Highly porous chitosan based magnetic polymeric nanocomposite (PNC) for the removal of radioactive, Cs(I) and Sr(II) ions from aqueous solution
⁎Corresponding authors. tahamed@ksu.edu.sa (Tansir Ahamad), alshehri@ksu.edu.sa (Saad M. Alshehri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
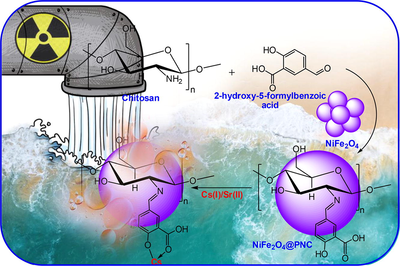
In the present study highly porous magnetic polymeric schiff base nanocomposite was fabricated using chitosan and 2-hydroxy-5-formylbenzoic acid in the presence of NiFe2O4. The analytical analysis (FTIR, TGA, XRD, BET, SEM, TEM and XPS) results support the successfully fabrication of NiFe2O4, polymeric matrix (PNC) and magnetic polymer nanocomposite (NiFe2O4@PNC). The surface area of NiFe2O4, and NiFe2O4@PNC was found to be 78.12, and 254 m2/g for NiFe2O4, and NiFe2O4@ PNC respectively. The NiFe2O4@PNC used for the adsorption of radioactive metal ions, Cs(I) and Sr(II) and the adsorption capacity was found to 232.12 mg/g and 212.5 mg/g respectively. The interaction between the NiFe2O4@PNC and both the metal ions were determine using adsorption kinetics and adsorptions isotherms models, which support the pseudo-second order and Langmuir adsorption models take part. The regeneration results revealed that the NiFe2O4@PNC retain 94.1 and 92.0 % adsorption capacity after six cycles with Cs(I) and Sr(II). These results demonstrate that the fabricated NiFe2O4@PNC can be used as an advanced and efficient adsorbent for adsorbing toxic inorganic and organic pollutants from contaminated water.
Keywords
Chitosan
Polymer nanocomposite
Radioactive
Adsorption
1 Introduction
The demand for energy is increasing and energy sources are being reduced day by day to overcome these problems alternative energy sources such as nuclear power play a key role in meeting the energy demand. However, it was noticed that the, radioactive waste including U(VI), Cs(I), Sr(II), and many more ions were discharged into the natural environment without any form of treatment and resulting contaminate the water sources such as well, rivers, and sea (Tang et al., 2020; Munthali et al., 2015; Johansen et al., 2018). These radionuclides are toxic and have a long half-life. Long-term exposure to radionuclides can cause serious health problems such as cancer, infertility, neurological disorders, and birth death (Ivanets et al., 2020; Tachibana et al., 2020; Uematsu et al., 2020).
Therefore, the removal of these radioactive ions form an aqueous solution is urgently required. To date, various techniques such as liquid–liquid extraction, chemical precipitation, electrochemical treatment, membrane separation and adsorption have been used to remediate radioactive ions from contaminated water (Asgari et al., 2019; Liu and Wang, 2020; Sun et al., 2020; Wang et al., 2019). Besides these methods, adsorption and photocatalytic degradation are the most effective method for treating contaminated water, even for organic and inorganic pollutants, due to its low cost, ease of use and great accessibility (Ahamad et al., 2021; Ahamad et al., 2020; Ahamad et al., 2020; Ahamad et al., 2020). Several nano-systems have been used for the treatment of other toxic pollutants including dye, organic nutrients, antibiotics etc. from contaminated water (Kaur et al., 2021; Singh et al., 2022; Chakraborty et al., 2020; Chakraborty et al., 2021). Among these adsorbents polymeric resins and their nanocomposites have potential application due to their high surface area, tuning the selectivity and adsorption capacity with functional groups (Ghalami et al., 2019; Ogata et al., 2018; Bisla et al., 2020; Shi et al., 2020). However, the uses of these polymers as adsorbents are limited because of their separation and recovery after the adsorption of toxic pollutants is time consuming and costly.
Realizing these drawbacks, a series of investigations and study were exposed. Among them, the production of magnetic polymer nanocomposite and its use to remove toxic pollutants from aqueous solution is the most effective technique to solve the above problems. Previously, several magnetic nanocomposites have been used for the treatment of polluted aqueous solution. As we mention above that the porosity and the functional groups of the polymer nanocomposites can be tuned according to the desired applications. Therefore, in the present study, a highly porous magnetic polymeric nanocomposite was fabricated using chitosan, salicylaldehyde and NiFe2O4. The fabricated nanocomposite was well characterized and used for the adsorption of radioactive ions including Cs(I) and Sr(II) from aqueous solution. The interaction between the NiFe2O4@PNC and both the metal ions were determine using adsorption kinetics and adsorptions isotherms models. The regeneration results revealed that the NiFe2O4@PNC show promising adsorption capacity after six cycles with Cs(I) and Sr(II). The overall results, demonstrate that the fabricated NiFe2O4@PNC can be used as an advanced and efficient adsorbent for adsorbing toxic inorganic and organic pollutants from contaminated water.
2 Experimental
2.1 Materials
Chitosan (low molecular weight < 50 kDa, degree of deacetylation 70–80%), 2-hydroxy-5-formylbenzoic acid, nickel(II) chloride hexahydrate (NiCl2·6H2O), Iron(III) chloride hexahydrate (FeCl3·6H2O), cesium nitrate, and strontium nitrate were purchased from Sigma Aldrich. Other chemicals were also used analytic grade without any purification. The magnetic NiFe2O4 nanoparticles were fabricated using co-precipitation method using ammonia solution.
2.2 Fabrication of the adsorbent (NiFe2O4@PNC)
To fabricate the magnetic nanocomposite 1 g of chitosan (Low molecular weight, <50 kDa) was dissolved in ethanol with help of dil. CH3COOH, the emulsion was sonicated well and added 0.83 g of 2-hydroxy-5-formylbenzoic acid dissolved in ethanol to this solution slowly. The resulting mixture was stirred and heated at 60 °C for 1 h and then 0.5 g of NiFe2O4 nanoparticles was added and stirred and heated at 60 °C for 3 h. After that the pH of the solution was neutralized using aqueous NaOH solution and resulting yellow precipitate of NiFe2O4@PNC nanocomposite was filtered and washed with distilled water and separated with an extranet magnate and dried and storage in vacuum for further characterization and used. The details of characterization such as FTIR, TGA, XRD, BET, SEM, TEM, XPS and adsorption assay are given in supporting information.
3 Results and discussion
The magnetic adsorbent was fabricated using NiFe2O4 nanoparticles embedded into chitosan and 2-hydroxy-5-formylbenzoic acid based polymeric matrix. The polymer matrix was fabricated under acidic solution at 70 °C temperature and the synthetic route is illustrated in Fig. 1.
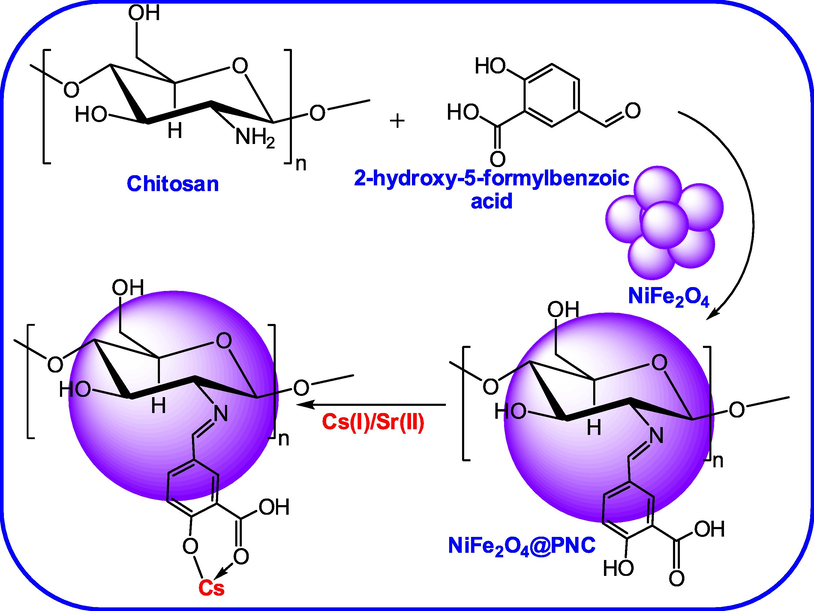
- The synthesis routes for the synthesis of NiFe2O4@PNC.
The FTIR spectra of the polymer (PNC), NiFe2O4 nanoparticles and the magnetic polymer nanocomposite (NiFe2O4@ PNC) are illustrated in Fig. 2(a). The FTIR spectra of the PNC shows several FTIR peaks at 3340–3481, 3060, 2945–2841, 1725, 1687, 1548, 1440 and 1178 cm−1 which assigned to –OH, =C–H, C–H, C=N, C=C, C-O, C-N stretching vibration, respectively (Shanavas et al., 2021). While in the case of the NiFe2O4@PNC the broadness of the O–H region was increased and additional peaks at 1665 cm−1 was observed due to presence of OH groups on the surface of NiFe2O4. Additionally, two peaks at 549 and 614 cm−1 were assigned to the Ni-O and Fe-O of the spinal structure of NiFe2O4. TGA curves of the polymer, NiFe2O4 and the NiFe2O4@PNC were recorded under the flow of air and recorded from room temperature to the 900 °C and the results are illustrated in Fig. 2(b). In the case of pure NiFe2O4 only 3.5 % weight loss was noticed and this may be due to the loss of adsorbed O–H groups up to 200 °C. However, in the case of the PNC and the NiFe2O4@PNC, the weight loss up to 200 °C were found to be 5.80% and 4.81 % respectively. The main degradation was carried out in second and third stages, the second stage, between 200 and 325 °C, and in this stage about 35.67 and 30.34 % weight loss was noticed. Moreover, the third degradation stage in the case of polymer is also fast and completely decomposed at 600 °C, while in the case of the NiFe2O4@PNC, he residue weight was found to be 24.88 %, due to the presence of the metal oxide (Ubaidullah et al., 2021; Alshehri et al., 2016). The crystalline nature and the purity of the NiFe2O4 nanoparticles and NiFe2O4@PNC was determined using X-ray diffraction patterns analysis. As shown in Fig. 2(c), the XRD results of the pure NiFe2O4 nanoparticles show the diffraction peaks at 2ϴ = 29.95, 35.45, 37.50, 43.05, 53.65°, 56.89 and 62.89 with crystal planes (2 2 0), (3 1 1), (2 2 2), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) respectively and support the spinel structure of NiFe2O4 (JCPDF-74-2061) (Gao et al., 2020; Lu et al., 2021). The XRD spectra of the NiFe2O4@PNC shows similar diffraction peaks without changing their position, however the peaks become broad and less instance, this is because the polymer is monocrystalline in nature (Al-Enizi et al., 2018). These outcomes additionally support the formation of the NiFe2O4 and maintain its originality in the NiFe2O4@PNC. The porosity and the surface area of the NiFe2O4, and NiFe2O4@ PNC was determined using N2 adsorption–desorption isotherm. As shown in Fig. 2(d), the CSRs and NiFe2O4@ PNC show the mesoporous nature with the type IV hysteresis loop based on IUPAC classification (Ahamad et al., 2021). The surface area was determine using BET and observed about to be 78.12, and 254 m2/g for NiFe2O4, and NiFe2O4@ PNC respectively. Additionally, the average pore volume was calculated using the BJH equation and in the case of the NiFe2O4, and NiFe2O4@PNC it was found to be 15 nm, and 28 nm respectively.
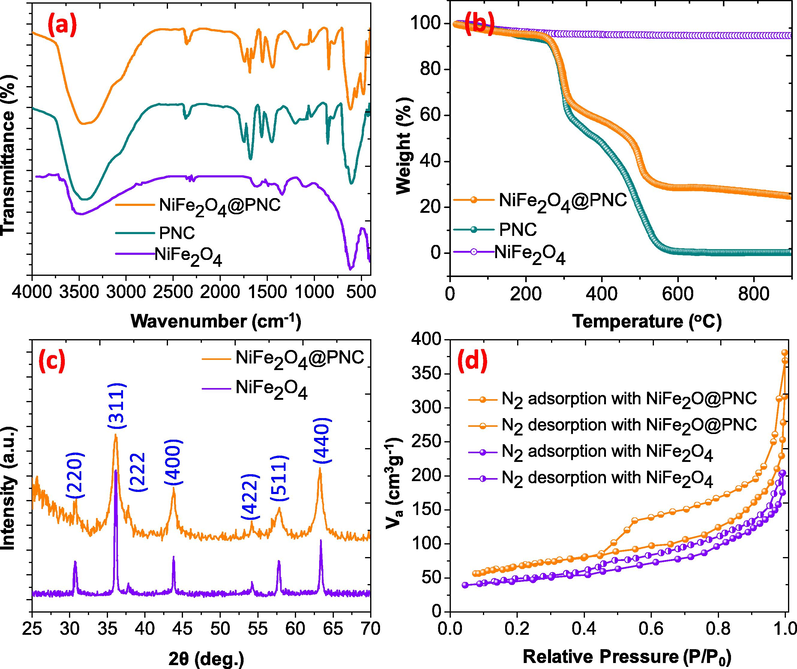
- (a) FTIR spectra of NiFe2O4, PNC and NiFe2O4@PNC (b) TGA/DTA curves of NiFe2O4, PNC and NiFe2O4@PNC (c) XRD of NiFe2O4, and NiFe2O4@PNC (d) N2 adsorption and desorption of NiFe2O4, and NiFe2O4@PNC.
The morphology of the NiFe2O4 and NiFe2O4@ PNC was determine using SEM images, as shown in Fig. 3(a) (b), the SEM image display that the NiFe2O4 nanoparticles are spherical shape with a particles size range of 8–12 nm. The SEM image of the NiFe2O4@PNC revealed that the nanoparticles are embedded uniformly into the polymer matrix of PNC. However, the presence of the polymer in nanocomposites reduces the aggregation of the magnetic nanoparticles. Further, the morphology of the NiFe2O4@PNC was determined using TEM technique as shown in Fig. 3(c). And the results revealed that the surface of the nanoparticles was smooth spherical shape and uniform in size with average diameter about 9 nm and embedded in to the polymer matrix. The HRTEM image of the nanocomposites display the crystalline nature of the magnetic NiFe2O4 nanoparticles and the lattice fringes was clearly identified with d- spacing 2.96 nm and 4.87 nm due to the (2 2 0) and (1 1 1) plane of the NiFe2O4 (Pang et al., 2021; Ren et al., 2014) as displayed in Fig. 3(d).
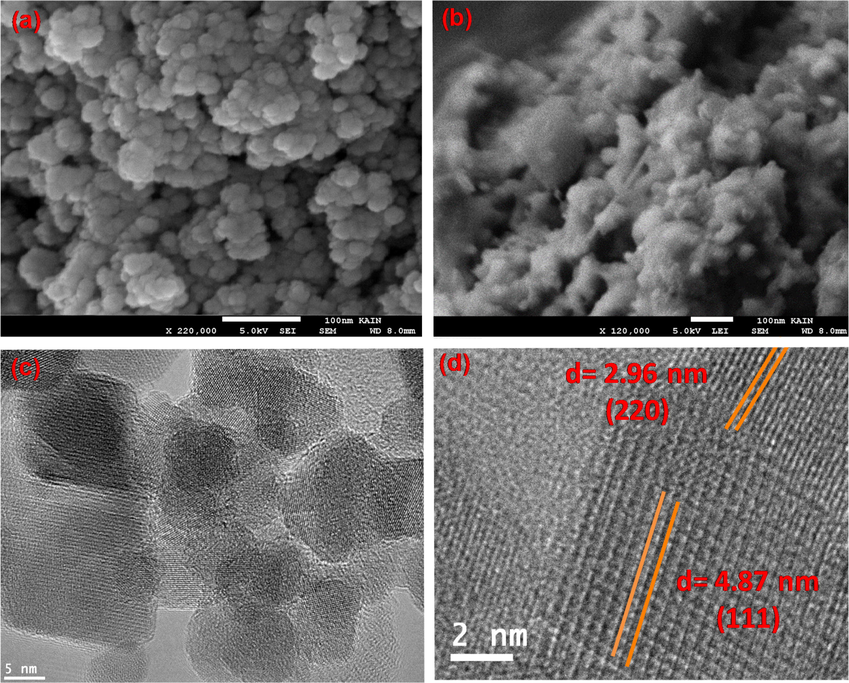
- (a) SEM image of NiFe2O4 (b) SEM image of NiFe2O4@PNC (a) TEM image of NiFe2O4@PNC (d) HRTEM image of NiFe2O4@PNC.
The present of elements on the surface and their oxidation were determined using X-ray photoelectron spectroscopy (XPS). The XPS spectrum of the NiFe2O4@PNC displays the presence of C, N, O, Ni, and Fe elements without any impurities, however there is no peaks for Ni, and Fe were found in the XPS spectra of NPC, as illustrated in Fig. 4(a). The high-resolution spectra of the Fe 2p is illustrated in Fig. 4(b) and split into two central peaks and appear at binding energy of around 711.14 and 725.23 eV, due to Fe 2p3/2 and Fe 2p1/2 respectively. While, another peak at 718.43 eV is assigned to the satellite pack belonging to the Fe 2p3/2 and support the presence of Fe3+ ions. The Ni 2p spectrum of the nanocomposite display two core peaks with two satellite peaks, the core peaks appear at binding energy 855.74 eV and 873.2 eV and assigned to the Ni 2p3/2 and Ni 2p1/2, respectively as displayed in Fig. 4(c). While the peaks at 862.1 and 880.7 eV appear due to the satellites peak of Ni 2p3/2 and Ni 2p1/2, respectively and support the existence of Ni2+ in the nanocomposite. These results support that the nanoparticles and the nanocomposite contain Ni2+ and Fe3+ and their atomic ratio was found to be 1:2, being consistent with the stoichiometry of NiFe2O4.
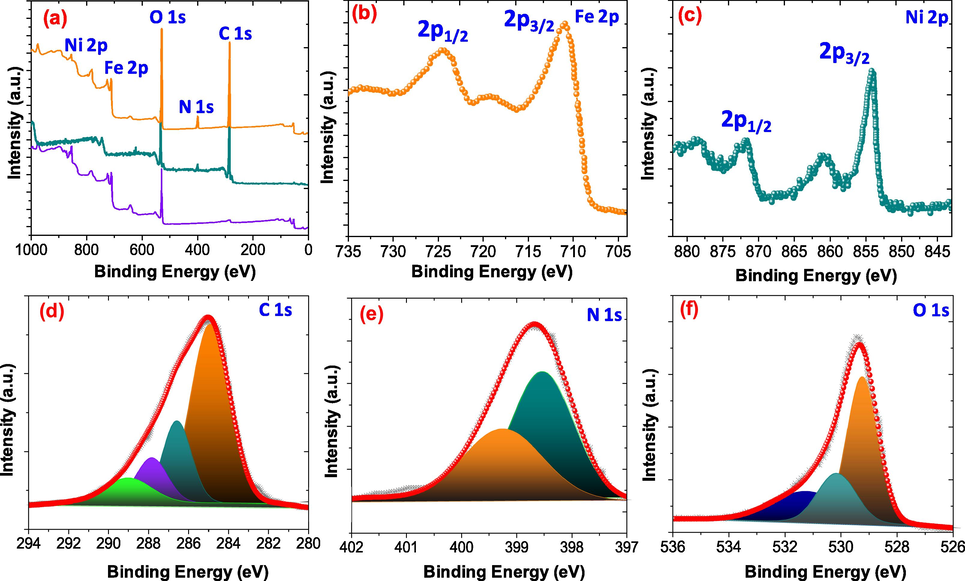
- (a) A wide XPS spectra for NiFe2O4, PNC and NiFe2O4@PNC (b) Fe2p, (c) Ni 2p (d) C 1s, (e) N1s (f) O1s.
As shown in Fig. 4(d), the high resolution spectra of the C 1s was deconvoluted into four peaks and appear the binding energy at 283.72 eV, 285.82 eV, 286.85 eV and 287.92 eV which were allocated to the C–C/C=C, C=O, C=N and C-O respectively(Ahamad et al., 2021). The XPS spectrum of N1s was split into two main pecks and appeared at binding energy of 398.60 eV, and 399.024 eV and support the formation of the C=N, - and –NH functional groups in the polymer matrix respectively as shown in Fig. 4(e). As displayed in Fig. 4(f), the O1s spectrum was deconvoluted into three peaks and appear at binding energy of 529.29 eV, 530.31 eV and 531.85 eV which were assigned to Fe-O/Ni-O, C-O/C=O, and O–H respectively.
The magnetic properties of the nanocomposite and NiFe2O4 nanoparticles were recorded using VSM analysis at room temperature and the observed magnetic curves were illustrated in Fig. 5(a). It was noticed that the saturation magnetization of the pure magnetic NiFe2O4 nanoparticles was reduced from 46.84 emu/g to 32.02 emu/g after the formation of the NiFe2O4@PNC. This may be due to weight of the nonmagnetic polymer, however the superparamagnetic properties of the NiFe2O4 retained in the nanocomposite and can be simply removed from the contaminated solution using an external magnate (Naushad et al., 2019). The zero-charge point of the NiFe2O4 and NiFe2O4@PNC was determined illustrated in Fig. 5(b). It was noticed that the zero-charge point was found at pH 7.12 and 6.54 for NiFe2O4 and NiFe2O4@PNC respectively.
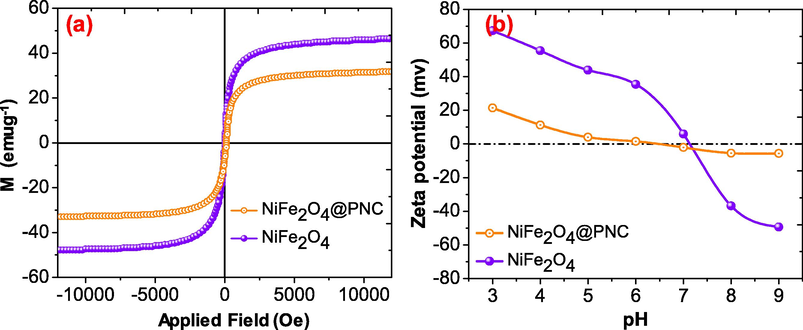
- (a) Magnetic measurements and (b) Zeta potential of NiFe2O4, and NiFe2O4@PNC.
3.1 Batch adsorption studies
To determine the adsorption capacity and the optimum adsorption conditions, the adsorption process was carried out by changing several factors such as pH, initial concentration, time and temperature (Pathania et al., 2017). The pH of aqueous solution is one of the main reason, which affect the adsorption capacity of the adsorbents. As shown in Fig. 6(a), when the pH of the solution was improved form 2 to 7 the adsorption of Cs(I) and Sr(II) was increased and the absorption capacity of NiFe2O4@PNC at pH 7 were found to be 232.5, and 212.2 mg/g respectively.
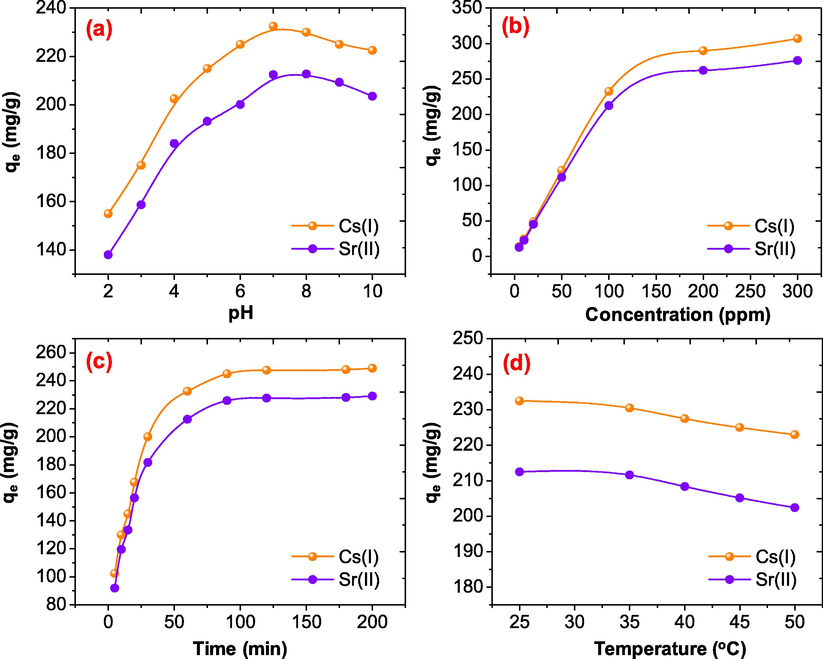
- Effect of (a) pH (b) concentration (c) time and (d) temperature on the adsorption of Cs(I) and Sr(II) onto NiFe2O4@PNC.
On the other hand, when the pH was improved more than 7 the adsorption of both the ions was slightly declined and at pH 10 the adsorption capacity was found to be 222.4 and 203.5 mg/g against Cs(I) and Sr(II) respectively at room temperature. This may be because, at lower pH the surface of the NiFe2O4@PNC was protonated resulting decreased the adsorption capacity. However, when the pH was more then 7, the metal ions were formed it corresponding hydroxides and reduced the adsorption capacity of the NiFe2O4@PNC at room temperature (Naushad et al., 2020). Based on these outcomes, the optimum pH was used 7 in all other adsorption experiment. The initial concentration of the Cs(I) and Sr(II) also affect the adsorption capacity of the NiFe2O4@PNC and the adsorption was run with a wide range of metal ions, from 5 mg/L to 300 mg/L were tested. It was observed, that when the initial concentrations of the Cs(I) and Sr(II) ions were increased form 5 mg/L to 100 mg/L the percentage adsorption and adsorption capacity was increased. However, when the initial concentration was further increased, up to 300 mg/L minor increment were noticed and the adsorption capacity and were found to be 249.75, and 229.70 mg/g against Cs(I) and Sr(II) respectively at room temperature and at pH 7. These outcomes support that the as the initial concentrations of the metal ions were increased the adsorption site of the adsorbent was decreased and at 100 mg/L concentration most of the active sites were occupied with the metal ions. Therefore, the initial concentration 100 mg/L of Cs(I) and Sr(II) were used as the optimum initial concentration. To determine the effect of the contact time the adsorption process, the experiment was carried out with different contact time range, from 5 to 200 min, at pH 7, initial concentration 100 mg/L and at room temperature and results are illustrated in Fig. 6(c). It was observed that when the time was increased form 5 min to 30 min the adsorption of all the metal ions were increased sharply and after 30 min the adsorption capacity of NiFe2O4@PNC was found 240.21 and 219.92 mg/g and the equilibrium was occurring after 60, min for both the Cs(II) and Sr(II) ions. To find out the optimum temperature for the adsorption of both the metal ions Cs(I) and Sr(II) ions, the experiments were carried out at 25, 35, 40, 45 and 50 °C. and the adsorption capacities were found to be 232.12, 230.4, 227.5 and 225.21 mg/g for Cs(I), while in the case of Sr(II) were found to be 212.5, 211.6, 207, 205.2 and 200.1 respectively as illustrated in Fig. 6(d). These outcomes revealed that the adsorption of both metal ions were decreased with increasing the temperature of the solution.
3.1.1 Adsorption kinetics and isotherm
The role of the adoption time with the interaction between the metal ions and the NiFe2O4@PNC, and the adsorption kinetics determine the rate at which the adsorption occurs. The kinetic results and equilibrium adsorption of both the metal ions are showed in Fig. 7(a) and 7(b), and the parameters were concise in Table 1.
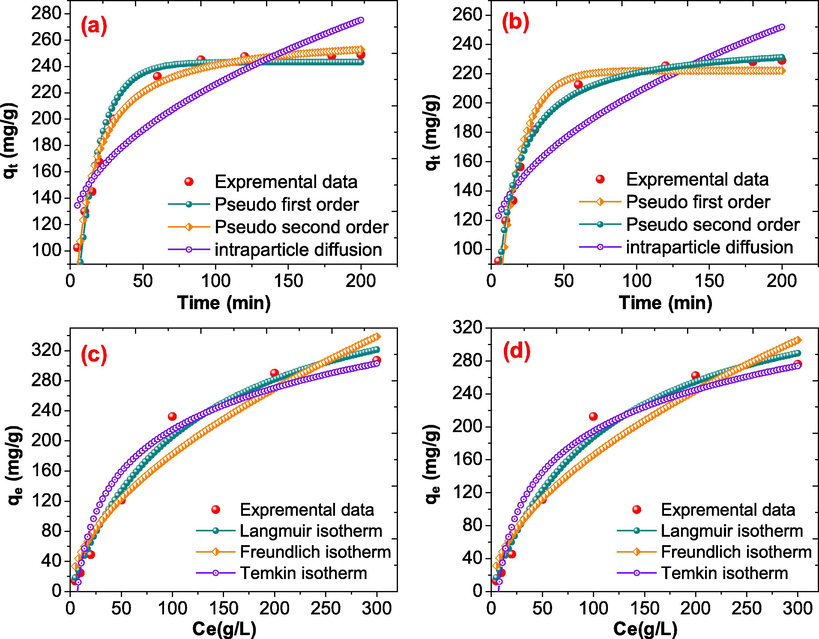
- (a) Adsorption kinetics of Cs(I) (b) Adsorption kinetics of Sr(II) (c) Adsorption isotherms for the adsorption of Cs(I) (d) Adsorption isotherms for the adsorption of Sr(II) on NiFe2O4@PNC.
| Metal Ions | Kinetic models | Parameters | |
|---|---|---|---|
| Cs(I) | PFO model | qe (mg·g−1) | 243.20 |
| k1 (min−1) | 0.6758 | ||
| R2 | 0.9329 | ||
| PSO model | qe (mg·g−1) | 265.81 | |
| k2 (g·mg−1·min−1) | 3.64x10-4 | ||
| R2 | 0.9914 | ||
| Intra-particle diffusion | C | 10.8 | |
| Kdif (mg g−1 min−1/2) | 11.81 | ||
| R2 | 0.8232 | ||
| Cd(II) | PFO model | qe (mg·g−1) | 222.10 |
| k1 (min−1) | 0.0683 | ||
| R2 | 0.9387 | ||
| PSO model | qe (mg·g−1) | 242.95 | |
| k2 (g·mg−1·min−1) | 4.01X10-4 | ||
| R2 | 0.9854 | ||
| Intra-particle diffusion | C | 9.87 | |
| Kdif (mg g−1 min−1/2) | 1083 | ||
| R2 | 0.8432 | ||
The kinetics parameters were determine using nonlinear model of pseudo-first-order (PFO), pseudo-second-order (PSO) and intra-particle diffusion model (IPD) at room temperature. Details of these models are given in supplementary material. It was seen that the adsorption data of both the metal ions were fitted well with and follow the PSO reaction kinetics model. This is because in the case of pseudo-second-order model the correlation coefficient (R2) was found to be 0.9914 while it was found 0.9329 and 0.8232 for pseudo-first-order, and Intra-particle respectively during the adsorption of Cs(I) ions. While in the case of Sr(II) the R2 was found to be 0.9854 while it was found 0.9387 and 0.8432 for PSO, PFO and Intra-particle respectively. These outcomes revealed that the pseudo-second-order kinetic model support the chemisorption process for the adsorption of Cs(I) and Sr(II).
The adsorption isotherm supports the find out the interaction between the metal ions and the NiFe2O4@PNC at the equilibrium concentration of the Cs(I) and Sr(II) ions and the amount of adsorbate. Adsorption isotherm was carried out using the Langmuir, Freundlich, and Temkin isotherm model. Their details are given in supplementary material. The adsorption isotherm parameters for Langmuir, Freundlich, and Temkin isotherm model are summarized in Table 2. The nonlinear fitting of the experimental data is illustrated Fig. 7(a) and 7(b), it was noticed that the higher correlation coefficient (R2) of Langmuir isotherm was fitted well batter then that of the Freundlich, and Temkin models and their value was found to be 0.9854, 0.9306, and 0.9365 respectively during the adsorption of Cs(I). While with Sr(II), the value of R2 was found to be 0.9792 in Langmuir isotherm model. These outcomes indicate that the adsorption of both the metal ions were carried out via monolayer homogeneous adsorption and the maximum adsorption capacity were found to be 242.21, and 214.23 mg/g, with the Cs(I) and Sr(II) respectively and the comparison with other adsorbents is summarised supporting table S-1.
| Metal Ions | Isotherm models | Parameters | |
|---|---|---|---|
| Cs(I) | Langmuir model | qm (mg·g−1) | 242.21 |
| KL (L·mg−1) | 0.0085 | ||
| R2 | 0.9854, | ||
| Freundlich model | Kf (mg1−1/n·L1/n·g−1) | 13.57 | |
| n | 1.77 | ||
| R2 | 0.9306, | ||
| Tamkin model | Kt (L/gm) | 0.1462 | |
| Bt | 80.08 | ||
| R2 | 0.9365 | ||
| Sr (II) | Langmuir model | qm (mg·g−1) | 214.23 |
| KL (L·mg−1) | 0.0089 | ||
| R2 | 0.9792 | ||
| Freundlich model | Kf (mg1−1/n·L1/n·g−1) | 17.76 | |
| n | 1.796 | ||
| R2 | 0.9551 | ||
| Tamkin model | Kt (L/gm) | 0.148 | |
| Bt | 72.24 | ||
| R2 | 0.9591 | ||
3.1.2 Adsorption thermodynamics
To determine the effect of temperature during the adsorption of the Cs(I) and Sr(II) on NiFe2O4@PNC the temperature was changed from 25 to 50 under optimum condition. The results revealed that the adsorption capacities of NiFe2O4@PNC were as the temperature was increased. To further determine the thermodynamics parameters such as entropy change (ΔS), free energy change (ΔG) and enthalpy change (ΔH), Van’t Hoff equation has been used as the details are given in supporting information. The value of the ΔS, ΔH were calculated with the liner fitting of Van’t Hoff as illustrated in figure-8(a). It was noticed that the positive values of the ΔS and ΔH support that the adsorption of both the metal ions were endothermic and the positive value of ΔG support the spontaneous adsorption.
3.1.3 Regeneration of NiFe2O4@PNC
Regeneration of adsorbents is an important parameter from application point of view. Thus, the regeneration was carried out with six cycles and NiFe2O4@PNC was washed after each adsorption cycles using dil HCl and deionised to use for next cycles to economize the production cost (Asgari et al., 2020; Mohammadabadi and Javanbakht, 2020). As shown in Fig. 8(b), it was noticed that the adsorption capacity of the NiFe2O4@PNC was found to be 94.1% and 92.0 % after six cycles, which suggests that as-fabricated NiFe2O4@PNC is an excellent cost-effective adsorbent for the adsorption of radioactive Cs(I) and Sr(II) form polluted aqueous solution.
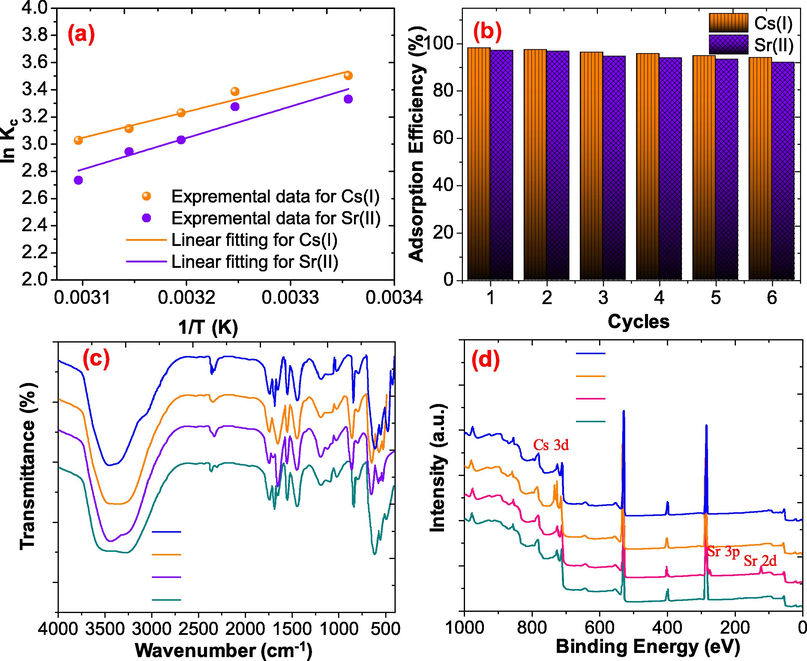
- (a) adsorption thermodynamic of Cs(I) and Sr(II) over NiFe2O4@PNC (d) regeneration behaviour of NiFe2O4@PNC (c) FTIR spectra of NiFe2O4@PNC after adsorption and desorption (d) XPS analysis of NiFe2O4@PNC after adsorption and desorption.
3.1.4 Adsorption mechanism
To determine the adsorption mechanism for the adsorption of Cs(I) and Sr(II) onto NiFe2O4@PNC FTIR and XPS were used. As shown in Fig. 8(c), the FTIR spectra after the adsorbent of Cs(I) and Sr(II) were compared with the spectra of fresh NiFe2O4@PNC and used NiFe2O4@PNC after 2 cycles. It was noticed that after the adsorption of Cs(I) and Sr(II), the FTIR band at 1687 cm−1 was shifted to lower frequency and observed at 1644 and 1641 cm−1 respectively, while after the desorption of the metal ions this peaks reached on its original position. These results support the coordination of metal ions with the C=N groups. The adsorption of Cs(I) and Sr(II) was further determine using XPS analysis and the results were illustrated in Fig. 8(d), the wild range XPS spectra of NiFe2O4@PNC-Cs(I) and NiFe2O4@PNC-Sr(II) show the presence of Cs(I) and Sr(II) ions at binding energy about 423–737 eV and 135.5 eV respectively. Moreover, the XPS spectrum of adsorbent NiFe2O4@PNC after 2 cycles shown that there is no Cs(I) and Sr(II) elements are not presence on its surface and desorb completely and no change in the chemical composites of the adsorbent was notices after regeneration.
4 Conclusions
In the present study, we have prepared NiFe2O4, PNC and NiFe2O4@PNC, and all the prepared compounds were well characterised using several analytical techniques. The adsorption of Cs(I) and Sr(II) on NiFe2O4@PNC were studied and the adsorption capacity was found to 232.12 mg/g and 212.5 mg/g respectively. The adsorption kinetics and adsorptions isotherms models support the pseudo-second order and Langmuir adsorption models respectively. The regeneration was carried out with the help of dil HCl and the results revealed that the NiFe2O4@PNC retains 94.1 and 92.0 % adsorption capacity after six cycles with Cs(I) and Sr(II). The interaction between the metal and the adsorbent was discussed and the overall out comes suggest that the fabricated adsorbent can be used for effective removal of radioactive models Cs(I) and Sr(II) ions form contaminated water.
Acknowledgement
The authors extend their appreciation to the King Saud University, Saudi Arabia for funding this research work through the project number RSP-2021/29.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation of porous chitosan/carboxylated carbon nanotube composite aerogels for the efficient removal of uranium(VI) from aqueous solution. Int. J. Biol. Macromol.. 2020;160:1000-1008.
- [Google Scholar]
- Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J. Asian Ceram. Soc.. 2015;3(3):245-250.
- [Google Scholar]
- Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm. J. Environ. Radioact.. 2018;190–191:130-133.
- [Google Scholar]
- Sorption of stable and radioactive Cs(I), Sr(II), Co(II) ions on Ti–Ca–Mg phosphates. J. Radioanal. Nucl. Chem.. 2020;324(3):1115-1123.
- [Google Scholar]
- Combined use of tannic acid-type organic composite adsorbents and ozone for simultaneous removal of various kinds of radionuclides in river water. Water Res.. 2020;182
- [Google Scholar]
- Removing Sr(II) and Cs(I) from the aqueous phase using basil seed and elucidating the adsorption mechanism. Sustainability (Switzerland). 2020;12(7)
- [Google Scholar]
- Nd-BTC metal-organic framework (MOF); synthesis, characterization and investigation on its adsorption behavior toward cesium and strontium ions. Microchem. J.. 2019;150
- [Google Scholar]
- Adsorptive removal of Sr2+ and Cs+ from aqueous solution by capacitive deionization. Environ. Sci. Pollut. Res. 2020
- [Google Scholar]
- Highly selective recovery of lanthanides by using a layered vanadate with acid and radiation resistance. Angew. Chem. – Int. Ed.. 2020;59(5):1878-1883.
- [Google Scholar]
- Effective and rapid adsorption of Sr2+ ions by a hydrated pentasodium cluster templated zinc thiostannate. Inorg. Chem.. 2019;58(15):10184-10193.
- [Google Scholar]
- Analysis of degradation pathways and intermediates products for ciprofloxacin using a highly porous photocatalyst. Chem. Eng. J.. 2021;417:127969
- [Google Scholar]
- Photocatalytic degradation of bisphenol-A with g-C3N4/MoS2-PANI nanocomposite: kinetics, main active species, intermediates and pathways. J. Mol. Liq.. 2020;311:113339
- [Google Scholar]
- Fabrication of MoS2/ZnS embedded in N/S doped carbon for the photocatalytic degradation of pesticide. Mater. Lett.. 2020;263:127271
- [Google Scholar]
- Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: kinetic and thermodynamic studies. Int. J. Biol. Macromol.. 2020;147:258-267.
- [Google Scholar]
- Advanced green analytical chemistry for environmental pesticide detection. Curr. Opin. Green Sustainable Chem.. 2021;30:100488
- [Google Scholar]
- Green-monodispersed Pd-nanoparticles for improved mitigation of pathogens and environmental pollutant. Mater. Today Commun.. 2022;30:103106
- [Google Scholar]
- A flower-like ZnO–Ag2O nanocomposite for label and mediator free direct sensing of dinitrotoluene. RSC Adv.. 2020;10(46):27764-27774.
- [Google Scholar]
- Microwave-assisted assembly of Ag2O-ZnO composite nanocones for electrochemical detection of 4-Nitrophenol and assessment of their photocatalytic activity towards degradation of 4-Nitrophenol and Methylene blue dye. J. Hazard. Mater.. 2021;416:125771
- [Google Scholar]
- Highly efficient capturing and adsorption of cesium and strontium ions from aqueous solution by porous organic cage: a combined experimental and theoretical study. Appl. Surf. Sci.. 2019;471:726-732.
- [Google Scholar]
- Biomass potential of virgin and calcined tapioca (cassava starch) for the removal of Sr(II) and Cs(I) from aqueous solutions. Chem. Pharm. Bull.. 2018;66(3):295-302.
- [Google Scholar]
- Green and novel adsorbent from rice straw extracted cellulose for efficient adsorption of Hg (II) ions in an aqueous medium. Int. J. Biol. Macromol.. 2020;161:194-203.
- [Google Scholar]
- High-capacity adsorption of Cr(VI) by lignin-based composite: characterization, performance and mechanism. Int. J. Biol. Macromol.. 2020;159:839-849.
- [Google Scholar]
- A facile microwave route for fabrication of NiO/rGO hybrid sensor with efficient CO2 and acetone gas sensing performance using clad modified fiber optic method. Optik. 2021;226:165970
- [Google Scholar]
- Fabrication of highly porous N-doped mesoporous carbon using waste polyethylene terephthalate bottle-based MOF-5 for high performance supercapacitor. J. Storage Mater.. 2021;33:102125
- [Google Scholar]
- Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr. Polym.. 2016;138:229-236.
- [Google Scholar]
- Morphology-controllable synthesis of NiFe2O4 growing on graphene nanosheets as advanced electrode material for high performance supercapacitors. J. Alloy. Compd.. 2020;826:154088
- [Google Scholar]
- A novel preparation of GO/NiFe2O4/TiO2 nanorod arrays with enhanced photocatalytic activity for removing unsymmetrical dimethylhydrazine from water. Mater. Sci. Semicond. Process.. 2021;121:105448
- [Google Scholar]
- Cellulose gum and copper nanoparticles based hydrogel as antimicrobial agents against urinary tract infection (UTI) pathogens. Int. J. Biol. Macromol.. 2018;109:803-809.
- [Google Scholar]
- Enhanced photovoltaic performance of dye-sensitized solar cells based Ag2O doped BiFeO3 hetrostructures. Sol. Energy. 2021;220:758-765.
- [Google Scholar]
- Heterogeneous FeNi3/NiFe2O4 nanoparticles with modified graphene as electrocatalysts for high performance dye-sensitized solar cells. Chem. Eng. J.. 2021;405:126944
- [Google Scholar]
- Enhanced visible-light-driven photocatalytic activity for antibiotic degradation using magnetic NiFe2O4/Bi2O3 heterostructures. Chem. Eng. J.. 2014;258:301-308.
- [Google Scholar]
- A highly porous nanocomposite (Fe3O4@BFR) for the removal of toxic Cd(II) ions from aqueous environment: adsorption modelling and regeneration study. Compos. B Eng.. 2019;172:179-185.
- [Google Scholar]
- Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arabian J. Chem.. 2017;10:S1445-S1451.
- [Google Scholar]
- Development of a polymeric nanocomposite as a high performance adsorbent for Pb(II) removal from water medium: equilibrium, kinetic and antimicrobial activity. J. Hazard. Mater.. 2020;407:124816
- [Google Scholar]
- Application of the Fe3O4-chitosan nano-adsorbent for the adsorption of metronidazole from wastewater: optimization, kinetic, thermodynamic and equilibrium studies. Int. J. Biol. Macromol.. 2020;164:694-706.
- [Google Scholar]
- Lignin extraction from barley straw using ultrasound-assisted treatment method for a lignin-based biocomposite preparation with remarkable adsorption capacity for heavy metal. Int. J. Biol. Macromol.. 2020;164:1133-1148.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102036.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







