img/10.1016_j.jksus.2023.103038-fig1.png)
- GC–MS spectrum showing the abundance and peak retention time of the compounds present in the flower extracts of C. gigantea. Six compounds with short retention [RT] and abundance are seen in the graph.
| Compounds | Mol. Structure | Formula |
Mol. Wt [ g/mol] |
RT* [min] |
|---|---|---|---|---|
| Butanoic acid, 2[(phenylmethoxy)imino] trimethylsilyl ester |
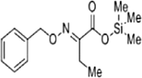
|
C14H21NO3Si | 279.41 | 3.933 |
| Butanoic acid, 3-[(phenylmethoxy)imino] trimethylsilyl ester |
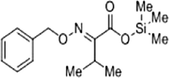
|
C15H23NO3Si | 262.49 | 3.933 |
| Acetic acid 2-[(phenylmethoxy) imino]trimethylsilyl ester |
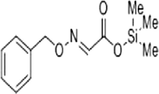
|
C12H17NO3Si | 251.35 | 3.933 |
| Hexadecanoic acid methyl ester |
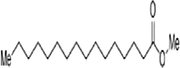
|
C17H34O2 | 270.0 | 22.894 |
| Pentadecanoic acid,14-methyl 1-methyl ester |
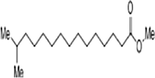
|
C17H34O2 | 270.4507 | 22.894 |
| Benzene propanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy methyl ester |
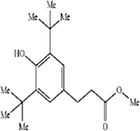
|
C18H28O3 | 292.4131 | 23.142 |
3.2 Effect of flower extract on cell viability and morphology
Human lung cancer cells A-549 were exposed to different concentrations viz., 1, 5, 10, 25, 50, and 100 μg/ml of oil soaked of C. gigantea ash powder extract for 24 h to study the effect on cell viability. From the MTT assay, it was found that the percent cell viability ranged between 30 % and 100 %. (Fig. 2). There was no cytotoxic response was observed at concentrations 1 and 5 μg/ml and only 5 % of cell death was observed at a concentration of 10 μg/ml. The highest cytotoxic effect was observed at 100 μg/ml, wherein 70 % of cell death occurred.
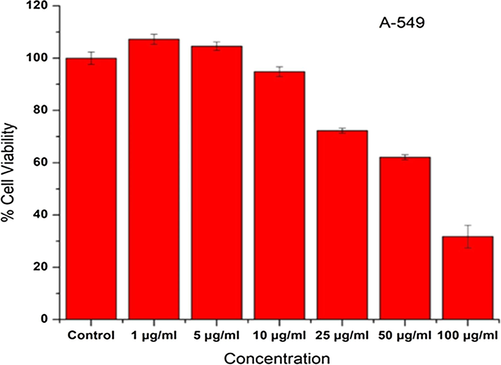
- Viabilities of cells (A-549 cell line) exposed to C. gigantea flower methanol extract at different concentrations (0 to 100 µg/ml) for 24 h.
From the examination of A-549 cell morphology at 24 h after treatment with methanolic flower extract, it was observed that the extracts suppressed the growth and caused morphological damage to the cells. As depicted in Fig. 3, a concentration-dependent effect was observed on cells treated with 5–100 μg/ml of methanolic flower extract. The cells started shrinking showing the symptoms of cell death. All the cells incubated with 10 μg/ml concentrations and above become rounded and lose their typical morphology.
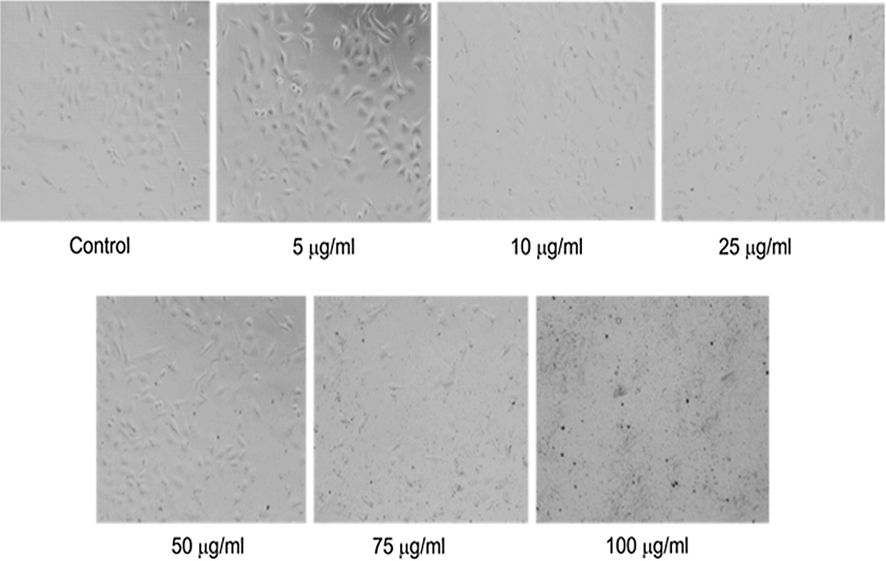
- Morphological alterations in A-549 cell line exposed to C. gigantea flower methanol extract at different concentrations (5 to 100 µg/ml) for 24 h. Images were taken using a phase contrast inverted microscope at 20x magnification.
3.3 Molecular docking of bioactive compounds
The C. gigantea flower ash powder was utilized for molecular docking which was the evidence of our material is potent to reach the targeted region for a specific disease. The action of material was observed binding capacities of protein interactions. In silico studies are anticipated to act as a major role to exhibit diversity and ligand-receptor interaction in the macrostructure. The identified phytoconstituents from C. gigantea ash were tested in the molecular docking in anticancer proteins and the first active compound 1 T69 binding amino acid of HIS 1180 side-chain interaction with pi-pi stacking (Fig. 4(a–d) and Table 2). Second compound IT69 with active site binding with HIS143 sidechain interaction (Fig. 5(a–d) and Table 2). The third compound of active site at IT69 interaction with hydrogen bonds TYR306 and PHE152 Fig. 6(a–d) and Table 2). The docking test was performed with a different angle of polymer chains and was expected significant binding site mode of ligand-target interactions. The binding score/binding energy, which depicts the binding affinity of the ligand and protein of interest, is represented in kcal/mol Table 2). It is reported that if the binding energy is very minimum, the binding affinity increases. The binding score of the tested compounds 3, 2 and 1 were-5.18, −4.576, and --4.479 kcal/mol, respectively. The active binding amino acids of HIS 180 side-chain interaction, HIS 143 pi-pi stacking with Compound 1, and Compound 2 has hydrophobic interaction with the selected binding site. Compound 3 has hydrogen bonding interaction with TYR306 and pi-pi stacking with PHE152. Binding energy with lead compounds of C1, C2, and C3 were −28.52, −29.32, and −37.44 kcal/mol. The interaction of the compound C3 was good with the cancer target macromolecule, histone deacetylase enzyme 8, with a glide XP score of −5.18.
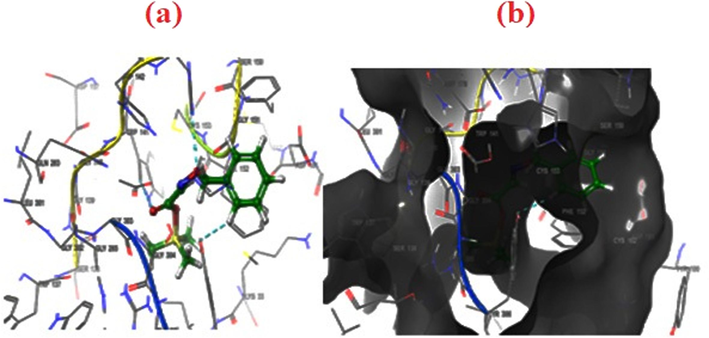
- (a–b). 2D & 3D docking pose of compound 2 at IT69 active site, showing hydrophobic bonding interaction with selected sites. The active binding amino acids of HIS 180 side-chain interaction with pi-pi stacking.
| Compound | Energy kcal/mol | docking score | Glide ligand efficiency | Glide ligand efficiency sa | Glide ligand efficiency ln |
XP GScore |
Glide Gscore | Glide Vander Waals energy | Glide coulo-mb energy | Glide energy | Glide internal | Glide model | XP HBond | Glide conf | Glide pose |
xp pose rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C3 | 31.327 | −5.18 | −0.259 | −0.703 | −1.296 | −5.18 | −5.18 | −28.062 | 0.542 | −27.52 | 6.977 | 4.187 | −0.377 | 1 | 1 | 1 |
| C2 | 22.12 | −4.576 | −0.269 | −0.692 | −1.194 | −4.576 | −4.576 | −29.481 | 0.159 | −29.323 | 12.799 | –33.892 | 0 | 1 | 9 | 1 |
| C1 | 25.617 | −4.479 | −0.236 | −0.629 | −1.135 | −4.479 | −4.479 | −37.823 | 0.375 | −37.448 | 5.188 | −49.103 | −0.117 | 1 | 1 | 1 |
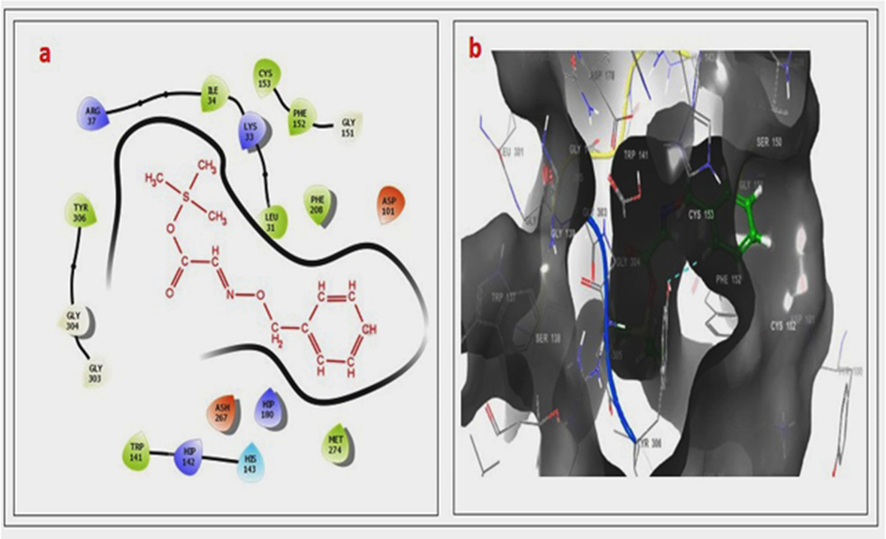
- (a–b). 2D & 3D docking pose of compound 2 at IT69 active site, showing hydrophobic bonding interaction with selected sites. The active binding amino acids of HIS 143 side-chain interaction with pi-pi stacking.
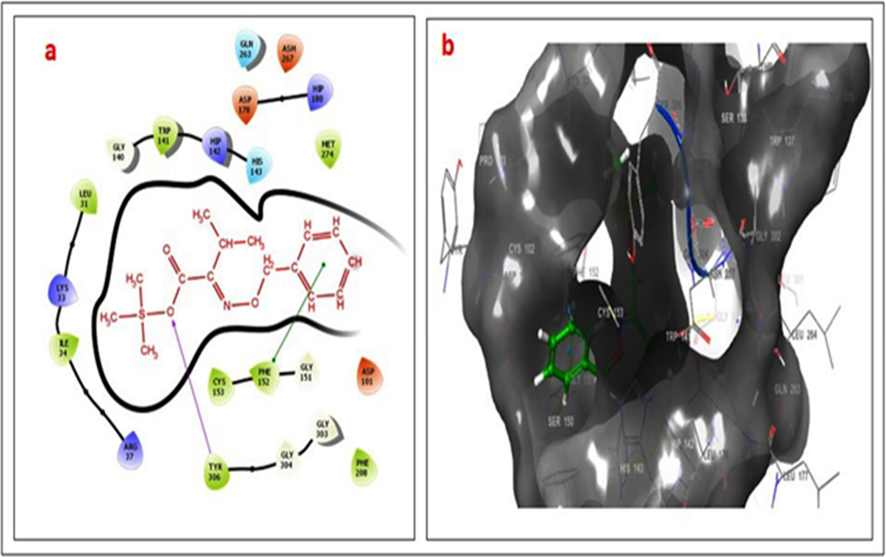
- (a–b). 2D & 3D docking pose of compound 3 at IT69 active site, showing hydrogen bonding interaction with TYR306 (rose line) and pi-pi stacking with PHE152 (green line).
4 Discussion
Bioactive compounds from plants are researched to discover therapeutic solutions to many ailments as they are believed to have more numerous significance as compared to synthetic drugs. Medicinal plants outcomes-based remedies are safer than artificial drugs. C. gigantea and C. procera are twin members of the genus Calotropis with medicinal, ritual, and commercial importance. This genus is a main source of therapeutically dynamic phytoconstituents (Pathania et al., 2020). These phytochemical constituents show good analgesic, antitumor, antihelminthic, antioxidant, and several other activities (Uthirasamy, et al., 2021). The methanolic extracts of C. gigantea induce apoptosis in breast cancer cells. Such action is done by accumulating phosphatidyl serine on the cell membrane, recruitment of poly-caspases, DNA fragmentation, and gene expression (Kharat and Kharat, 2019).
The first step in exploring any medicinal plant's significance is to look for its biologically active components, as this provides a general understanding of the type of natural compounds present in it. The preliminary phytochemical evaluation revealed the presence of several compounds, including flavonoids and phenolic derivatives. Flavonoids are a group of naturally occurring compounds widely distributed as secondary metabolites in the plant kingdom and they possess an influence on different anticancer activities. They modulate reactive oxygen species-scavenging enzyme and it block the cell cycle, stimulates apoptosis, and suppresses cancer cell proliferation (Kopustinskiene et al., 2020). A previous study by Divya and Manimegalai reported the presence of alkaloids, tannin, flavonoids, phenol, sterol, and anthraquinones in the flowers of C. gigantea, while terpenoids and saponin were absent (Dhivya and Manimegalai, 2013). The biomedical importance of flavonoids and other phenolic compounds of plant origin is well documented as an attractive alternative source for pharmaceutical and medicinal applications (Tungmunnithum, et al., 2018). Patel et al. reported the presence of flavonoids and alkaloids in the flowers and their antioxidant activity (Patel, et al., 2019). Further, flavonoids and phenolic compounds influence immune system function and inflammatory responses (Locatelli et al., 2018).
The phenolic acids and flavonoid derivatives are the greatest players in scavenging free radicals to promote human health and improve the bio-valorization of waste in the environment (Yu, et al., 2021). Phenolic compounds target oxidative stress and cancer development (Wahle, et al., 2010). The phenolic compounds in herbal food and medicine are the major champion for removing free radicals and preventing related cancer origin in the human system (Basli, et al., 2017). The rich presence of flavonoid and phenolic compounds justifies the use of the flower of C. gigantea for treating various health issues by traditional practitioners.
Several therapeutically relevant anticancer medications have recently benefited from the usage of plants, and it has been established that more than 60 % of currently used antiproliferative chemicals are derived directly or indirectly from natural sources. In traditional medical treatment, the role of herbal medicine is considerable; thus, recently, many studies have been conducted to screen and evaluate new plant-derived natural compounds (El-Naggar, et al., 2015; Naz, et al., 2017). In the present study, the cytotoxic effect of methanol extract of C. gigantea flowers against lung cancer cell line A-549 was reported. The morphological changes in cells clearly indicate that cells undergo apoptosis after 24 h incubation with different concentrations of flower extract (5–100 μg/ml) of C. gigantea based on the MTT assays. Cell morphologies became more rounded and interacted less with the surrounding cells after treatment with 10 µg/ml of extract compared to cells in control and less than 10 µg/ml added concentrations.
Lee and his research group also reported that the ethanol extract of C. gigantea caused the inhibition of the growth of A-549 human lung cancer cells (Lee, et al., 2019). They found that the cytotoxicity of C. gigantea plant extract against lung cancer cells was due to the apoptotic effect that was elucidated through three different mechanisms. Firstly, C. gigantea collectively caused inhibitory effects in the cell cycle of A-549, which stopped the growth of the cells and induced apoptosis. Secondly, the flower extracts induced both the extrinsic and intrinsic apoptotic signaling pathways, which were mediated via death receptors, cytochrome c, and caspases, and this was followed by the downregulation of the DNA damage repair protein, poly (ADP-ribose) polymerase in A-549 cells which led to apoptosis. Thirdly, the plant extracts generated reactive oxygen species (ROS) under metabolic stress conditions in A-549, and this ROS stress led to cancer cell death. The phytochemicals such as flavonoids, glycosides, triterpenes, steroids, heterocyclic and phenolic compounds in the ethyl acetate flower extract of C. gigantea are known to possess potent antitumor activities (Habib, et al., 2010).
GC–MS analysis showed the presence of six major compounds and a few of them showed antibacterial, anticancer, antioxidant, and antifungal activities (Nguyen,et al., 2020). Computer-aided drug discovery (CADD) has become an increasingly important tool for drug discovery and faster, cheaper, and more effective drug design. The molecular docking-based drug development for SARS-CoV-2 helped to develop vaccines and drugs (Eweas et al., 2021). As molecular docking is an effective quick tool to predict the action of the drugs on the specific target proteins, in the present study three most promising compounds identified in the extracts were subjected to in silico analysis. The ligands prepared for the drug compounds were docked to target anti-lung cancer protein IT 69. The binding of ligands confirms the drug's action with target macromolecules. The free energy in ligand-receptor binding and the intermolecular recognition confirmed the drug s action. Today, various docking algorithms are available to trace the pharmacological importance of natural products (Ferreira,et al.,2015).
In the present study, molecular docking was done for three compounds chosen from the six compounds for molecular docking to confirm anticancer activities based on their rich presence, retention time, and a previous report. The molecular docking of these compounds was done with anticancer protein complex IT69. The binding of the drug molecule with 1 T69 and its orientation and position are similar to previous reports (Alqahtani, et al., 2020, Gul, et al., 2019). Also, the compound Butanoic acid, 3-[(phenyl methoxy)imino] trimethylsilyl ester exhibits H-bonding between the target and ligand. The perfect interaction between the compound C3 and histone deacetylase enzyme 8 with a glide XP score of −5.18 indicates the drug's anticancer action. The result indicates good anti-lung cancer activities of compounds in the flowers of C. gigantea.
5 Conclusion
Calotropis gigantea is a weed plant commonly found in Saudi Arabia. The qualitative screening of the extract of the flower showed the presence of several chemicals, including anti-cancerous flavonoids and phenols. The significant cytotoxic action of the extract on the lung cancer cell lines, A-549 proved inhibition of the proliferation of the cancer cells. The morphological changes in cells indicate that cells undergo apoptosis after 24 h of exposure with different concentrations of flower extract. Of the six compounds identified in the GC–MS study, three compounds were chosen for molecular docking for anticancer properties. The in silico study using GLIDE-XP-SP version on the target anticancer protein IT 69, the GLIDE XP score of −5.18 for the compound Butanoic acid, 3-[(phenyl methoxy)imino] trimethylsilyl ester indicates a good anti-lung cancer activity. The flower of this plant is enriched with several pharmaceutically active compounds it could be good remedy for cold, asthma and hematopoiesis. Hence the identified compound opens an avenue for further chemotherapeutic application to combat lung cancer.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research. (IFKSURC-1-0706)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of the phytochemical, antioxidant, enzyme inhibition, and wound healing potential of Calotropis Gigantea (L.) dryand: a source of a bioactive medicinal product. Front. Pharmacol.. 2021;12:701369
- [Google Scholar]
- Documentation of bioactive principles of the flower from Caralluma Retrospiciens (Ehrenb) and in vitro antibacterial activity-part B. Arab. J. Chem.. 2020;13:7370-7377.
- [Google Scholar]
- Synthesis of silver nanoparticles using plant derived 4-N-Methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci.. 2019;26:970-978.
- [Google Scholar]
- Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42.
- [Google Scholar]
- International natural product sciences taskforce. Nat. Rev. Drug Discov.. 2021;20:200-216.
- [Google Scholar]
- Health benefits of phenolic compounds against cancers. Phenolic Compd.-Biol. Act. Lond. Intechopen. 2017;8:193-210.
- [Google Scholar]
- Highly sensitive multiplex detection of MicroRNA by competitive DNA strand displacement fluorescence assay. Talanta. 2019;200:487-493.
- [CrossRef] [Google Scholar]
- Anti-VCAM-1 and Anti-IL4Rα Aptamer-conjugated super paramagnetic iron oxide nanoparticles for enhanced breast cancer diagnosis and therapy. Molecules. 2020;25
- [CrossRef] [Google Scholar]
- Phytochemical screening and evaluation of cytotoxic activity of Calotropis Gigantea leaf extract on MCF7, HeLa, and A549 cancer cell lines. J. Nat. Sci. Biol. Med.. 2019;10:131-138.
- [Google Scholar]
- Devanesan, S., AlSalhi, M.S., Devanesan., Periyasami, G., Ali Kanakhir, A., 2018. Method of preparing biologically active derivatives from Calotropis gigantea flowers. US. Patent Number: US10111918B1.
- Preliminary phytochemical screening and Gc-Ms profiling of ethanolic flower extract of Calotropis Gigantea Linn. (Apocyanaceae) J. Pharmacogn Phytochem.. 2013;2:28-32.
- [Google Scholar]
- Natural products: an upcoming therapeutic approach to cancer. Food Chem. Toxicol.. 2019;128:240-255.
- [Google Scholar]
- Metabolomic profiling, antioxidant capacity and in vitro anticancer activity of some compositae plants growing in Saudi Arabia. Afr. J. Pharm. Pharmacol.. 2015;9:764-774.
- [Google Scholar]
- Molecular docking reveals ivermectin and remdesivir as potential repurposed drugs against SARS-CoV-2. Front. Microbiol.. 2021;11:3602.
- [Google Scholar]
- Molecular docking and structure-based drug design strategies. Molecules. 2015;20:13384-13421.
- [Google Scholar]
- Rapid colormetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J. Immunol. Methods. 1984;70:257-268.
- [Google Scholar]
- Molecular docking and Quantitative Structure Activity Relationship (QSAR) studies of some newly synthesized Poly (Azomethine) esters. Int. J. Polym. Sci. 2019
- [Google Scholar]
- Spectroscopic signature, antibacterial and anticancer properties of Calotropis Gigantea (Linn.) Flower. Int. J. Pharm. Sci. Res.. 2016;7:1686.
- [Google Scholar]
- Habib, M.R., Aziz, M.A., Karim, M.R. Inhibition of Ehrlich’s Ascites Carcinoma by Ethyl Acetate Extract from the Flower of Calotropis Gigantea L. in Mice. J. Appl. Biomed. 2010, 8, 47–54, doi:10.2478/v10136-009-0007-7.Saudi J. Biol. Sci. 2023, 30, 103622.
- A Guide to Modern Techniques of Plant Analysis. Chapman and Hall; 1973. ISBN 0-412-10540-3.
- Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. springer science & business media; 1998. ISBN 0-412-57270-2
- Hassan, S., Atef, A., Ali, H.M., Alshamrani, R., Ramadan, A., 2013. Calotropis Procera Accumulates Uzarigenin and Calotropagenin in Response to Environmental Lighting and Drought.
- The Calotropis Gigantea methanolic extract induces apoptosis in human breast carcinoma cells. Iran. J. Med. Sci.. 2019;44:483.
- [Google Scholar]
- Calotropis Gigantea extract induces apoptosis through extrinsic/intrinsic pathways and reactive oxygen species generation in A549 and NCI-H1299 non-small cell lung cancer cells. BMC Complement. Altern. Med.. 2019;19
- [CrossRef] [Google Scholar]
- Graminex Pollen: phenolic pattern, colorimetric analysis and protective effects in Immortalized Prostate Cells (PC3) and rat prostate challenged with LPS. Molecules. 2018;23:1145.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [Google Scholar]
- Natural products as reservoirs of novel therapeutic agents. Excli. J.. 2018;17:420.
- [Google Scholar]
- Enhancement of cell migration and wound healing by nano-herb ointment formulated with biosurfactant, silver nanoparticles and Tridax procumbens. Front. Microbiol.. 2023;14:1225769.
- [Google Scholar]
- Antioxidant, antimicrobial and antiproliferative activities of peel and pulp extracts of red and white varieties of Ipomoea Batatas (L) Lam. Trop. J. Pharm. Res.. 2017;16:2221-2229.
- [Google Scholar]
- Calosides A-F, cardenolides from Calotropis Gigantea and their cytotoxic activity. J. Nat. Prod.. 2020;83:385-391.
- [Google Scholar]
- Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan. 2016;31:984-991.
- [Google Scholar]
- In vitro anti-influenza virus activities of a new lignan glycoside from the Latex of Calotropis Gigantea. PloS One. 2014;9:e104544
- [Google Scholar]
- Comparative analysis of exosome isolation methods using culture supernatant for optimum yield. Purity Downstream Appl.. Sci. Rep.. 2019;9:1-10.
- [Google Scholar]
- Genus Calotropis: a hub of medicinally active phytoconstituents. Curr. Tradit. Med.. 2020;6:312-331.
- [Google Scholar]
- Qureshi, R., Ahmad, I., Ishtiaq, M., 2006. Ethnobotany and Phytosociological Studies of Tehsil Gujar Khan.
- Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93.
- [Google Scholar]
- Identification of bioactive constituents in Calotropis Gigantea leaves by GC-MS, HPLC and FTIR techniques. Asian. J. Adv. Res. 2021:1-8.
- [Google Scholar]
- Plant phenolics in the prevention and treatment of cancer. Bio-Farms Nutraceuticals Funct. Food Saf. Control Biosens. 2010:36-51.
- [Google Scholar]
- World Health Organization WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All, 2020.
- Calotropis Gigantea stem bark extract induced apoptosis related to ROS and ATP production in colon cancer cells. Plos One. 2021;16:e0254392
- [Google Scholar]
- Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep.. 2021;11:10041.
- [Google Scholar]







