Translate this page into:
Highly functionalized dispiropyrrolidine embedded indandione hybrids as potent cholinesterase inhibitors
⁎Corresponding author. anatarajan@ksu.edu.sa (Natarajan Arumugam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
AChE interacting with spiropyrrolidines, 4h.
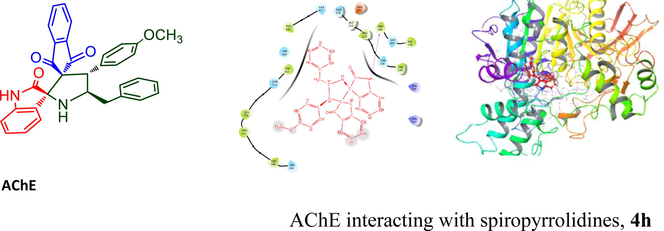
Abstract
New class of highly functionalized dispiropyrrolidine tethered indandione heterocycles in good yield were obtained through stereo- and regioselective single-pot three component cycloaddition methodology between benzylidene-indandione and non-stabilized azomethine ylide. The ylide generated from active diketone and aminophenylpropanoic acid via dehydration/decarboxylative pathway. The synthesized dispiropyrroldine comprising indandiones were elucidated through 1H and 13C NMR spectroscopic analysis, the stereo and regioselective formation of the dispiropyrrolidine were unequivocally assigned by X-ray diffraction analysis. Compound thus synthesized were assayed as potential inhibitor for the treatment of AD. Spiropyrrolidine 4h that bearing methoxy group on the phenyl ring showed significant activity, IC50 of 3.24 ± 0.25 and 10.25 ± 0.16 μM against acetyl and butyryl cholinesterase enzyme, respectively. The lead compound 4h executed to investigate for their docking simulation that showed strong binding affinity with binding site of the cholinesterase protein.
Keywords
Spiropyrrolidines
Cycloaddition reaction
Cholinestrase inhibitory activity
Docking simulation
1 Introduction
Alzheimer’s disease (AD) is most common lethal irreversible progressive neurodegenerative disease, affecting more than fifteen millions of individual of a particular age globally and expected to double every 20 years as results of an increasing life span (World Alzheimer Report, 2009). AD is characterized by personality changes, memory loss, a deterioration in language ability, inability to perform routine activities (Salloway and Correia, 2009; De la Torre, 2004; Selkoe, 1996; Selkoe, 2004), neuropsychiatric symptoms (Scheltens et al., 2016) such as depression, confusion, hallucinations, ultimately lead to death (Hardy, 2006). The pathogenesis has not been fully elucidated due to its various pathogenetic hypothesis (Talesa, 2001) such as amyloid cascade hypothesis (Hardy and Selkoe, 2002), oxidative stress hypothesis (Barnham et al., 2004), cholinergic hypotheses, metal dyshomeostasis hypothesis (Hegde et al., 2009; Bolognin et al., 2009) and multifactorial complex etiology. Among them, the hypothesis of cholinergic deficiency has been widely accepted by scientist. Ultimately, the theory recommends that AD suffers from the cognitive impairment, memory and learning are closely related to the low acetylcholine level in human brain (Francis et al., 1999; Green et al., 2009).
Acetyl and butyryl cholinesterase are present in the human brain which are the key factor in AD problems and mainly play a role in breakdown ACh, and inhibiting the proteins can escalation the effectiveness of medications and widen signals. Therefore, increasing ACh levels by the supporting AChE inhibitors is a great approach for the representative treatment of AD patients. The available medication of AD that include cholinesterase (ChE) inhibitors such as galantamine, donepezil, tacrine, rivastigmine (Yang et al., 2009), and many other drug candidates are at various stage of under development (Rampa et al., 2001). Currently available AChE inhibitor is limited by their biological action with low duration, low bioavailability, high toxic and less therapeutic effects. Therefore, an invention and evolution of new cholinesterase medications that potentially inhibit ChE is necessary for cure. Moreover, AD was reported as one of the top ten cause of death, prompting the chemist to develop new hit compounds as drug candidates.
Heterocyclic hybrids play a protuberant place in drug discovery as most drug candidates have heterocycles as the active moiety. In this context, multicomponent reactions encompassing the [3 + 2] cycloaddition reaction of in situ 1,3-dipole with an activated olefin provide a concise approach to heterocyclic hybrids in a stereospecific fashion (Arumugam et al., 2018; Arumugam et al., 2021; Almansour et al., 2020). Among them, spiropyrrolidines have a significant biological and pharmaceutical profile, since their moiety is an essential part of biologically relevant natural and synthetic compounds, which are valuable prototypes for pharmaceutical drug design. Recent past year, our research team reported structurally intriguing hybrid heterocycles comprising spiropyrrolidines employing [3 + 2] cycloaddition reaction, in which some of spirocompounds showed potent cholinesterase inhibitory activities even some of the compounds better activity than standard drugs. In this perspective, the synthesis of highly factionalized pyrrolidines is of great attention in synthetic and pharmaceutical chemistry. Our recent reported publication diplayed that spiropyrrolidines are able to inhibit both cholinestrase enzymes, and are potential candidates for Alzheimer’s treatment (Arumugam et al., 2018; Almansour et al., 2020; Arumugam et al., 2019).
In view of the remarkable biological precedents mentioned above, we anticipated that compounds containing spirooxindolopyrrolidine and indandione structural unit would have immense importance in the discovery of pharmaceutical agents. In addition, the incorporation of indandione into spiro compounds has often resulted in an enhanced in the biological potential of the compounds. Considering the above facts in mind, we herein report a facile approach to indandione integrated spirooxindolopyrrolidine via [3 + 2]-cycloaddition strategy and studied for their cholinesterase inhibitory activity against acetyl and butrylcholinestarase enzyme.
2 Materials and methods
Proton NMR were measured (s = singlet, d = doublet, t = triplet and m = multiplets) Mercury JEOL500 NMR spectrometers in deuterated chloroform using tetramethylsilane as reference. Carbon spectra were verified on 125 MHZ JEOL spectrometer. CHN calculation were analyzed on a Perkin Elmer instrument. The reaction were mornitored by thin layer chromatography (Merk precoated UV silica gel). Commercially available chemical were used without further purification. Molecular weight of component were recorded on a instrument furnished with an EIS coupled with Ultra Performance Liquid Chromatography system.
2.1 General preparation of spiropyrrolidine integrated indandione hybrids, 4a-j
A mixture of alkene 3 (1.36 mmol), amino acid 2 (245 mg, 1.49 mmol) and ketone 1 (147 mg, 1.36 mmol) was refluxed in MeOH (10 ml) for 2 h. The solvent was completely removed under vacuum and Et2O was added to crude mixture and impurities were removed using Et2O and the solvent decanted to give a cycloadduct 4 in excellent yield. The required NMR data of the compounds and spectrum have been provided in the supplementary section.
Spirocompound, 4h: 1H NMR: δH 2.89–2.92 (1H, m), 3.20–3.25 (1H, m), 3.64 (3H, s), 4.73–4.75 (1H, m), 4.92 (1H, d, J = 10.0 Hz), 6.55–6.69 (3H, m, ArH), 6.97–7.26 (11H, m, ArH), 7.52–7.63 (3H, m, ArH); 13C NMR δC 41.5, 55.1, 55.4, 63.8, 72.1, 73.3, 109.9, 113.9, 122.2, 122.7, 122.9, 125.3, 125.7, 125.9, 126.5, 128.3, 129.5, 129.9, 130.3, 135.5, 135.7, 140.9, 141.3, 142.2, 142.4, 158.8, 178.9, 197.9, 199.1; LC/MS(ESI): m/z = 514 (M+); CHN Analysis for C33H26N2O4: C, 77.03; H, 5.09; N, 5.44; Found C, 77.11; H, 5.18; N, 5.52.
2.2 Pharmacology
The spirocompounds synthesized were assessed for their ChE activity. For screening of AChE inhibition, 0.1 M Na3PO4 buffer (140 μL, pH 8) was added initially to each well of a 96-well microplate and then mixted with test samples (20 μL) and acetylcholinesterase enzyme (20 μL, 0.09 units/mL) from Electrophorus electricus Samples was incubated at 25 °C, for fifteen minutes then 10 mM DTNB (10 μL) and acetylthiocholine iodide (10 μL, 14 mM). After thirteen minutes, the absorbance of the colored final product was measured by Power Wave X 340 microplate spectrophotometer at 412 nm. Similarly, BChE assays has been done except using butyrylcholine esterase. Galantamine and compounds were made up in DMSO. Enzyme inhibition percentage was examined using below formula:
3 Results
3.1 Chemistry
The methodology for the construction of indandione integrated spirooxindolopyrrolidines is based on a intermolecular [3 + 2] cycloaddition cascade reaction protocol using benzyledine 3a-j, active ketone 1, and amino acid 2 containing a key [3 + 2]-cascade reaction process (Alaqeel et al., 2021), as described in Scheme 1. The dipolarophile 3 were synthesized based on the reported procedure (Kouzi et al., 2019) by the reaction of indandione and suitable aryl aldehyde in presence of piperidine base as shown in Scheme 1. Firstly, an intermolecular three-component cycloaddition methodology was performed under optimized conditions using dipolarophile 3, ketone 1, and compound 2 in different solvents such as EtOH, MeOH, 1,4-dioxane, dioxane: MeOH, and MeCN. The reaction of compound 1 and 2 via decarboxylative/dehydration pathway furnished the 1,3-dipole, that added regioselectivity to 3. In a representative case, a mixture of dipolarophile 3b, diketone 1, and amino acid 2 was dissolved in EtOH which was refluxed for 2 h. When all the reactants had completely disappeared, the resulting solid was filtered and then washed with Et2O to afford 4b in 67% yield. The results indicate that MeOH is suitable solvent for this cascade reaction protocol when compared to other solvents. The dispiropyrrolidines possessed four contiguous stereocenter out of four, two spiro carbons and the creation of the reaction by two carbon-carabon and one carabon-nitrogen bonds in single transformation.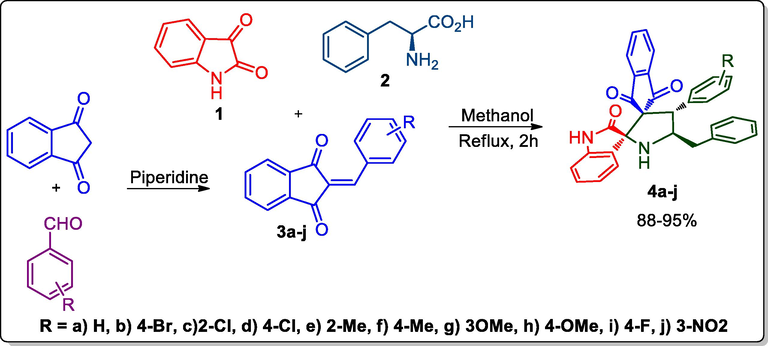
Spirooxindolopyrrolidine integrated indandiones, 4a-j.
The structure of dispiropyrrolidine heterocyles were elucitated by spectroscopic studies as described in Fig. 1A. As a representative case, compound 4b showed a two doublet of doublets at δ 2.90–2.93 and δ 3.23–3.28 ppm and was assignable to H-6 protons showing the following correlations (i) proton-COSY with multiplets at δ 4.73–4.78 was ascribable to pyrrolidine (H-5) proton (ii) HMBCs (Fig. 1B) with C-4 and C-5 at δ 55.0 and 63.7, respectively. Similarly, the H-5 proton shows (i) proton-COSY with doublet at δ 4.95 (J = 9.5 Hz) was due to benzyl (H-4) proton as evidenced by the formation of regioisomer 4b (ii) HMBCs with C-4 at δ 55.0 ppm. H-4 proton shows (i) HMBCs with spirocarbons (C-2, C-3), oxindole carbonyl carbon (C-2′) and indandione carbonyl carbons (C-1′′, and C-3′′) at 73.6, 71.8, 197.5, 198.8 and 179.0 ppm, respectively. Finally, the stereo and regiochemistry of dispiropyrrolidines 4c (Fig. 2A) and 4e (Fig. 2B) were clearly determined through single crystal XRD studies (CCDC 2158502 and 2158503).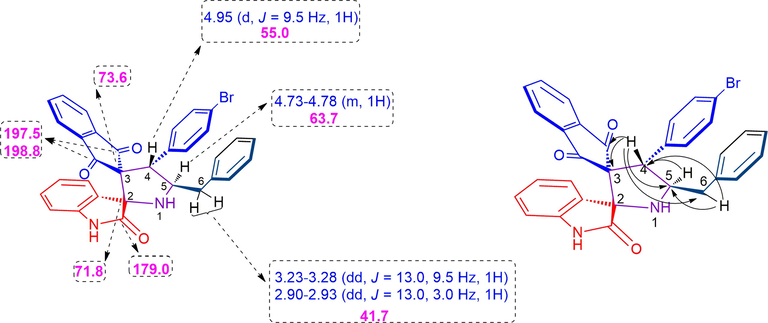
(A) Chemical shift of 4b (B) HMBC correlation of 4b.
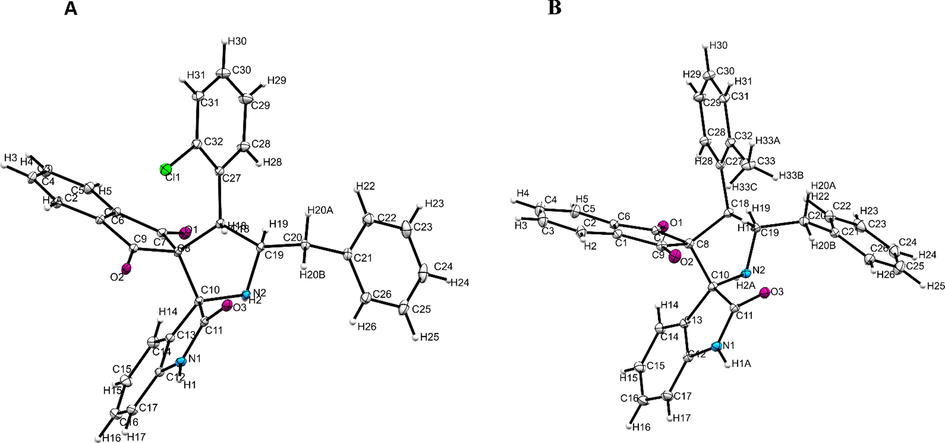
ORTEP of 4c (A) and 4e (B).
A likely mechanism justifying the generation of the final compund 4 is described in Scheme 2. Firstly, non-stabilized ylide 8 derived in situ from ketone 1, and amino acid 2 by the spontaneous dehydration and decarboxylation via intermediate 6 and 7. Accordingly, the ylide 8 with dipolarophile 3, would be generated via route A or B. The obtained regioselectivity is obvious from the regioisomeric product of 4 (route A), that 5 was not obtained. It is known that the carbon–carbon double bond is polarized with β-carbon in 3 that preferentially reacts with the electron-rich carbon of the ylide 8 to furnish 4. The creation of product through two carbon–carbon and one carbon–nitrogen bonds via a simple synthetic operation which creates up to four stereogenic center out of four, two are spiro carbons.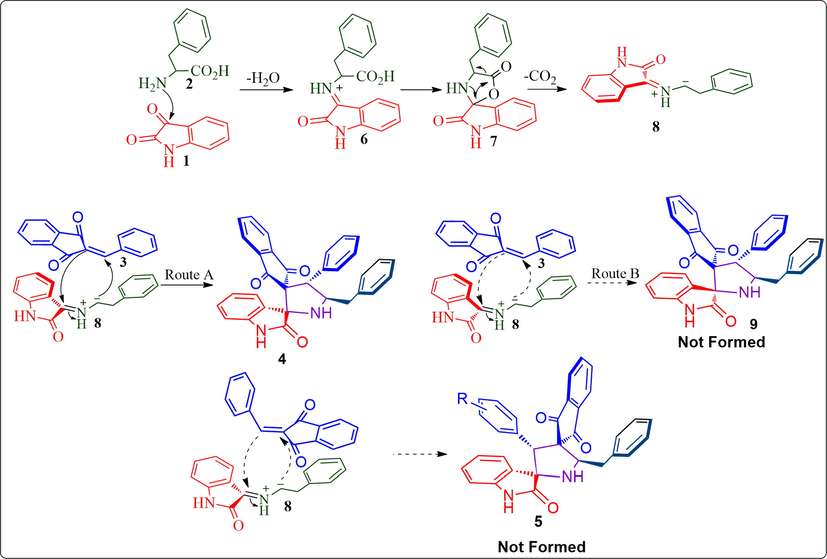
A plausible mechanism for the synthesis of dispirocompounds 4.
4 Discussion
4.1 Cholinesterase inhibitory activities
According to the cholinergic hypothesis, the cholinergic systems of the hippocampus and forebrain of these patients are harshly impaired, causing in a lower concentration of the ACh neurotransmitter in the synaptic clefts, ultimately leading to more memory loss and intellectual impairment. Both cholinestrase are answerable for the ordinance and interruption of acetylcholine in the body and widely examined as a main target for AD treatments AChE plays an important role in breakdown the transmission of nerve impulses from a nerve cell to postsynaptic membranes. During the inhibition of the ACHE enzyme, the concentration of acetylcholine was increased as the results improve memory impairment and cognitive dysfunction. It is pertinent to note that the important clinical method to treating AD is the use of ACh inhibitors viz. donepezil, that improve ACh level. Thus, new small molecules that can defeat AChE activity may play essential role with standard drug, donepezil as capable AD therapeutics. Furthermore, the role of BChE is unknown; this non-specific cholinesterase enzyme is thought to protects AChE by hydrolyzing harmful toxins that can damage or disable ACh. It is important to note that multi-target drug design has emerged as an attractive therapeutic modality for AD owing to different mechanisms of action and the multifactorial progression of AD.
In this context, the above newly synthesized dispiropyrrolidine hybrid heterocycles 4a-j were screened for their cholinesterase inhibitory effect and the results are shown in Table 1. Among them, four compounds namely, 4e-h that possessing IC50 values of less than 10 μM. For AChE, all the dispiropyrrolidines displayed significant activity with IC50 3.24 ± 0.25–22.18 ± 0.19 μM. It is obvious from Table 1 that the compound 4h carrying p-methoxy group on phenyl ring showed the most strong AChE inhibitory activity (IC50 = 3.24 ± 0.25 μM) followed by compound 4g carrying 3-methoxy unit (IC50 value of 5.81 ± 0.21 μM), which are comparable activity against galantamine (IC50 2.09 ± 0.11). Similarly, compounds 4f and 4e that having 4-methyl/2-methyl substituted on the aryl ring showed better activity (IC50 = 6.85 ± 0.25 and 7.36 ± 0.15 μM), respectively. Other substituted dispiropyrrolidines 4a-d and 4i-j exhibited moderate to good activity from 12.65 ± 0.15–22.18 ± 0.19 μM. In similar way, the dispiropyrrolidines 4a-j displayed moderate to excellent BChE activity (IC50 from 10.25 ± 0.16 to 28.20 ± 0.22). Compounds 4e-h showed better butrylchonestrase inhibitory activities than galatamine (19.34 ± 0.16), whereas other dispiropyrrolidines 4a-d and 4i-j lower activity than standard drug. The most potent BChE inhibitory activity were observed for compound 4h (10.25 ± 0.16), 4g (12.02 ± 0.07) and 4f (13.80 ± 0.20) bearing methoxy and methyl substituent on aryl ring. These results indicated that the presence of electron donating groups in either ortho or para position of the phenyl ring had notable inhibitory activities.
Entry
Compound
Yields
AChE Inhibition IC50 μM (±SD)
BChE Inhibition IC50μM
(±SD)Selectivity
AChE
BChE
1
4a (H)
20.14 ± 0.26
26.42 ± 0.06
1.31
0.76
2
4b (4Br)
18.14 ± 0.11
27.12 ± 0.15
1.50
0.67
3
4c (2Cl)
16.38 ± 0.15
21.44 ± 0.17
1.31
0.76
4
4d (4Cl)
15.27 ± 0.06
20.20 ± 0.11
1.32
0.76
5
4e (2Me)
7.36 ± 0.15
18.45 ± 0.18
2.51
0.40
6
4f (4Me)
6.85 ± 0.25
13.80 ± 0.20
2.01
0.50
7
4g (3OMe)
5.81 ± 0.21
12.02 ± 0.07
2.07
0.48
8
4h (4-OMe)
3.24 ± 0.25
10.25 ± 0.16
3.16
0.32
9
4i (4F)
12.65 ± 0.15
24.22 ± 0.22
1.91
0.52
10
4j (3NO2)
22.18 ± 0.19
28.20 ± 0.22
1.27
0.79
11
Galantamine
2.09 ± 0.11
19.34 ± 0.16
9.25
0.11
4.2 Docking studies
The ligands which chosen for docking studies were well optimized and characterized to move further computational docking studies. The proposed molecule named 4h structure were drown and 3D structural optimization was performed. The ligand 4h were prepared and initiated to computationally interact with two protein PDB ID of 2WIJ(butyrylcholinesterase) and 4EY6(Acetylcholinesterase) proteins with defined active binding grid covered amino acids in the structural domains. Schrodinger software 12 Glide modules were used to perform the SP and XP docking process (vide supplementary data, Table 2). Further regular computational flexible docking optimization method of glide tool will execute to identify the ligand binding flexible pose on macromolecule of two proteins. The predicted XP or G score were assinged in kcal/mol and in addition, protein–ligand hydrophobic interactions, interaction energies, hydrogen bonds, internal energy, π-π stacking interactions, and RMSD and many parameters to confirm the molecular interaction. Glide module of XP used to reduce the false positive interaction and to provide the best correlation to denote the score and pose. The theoretical characterization of molecular docking with the selective ligand with two different proteins were interreacted with different allosteric binding site in both the proteins. Amino acid interaction was well defined with the distance of 4 Amstrong. To characterize and prove the molecular interaction and scaling few very important parametric scores were considered for the analysis. Based on the computational docking Glide score 2WIJ protein has shown best interaction with ligand with Gscore of −6.292, Followed by another protein 4EY6 with ligand had interaction with Gscore of −5.459. Both protein molecule had hydrogen bonding interaction with respective amino acids of 2WIJ (ASP 375),4EY6(PRO368) and HIS 372 had pi-pi stacking interaction with respective ligand. The computational docking studies reveals that to confirm depends on the ligands electron donating and acceptor from the leads it holds better interaction (Figs. 3-6) to have good ligand interaction to show changes in the biological activity (Premnath et al., 2016; Premnath et al., 2021).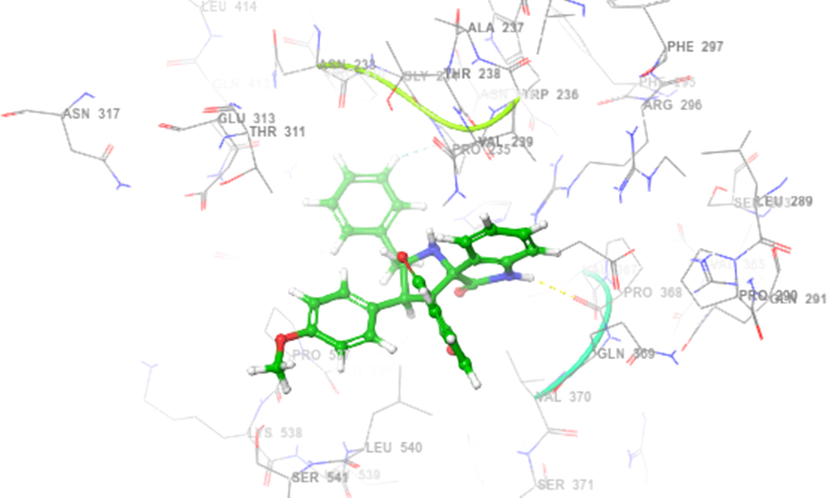
Interaction in spiropyrrolidines 4h with active site of human AChE.
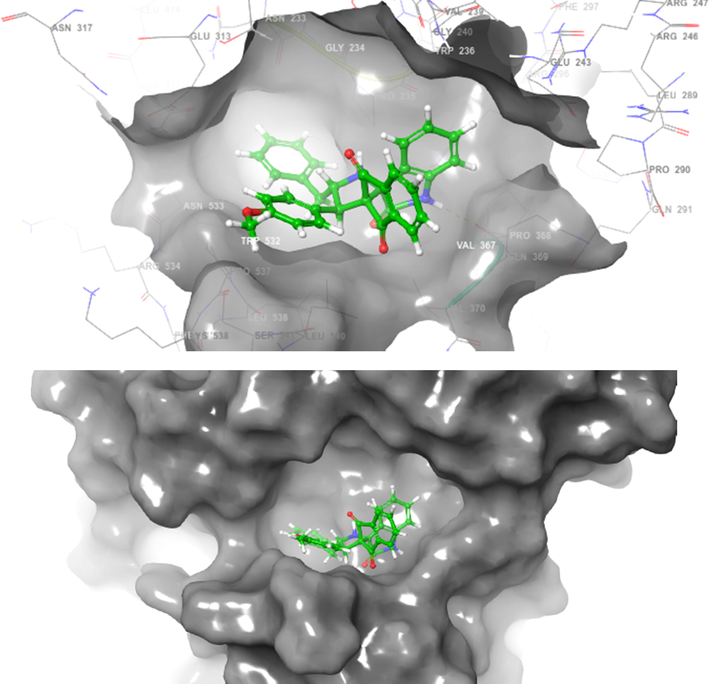
Spiropyrrolidines 4h docked with hAChE (surface view).
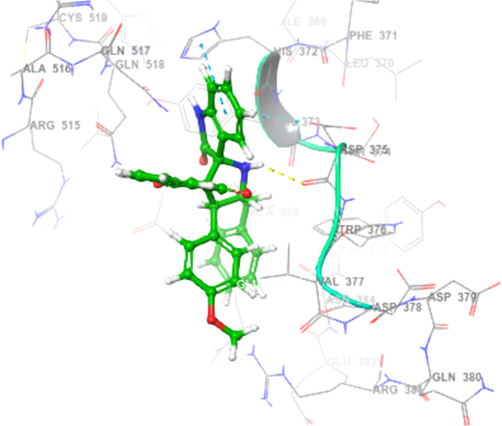
Spiropyrrolidines, 4h interaction in active site of human BChE.
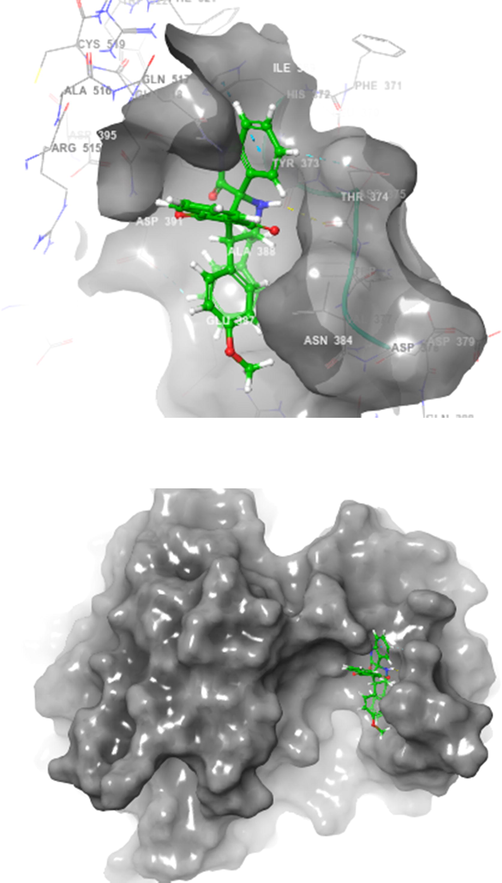
Spiropyrrolidine 4h docked with binding pocket of hBChE (Surface view).
5 Conclusion
A series of dispiropyrrolidine integrated indandione hybrids were synthesized in good to excellent yields employing stereo- and regioselective three component cascade cycloaddition protocol. The synthesized compounds were screened for their chlonestrase inhibitory activities. Among them compound 4h that bearing methoxy on the phenyl ring displayed the most potent activity with an IC50 of 3.24 ± 0.25 and 10.25 ± 0.16 μM against acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE) enzymes, respectively which are comparable activity against reference standard, galantamine (2.09 ± 0.11 (AChE) and 19.34 ± 0.16 (BChE). The results indicated that electron withdrawing substituents on the aryl ring of this particular dispiropyrrolidines displayed good to potent cholinesterase inhibitory activity. The potent dispiropyrrolidines were also studied for their molecular interaction with the active site of AChE and BChE enzyme to reveal their binding template to the cholinesterase enzyme, which strongly correlated with the in vitro results.
Acknowledgement
The project was funded by Researchers Supporting Project Number (RSP2023R143), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial activities of novel class of dispirooxindolopyrrolidine grafted indanedione hybrid heterocycles against carbapenemase producing Klebsiella pneumoniae (CKP) J. Infect. Public Health. 2021;14:1870-1874.
- [Google Scholar]
- Broad spectrum antimicrobial activity of dispirooxindolopyrrolidine fused acenaphthenone heterocyclic hybrid against healthcare associated microbial pathogens (HAMPs) J. Infec. Public Health. 2020;13:2001-2008.
- [Google Scholar]
- Design, synthesis and cholinesterase inhibitory activity of novel spiropyrrolidine tethered imidazole heterocyclic hybrids. Bioorg. Med. Chem. Lett.. 2020;30(2) Alzheimer’s Disease International World Alzheimer Report 2009; Alzheimer ’s disease International: London
- [Google Scholar]
- Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron. 2018;74:5358-5366.
- [Google Scholar]
- Spiropyrrolidine/spiroindolizino [6, 7-b] indole heterocyclic hybrids: stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study. Bioorg. Chem.. 2018;79:64.
- [Google Scholar]
- Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem.. 2019;27:2621.
- [Google Scholar]
- A stereo, regioselective synthesis and discovery of antimycobaterium tuberculosis activity of novel β-lactam grafted spirooxindolopyrrolidine hybrid heterocycles. Arab. J. Chem.. 2021;14:102938
- [Google Scholar]
- Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov.. 2004;3:205-214.
- [Google Scholar]
- Crystallographic data (including structure factors) for the compounds 4c and 4e in this article have been deposited with the Cambridge Crystallographic Data Centreas supplementary publication number CCDC 2158502 and 2158503. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, CambridgeCB2 1EZ, UK.
- Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol.. 2004;3:184.
- [Google Scholar]
- The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66:137-147.
- [Google Scholar]
- hChAT: A tool for the chemoenzymatic generation of potential acetyl/butyrylcholinesterase inhibitors. Chembiochem.. 2009;10:2191-2194.
- [Google Scholar]
- Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J. Alzheimer's Dis.. 2006;9:151-153.
- [Google Scholar]
- The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353-356.
- [Google Scholar]
- Challenges associated with metal chelation therapy in Alzheimer's disease. J. Alzheimer's Dis.. 2009;17:457-468.
- [Google Scholar]
- 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules. 2019;24:4411.
- [Google Scholar]
- Computational studies on T2Rs Agonist based anti-Covid-19 drug design. Front. Mol. Biosci.. 2021;8:690.
- [Google Scholar]
- Synthesis and spectroscopic characterization of fluorescent 4-aminoantipyrine analogues: Molecular docking and in vitro cytotoxicity studies. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2016;153:118-123.
- [Google Scholar]
- Acetylcholinesterase inhibitors: SAR and kinetic studies on omega-[N-methyl-N-(3- alkylcarbamoyloxyphenyl)methyl]aminoalkoxyaryl derivatives. J. Med. Chem.. 2001;44:3810-3820.
- [Google Scholar]
- Alzheimer disease: time to improve its diagnosis and treatment. Cleve. Clin. J. Med.. 2009;76:49.
- [Google Scholar]
- Amyloid beta-protein and the genetics of Alzheimer's disease. J. Biol. Chem.. 1996;271:18295.
- [Google Scholar]
- Alzheimer disease: mechanistic understanding predicts novel therapies. Ann. Intern. Med.. 2004;140:627.
- [Google Scholar]
- Acetylcholinesterase in Alzheimer's disease. Mech. Ageing Dev.. 2001;122:1961-1969.
- [Google Scholar]
- Modified TLC bioautographic method for screening acetylcholinesterase inhibitors from plant extracts. J. Sep. Sci.. 2009;32:3257-3259.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102706.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







