Translate this page into:
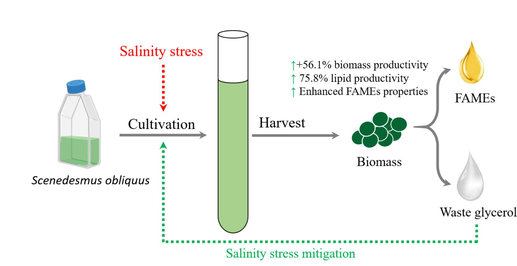
Harnessing waste glycerol to mitigate salinity constraints in freshwater microalgae cultivation for enhanced biodiesel recovery
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The current study investigated the synergistic effect of waste glycerol and salinity on Scenedesmus obliquus cultivation for enhanced biomass and biodiesel production. Optimal glycerol concentration was identified at 0.08 M supplementation, resulting in 23.1 % significant increase in biomass productivity. However, higher glycerol concentrations resulted in growth retardation. Salinity of +200 % NaCl showed positive impact on the growth, with the highest recorded dry weight of 1.76 g/L and biomass productivity of 0.206 g/L.day. However, further increases in salinity resulted in 53.4 % reduction in biomass yield at +800 % NaCl compared to +200 % NaCl. The combined treatment of the optimum glycerol concentration at different salinities (Glyc + Salin) showed superior growth performance up to +600 % NaCl, which confirmed mitigation of salinity inhibitory effects. Glyc + Salin exhibited the highest recorded nitrogen and phosphorus removal efficiencies (98.1 % and 96.6 %, respectively at day 8), dry weight (2.02 g/L), and biomass productivity (0.231 g/L.day). Notably, lipid content increased to 240.4 mg/g dw, and lipid productivity reached 56.0 mg/L.day, representing 76.1 % improvement over the control. Biodiesel characteristics, including cetane numbers and iodine values, showed also improvement, confirming the potential of Glyc + Salin for sustainable microalgal cultivation and biodiesel production.
Keywords
Green energy
Desalination
Waste recycling
Circular economy
Sustainability
1 Introduction
Petroleum currently serves as the primary global energy source, which was naturally derived from the remains of prehistoric animals and plants formed over thousands centuries. By 2030, it is anticipated that developing countries will experience increased energy consumption attributed to their growing populations and economic expansion, which is projected to contribute to the global oil consumption reaching approximately 17 million BTe (Abomohra et al., 2020b). Reliance on fossil fuels is a major contributor to excessive carbon dioxide emissions, with the transportation sector alone responsible for approximately 20 % of the global emissions. The adverse environmental impacts of fossil fuels, coupled with energy shortage due to depletion of the available petroleum reservoirs, have drawn increasing attention towards exploring alternative sustainable energy resources. Using edible oils for biodiesel raises the concerns about resource competition with human food and food-versus-fuel debate (Arshad et al., 2023). To address these issues, microorganisms capable of accumulating single-cell oils (SCO), i.e. those with lipid content exceeding 20 %dw, have gained attention as biodiesel feedstock.
Photosynthetic microalgae have been recognized as a versatile resource with various industrial applications including high-value food additives, pharmaceuticals, nanotechnology, aquaculture, wastewater treatment, micro-/nanoplastic removal, and biofuel production (Garrido-Romero et al., 2024). In addition, integrating microalgae-based biofuel production with wastewater treatment and pollutants removal could offer a cost-efficient method for cultivating microalgae (Abomohra and Hanelt, 2022). Nevertheless, the high cost of microalgae cultivation constitutes a significant portion of high biodiesel expenses, with nutrient additives and water being the key influencing factors (Leong et al., 2021). Hence, there is a pressing need to explore alternative cost-effective growth systems (Dasan et al., 2020), microalgae harvest (Suparmaniam et al., 2020), as well as growth media for competent microalgae cultivation (Beyer et al., 2023). In this context, lipid accumulation within the microalgal biomass can be induced under various stress conditions, including nutrient deficiency and high salinity (Abomohra et al., 2016b). For example, nitrogen shortage in the medium leads to lipid accumulation in the starved cells, but it was reported to simultaneously inhibit cellular growth, which results in low lipid productivity (Krzemińska et al., 2023).
Previous screening studies confirmed the higher growth rate of freshwater microalgae than those isolated from marine water (Abomohra et al., 2017, 2013). Numerous studies have confirmed lipid accumulation under salinity stress in freshwater microalgae such as Chlorella pyrenoidosa, Desmodesmus abundans, Chlamydomonas reinhardtii, and Scenedesmus obliquus. In addition, utilizing seawater for microalgae cultivation can be, therefore, developed as an economically-feasible technology for large-scale energy recovery from microalgae (Almutairi, 2022a). Salinity stress was reported to trigger specific mechanisms in microalgae to adapt, including osmo-protecting solutes accumulation, ion exchange regulation, antioxidants production, and shifting from active cellular division into lipids storage (Almutairi, 2022b). Thus, supplementation of osmo-protecting compounds to the growth medium can enhance the salinity tolerance of the cultivated freshwater microalga.
Glycerol is recognized as a significant osmolyte that accumulates in microbial cells, and can be also used as a carbon source. In addition, glycerol can promote lipid biosynthesis as a precursor in lipid anabolism. In this context, biodiesel production process from lipid conversion generates glycerol as a main by-product, constituting approximately 10 %, w/w (Yang et al., 2012). The increased production of biodiesel has led to a surplus of waste glycerol, estimated at about 4 billion gallons annually (Li et al., 2022). Therefore, recycling of waste glycerol for microbial growth might enhance the growth and lipid accumulation at elevated salinity, making biodiesel production more sustainable. This approach not only benefits the industry by enhancing efficiency and reducing waste, but also contributes to societal well-being by creating employment opportunities. The importance of such approach lies in its potential to foster sustainable practices in the biofuel industry while simultaneously supporting economic growth and job creation in the community.
There is a scarcity of research on the osmolytic impact of glycerol on freshwater microalgae subjected to salinity stress. Among freshwater microalgae, S. obliquus, also known as Tetradesmus obliquus, has emerged as a promising candidate for biodiesel production (Garrido-Romero et al., 2024). In a study by Abomohra et al. (2013), 13 freshwater microalgae were screened for fatty acid methyl ester (FAMEs) production, and S. obliquus Kützing SAG276-10 was identified as the most promising species for large-scale production due to its high biomass production contributed to elevated lipid and FAMEs productivity. Therefore, the present work aims to optimize the growth medium of S. obliquus with elevated salinity using glycerol to maximize the growth and lipid production. The optimum concertation of waste glycerol for enhanced biomass yield was identified, then it was supplemented as osmolyte at different salinities. The optimum combination of salinity and waste glycerol was applied, then lipid production and fatty acid profile of the produced FAMEs were analyzed.
2 Materials and methods
2.1 Organism and cultivation
S. obliquus (Kützing SAG276-10) was maintained in axenic culture by batch cultivation in 50 mL KC medium (Kessler and Czygan, 1970) with chemical composition shown in Table S1 (Supplementary data), using 100-mL filter-caped flasks. Experiments were conducted in glass tubular reactors using KC medium as described in the previous study (Almutairi et al., 2021). All cultures under different treatments (Section 2.2) were incubated for 14 days at 25 ± 1 °C at 100 μmol photons m−2 s−1 irradiance and 16:8h light/dark cycle according to Abomohra et al. (2013). To facilitate the mixing and provide aeration, sterile-filtered air was introduced at the reactor’s bottom through a thin glass aeration tube.
2.2 Treatments
Waste glycerol was obtained from waste cooking oil conversion into biodiesel and added to the medium at a certain concentration after pretreatment (Abomohra et al., 2018). The pretreatment aimed to remove the free fatty acids and soap which could influence the growth (Pyle et al., 2008). To evaluate the impact of waste glycerol on cellular growth and determine the optimum concentration, it was first added to the medium at various concentrations (0.0, 0.02, 0.04, 0.06, 0.08, 0.10, and 1.2 M). In addition, salinity of the medium was adjusted by changing the NaCl concentration, where 0.47 g/L is used as a typical concentration in KC medium as a control (Table S1). Different NaCl concentrations of −100 %, +100 %, +200 %, +400 %, +600 %, and + 800 % were used to evaluate the salinity effect on the growth and biomass production. The corresponding salinity and electrical conductivity values for each NaCl treatment are represented in Table S2 (Supplementary data).
In order to evaluate the glycerol impact on the salinity effect, the optimum glycerol concentration was supplemented to the medium at different salinities. Further, the growth, nutrients removal, lipid production, and biodiesel characteristics were evaluated for the optimum combination of salinity-glycerol comparing to the control, optimum glycerol, and optimum salinity.
2.3 Growth determination
The growth of the microalgae was assessed every 2 days by tracking the changes in the optical density at 680 nm (OD680). In addition, the cellular dry weight (dw, in g/L) was determined by filtering a specific volume (5–10 mL) of the culture through a 0.45 µm pore-size filter paper, followed by drying it in an oven at 80 °C until a constant weight was achieved. Biomass productivity during exponential growth was then calculated according to the modified method of Abomohra et al. (2013) using Eq. (1) as g dw/L.day:
2.4 Nutrients removal and salinity reduction
The changes in the concentration of the main nutrients including TP and TN were monitored photometrically using test kits for phosphorus TNT845 and nitrogen HR-TNT, respectively (Hach, USA). Salinity was determined by measuring the electric conductivity using conductivity meter (InLab 738-ISM, Mettler Toledo). Nutrients and salinity removal (%) were determined by Eq. (2). In addition, biomass potential to remove the measured parameters was calculated using Eq. (3);
2.5 Lipids and FAMEs analysis
For lipid extraction, a modified protocol based on Bligh and Dyer (1959) was employed to 20–30 mL of the culture as previously described (Almutairi et al., 2021). Cells were harvested by centrifugation at 3000g for 10 min. The resulting cell pellet was resuspended in 20 mL of chloroform/methanol mixture (2:1, v/v) and agitated on a shaker at 150 rpm for 2 h. Post-shaking, 4 mL of 0.9 % NaCl solution were added to the mixture and thoroughly vortexed. Following a gentle centrifugation at 200g for 2 min to separate the phases, the lower chloroform phase was siphoned and placed in a pre-weighed aluminum cup. After solvent evaporation, lipid content was quantified gravimetrically and calculated as a percentage of the dry weight (dw%). Lipid productivity during the exponential growth was then calculated as mg/L.day using Eq. (4);
For FAMEs analysis, 5 mL of the respective culture were collected at late exponential phase for lipid extraction followed by transesterification (Abomohra et al., 2016a). Before extraction, 100 µg of trinonadecanoylglycerol as internal standard was added to the cell pellet. FAMEs profile was determined using gas chromatography (GC-FID, Varian 3900, USA). The oven temperature was initially set to 175 °C for 5 min, then raised at a rate of 4 °C per minute until it reached 240 °C, where it was held for 2 min. The detector and injector temperatures were set at 260 °C and 240 °C, respectively. The essential biodiesel characteristics were determined as outlined earlier (Abomohra et al., 2020c) according to Eqs. 5–11;
2.6 Statistical analysis
All experiments were carried out in triplicates, and data are presented as the mean ± SD. Statistical analyses were conducted by SPSS (version 20, IBM) using one-way ANOVA followed by post-Hoc multiple comparison.
3 Results and discussion
3.1 Impact of glycerol
This section aimed to assess the glycerol impact on S. obliquus growth and biomass yield. It can be noted that all treatments, except 0.10 and 0.12 M glycerol, showed the start of stationary phase at day 12, where the optimal glycerol supplementation was 0.08 M (Fig. 1A). Treatment with high glycerol concentrations resulted in increasing of lag phase duration. This finding agrees with the results of Abomohra et al. (2018), where elongation of lag phase duration was attributed to the need of algal cells to acclimate with the presence of many complex compounds in waste glycerol. Cell density and cellular dry weight showed better results when glycerol concentration increased up to 0.08 M, reaching dry weight of 1.52 g/L (Fig. 1B). Therefore, treatment with 0.08 M glycerol showed the highest recorded biomass productivity of 0.181 g/L.day, which represented 23.1 % higher than the control. Enhancement of growth at relatively low levels of glycerol can be explained by glycerol utilization as additional carbon source (Li et al., 2022). However, the highest applied glycerol concentration of 0.12 M showed significant reduction in biomass productivity by 22.4 % below the control. In this context, elevated levels of waste glycerol were found to inhibit the cell cycle, primarily by promoting a shift towards lipid storage within the cells (Abomohra et al., 2018). Therefore, 0.08 M glycerol supplementation was considered as the optimal value and used in further experiments.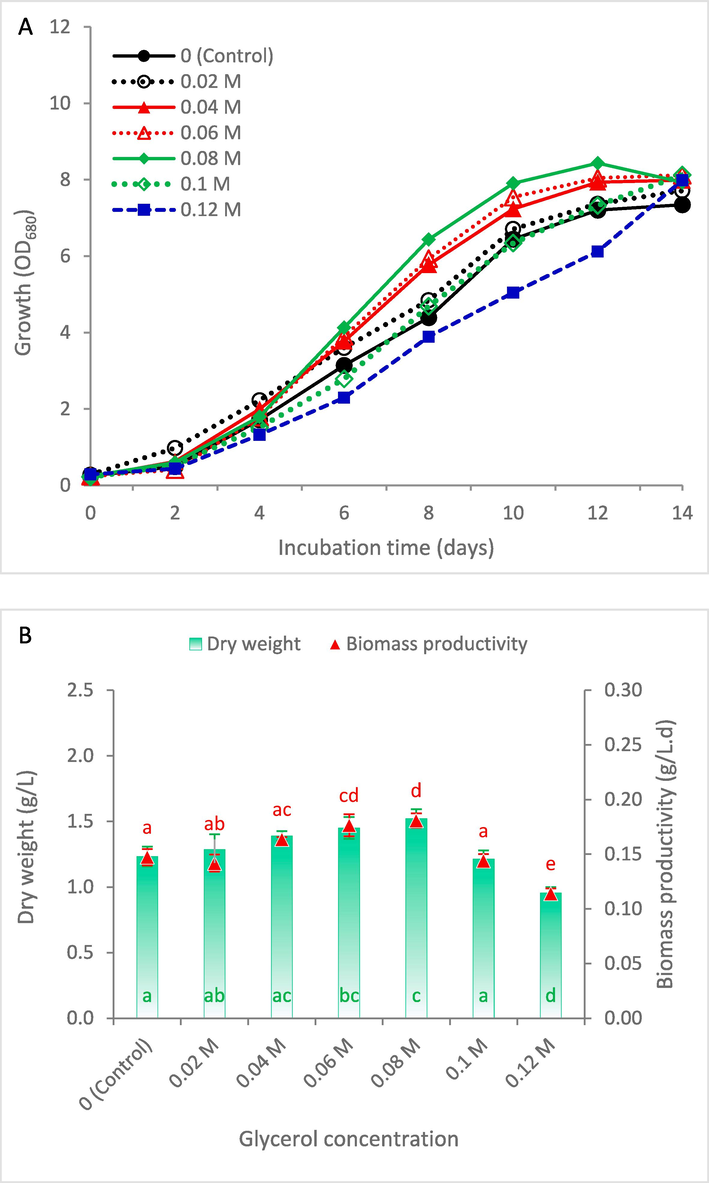
Growth curve (A) and biomass production (B) of Scenedesmus obliquus cultivated in KC medium supplemented with different concentrations of glycerol (0–0.12 M). The same letter in the same series indicates insignificant difference at P ≤ 0.05 using Tukey test.
3.2 Impact of salinity
The present results demonstrated that increasing of NaCl concentration up to + 400 % higher than the typical medium positively influenced the growth, while additional increase in NaCl concentration resulted in significant reduction in the growth (Fig. 2A). The complete elimination of NaCl from the medium showed the lowest recorded growth pattern. It can be noted from Fig. 2B that increasing of NaCl concentration by + 100 %, +200 %, and + 400 % showed dry weight and biomass productivity within the ranges of 1.63–1.76 g/L and 0.191–0.206 g/L.day, respectively, which showed insignificant differences between each other. However, increasing of NaCl concentration by + 600 % and + 800 % resulted in significant reduction in the biomass yield by 32.2 % and 50.3 %, respectively, compared to that at + 400 % NaCl. The increase in microalgal growth at higher salinity can be attributed to several factors including osmotic balance, stress-induced productivity, ion homeostasis, and the regulation of genes responding to salinity stress (El-Sheekh et al., 2013; Shetty et al., 2019). Low salinity levels were reported to enhance the microalgal growth. For instance, incorporating seawater at ratios of 25 % and 50 % in the culture medium resulted in significant increase in the growth of S. obliquus (El-Sheekh et al., 2013). However, the observed reduction in the growth under high salinity might be related to reactive oxygen species accumulation or photosynthesis inhibition due to reduction of chlorophyll content (Moradi and Ismail, 2007). Based on the present results, it can be confirmed that S. obliquus prefers higher salinity levels up to + 400 % NaCl more than that of the typical KC medium (470 mg/L), while levels above + 400 % NaCl result in significant growth retardation.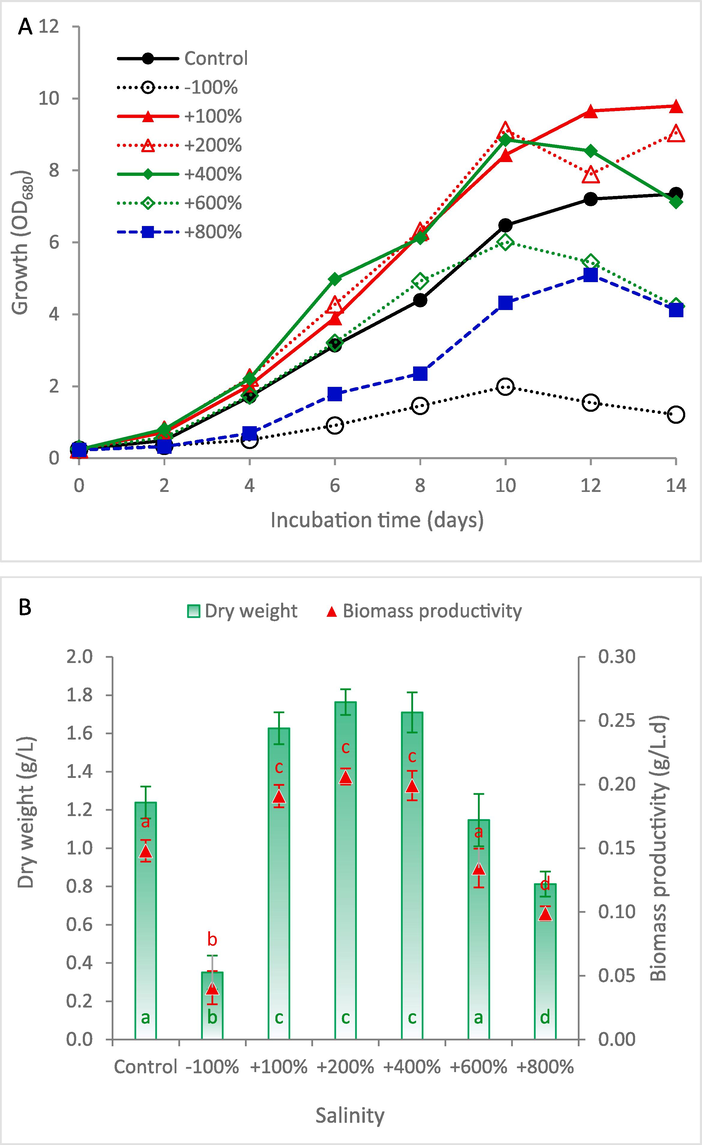
Growth curve (A) and biomass production (B) of Scenedesmus obliquus cultivated in KC medium at different salinities. NaCl concentrations (g/L) of the Control, −100 %, +100 %, +200 %, +400 %, +600 % and + 800 % were 0.47, 0, 0.94, 1.41, 1.88, 2.82, and 3.76, respectively. The same letter in the same series indicates insignificant difference at P ≤ 0.05 using Tukey test.
3.3 The role of glycerol to mitigate salinity stress
In order to evaluate the role of glycerol to alleviate the negative impact of high salinity, the optimum concentration of glycerol (0.08 M) was supplemented to the KC medium at different salinity levels (Fig. 3). It can be noted that complete elimination of NaCl from the medium results in significant reduction in growth, and glycerol can not compensate this inhibitory effect. However, glycerol supplementation enhanced the growth of S. obliquus at higher salinity levels, where all treatments up to + 800 % NaCl showed higher growth pattern compared to the control (Fig. 3A). The maximum recorded dry weight and biomass productivity were 2.02 g/L and 0.231 g/L.day at + 600 % NaCl, which represented 63.0 % and 56.1 %, respectively, over the control (Fig. 3B). This finding confirms the significant role of glycerol supplementation to enhance salinity resistance in microalgae. The pronounced decrease in biomass yield observed at + 800 % NaCl concentration highlights a crucial area for further research, suggesting that salt tolerance mechanism of S. obliquus is overwhelmed by such high salinity levels. To address this challenge, further research could include genetically modifying key stress response pathways, adopting adaptive evolutionary strategies to gradually accustom the cells to harsher saline environments, and exploring the use of additional compatible solutes to improve cellular osmotic balance.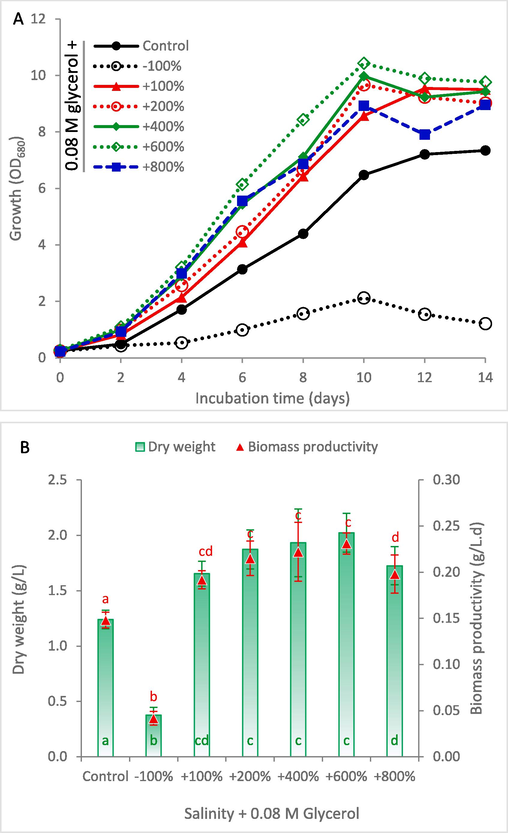
Growth curve (A) and biomass production (B) of Scenedesmus obliquus cultivated in KC medium supplemented by 0.08 M glycerol at different salinities. NaCl concentration (g/L) of the Control, −100 %, +100 %, +200 %, +400 %, +600 % and + 800 % is 0.47, 0, 0.94, 1.41, 1.88, 2.82, and 3.76, respectively. The same letter in the same series indicates insignificant difference at P ≤ 0.05 using Tukey test.
Fig. 4 compares the growth and biomass yield of S. obliquus at the three selected conditions of optimum glycerol supplementation of 0.08 M (Glyc), optimum salinity of + 200 % NaCl (Salin), and the combined effect of glycerol at highest salinity of + 600 % (Glyc + Salin) with the typical KC medium as a control. Results showed that Glyc + Salin enhanced the growth over all other treatments with shorter lag phase (Fig. 4A). In addition, it showed the highest recorded significant dry weight and biomass productivity (Fig. 4B). In this context, glycerol serves as a noteworthy example of the common and effective compatible solutes produced by many salt-sensitive algal species under high salinity stress. Glycerol is highly soluble, non-toxic, and its natural production/accumulation in the cells do not interfere with other metabolic pathways (Shetty et al., 2019). Glycerol accumulation in the cells enables the salt-stressed microalgae to restore the original cellular volume at extreme salt-stress conditions.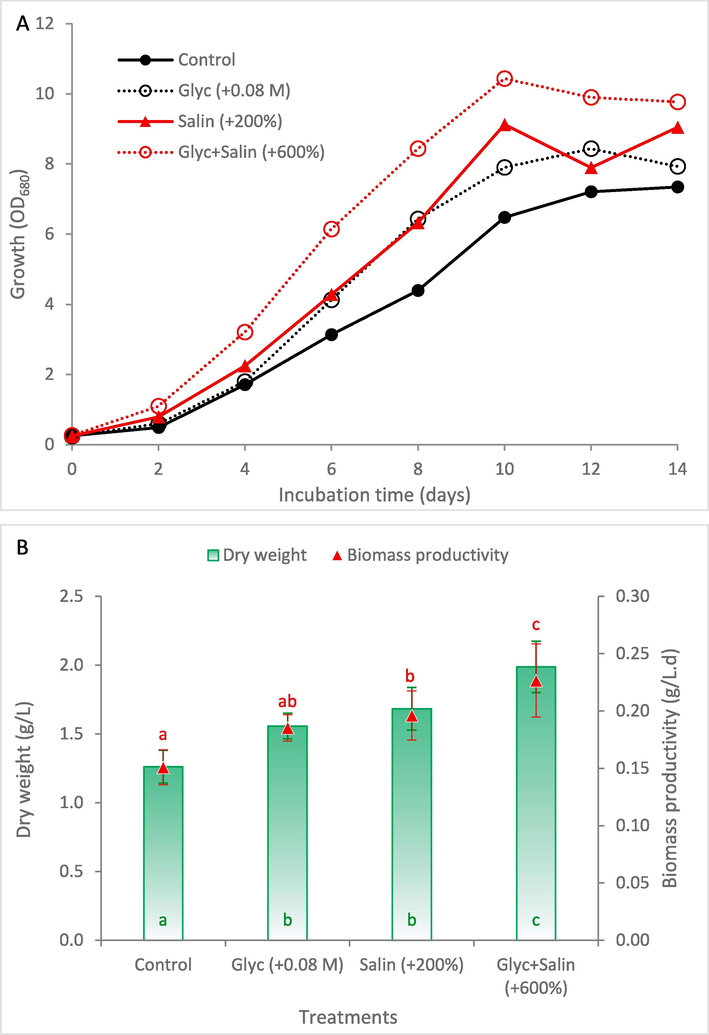
Growth curve (A) and biomass production (B) of Scenedesmus obliquus cultivated in KC medium at the optimum glycerol (Glyc), optimum salinity (Salin), and optimum combination (Glyc + Salin). NaCl concentrations (g/L) of the Control, +200 % and + 600 % were 0.47, 1.41, and 2.82, respectively. The same letter in the same series indicates insignificant difference at P ≤ 0.05 using LSD test.
3.4 Impact on nutrient removal
In general, cultures treated with Glyc + Salin exhibited higher removal efficiencies for all studied nutrients compared to the control (Fig. 5). Peaks of removal efficiencies of TN, TP, and conductivity typically occurred during the exponential phase of all cultures. For Glyc + Salin, 98.1 % removal efficiency of TN was recorded at day 8, which showed insignificant changes at day 12 (Fig. 5A). The removal efficiency of TN at day 8 using Glyc + Salin was 3.0 %, 8.4 %, and 19.3 % higher than that of Salin, Glyc, and control, respectively. At day 12, all treatments showed 100 % removal efficiency for TN, while control was only 92.6 %. Removal of TP showed similar pattern, where Glyc + Salin showed the highest removal efficiency of 96.6 % at day 8, which was 10.8 %, 5.8 %, and 17.9 % higher than that of Salin, Glyc, and control, respectively (Fig. 5B). The control showed only 92.6 % removal efficiency at the 12th day, while all other treatments showed complete phosphorus removal. Overall, the use of Glyc + Salin demonstrated higher rates of nutrient removal compared to those detected in a previous study using raw municipal wastewater (Han et al., 2016).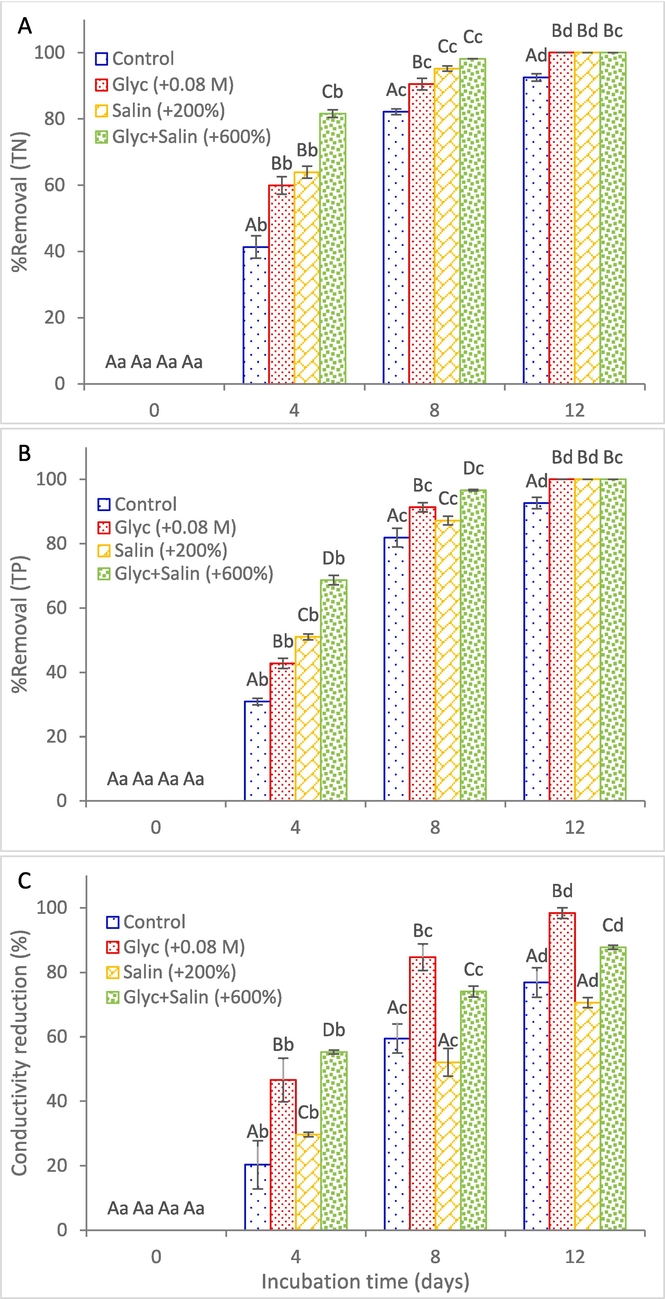
Removal efficiency of total nitrogen (A), total phosphorous (B) and reduction in conductivity (C) over incubation of Scenedesmus obliquus in KC medium at the optimum glycerol (Glyc), optimum salinity (Salin), and optimum combination (Glyc + Salin). NaCl concentrations (g/L) of the Control, +200 % and + 600 % were 0.47, 1.41, and 2.82, respectively. The same capital letter in different series at the same day, and the same small letter in the same series at different days represent insignificant differences at P ≤ 0.05 using Tukey test.
The combined application of glycerol supplementation at increased salinity potentially enhances nutrient removal efficiency through several synergistic mechanisms. As discussed earlier, glycerol acts as a compatible solute aiding in osmoregulation, which helps maintain cellular integrity and stabilize intracellular water content under saline conditions, ensuring continuous metabolic activities including nutrient uptake. This, coupled with the glycerol role as an organic carbon source, promotes the algal growth and increases the biomass production, thereby elevating the demand for TN and TP for new cellular synthesis. Moreover, physiological changes induced by the stresses might alter cell surface properties (Galluzzi et al., 2018), increasing nutrient absorption efficiency through changes in permeability and transporter protein expression. These findings confirm that combined Glyc + Salin has significant positive impact on nutrient removal that could help in wastewater treatment. In addition, the present results suggest that TN and TP are limiting factors for the growth of S. obliquus in Glyc + Salin cultures, and the growth might be further enhanced by increasing the initial TN and TP in the growth medium, which requires further validation.
Different from TN and TP, S. obliquus showed higher removal efficiency in salinity at Glyc, where the highest reduction in conductivity of 98.3 % was recorded at the 12th day of growth. It was 27.8 % higher than that recorded for the control. The higher salinity removal in Glyc might be attributed to the lower initial salinity of Glyc (which was similar to the control) and concurrent enhanced growth due to glycerol supplementation. Compared to Salin with salinity of + 200 %, the reduction of conductivity in Glyc + Salin at the 12th day was 24.2 % higher although it has higher initial salinity (+600 %), which is attributed to the higher growth rate. For all studied parameters, the highest salt removal potential of the biomass was recorded with Glyc + Salin at all days (Table 1), which is attribute to the higher initial salinity level in Glyc + Salin (+600 % NaCl) than that of Salin (+200 % NaCl). NaCl concentrations (g/L) of the Control, +200 % and + 600 % were 0.47, 1.41, and 2.82, respectively. The same capital letter in the same column for different treatments at the same day, and the same small letter in the same raw for the same measured parameter at different days represent insignificant differences at P ≤ 0.05 using Tukey test.
Treatments
TN (mg/g dw)
TP (mg/g dw)
Salinity (ms/g dw)
4 days
8 days
12 days
4 days
8 days
12 days
4 days
8 days
12 days
Control
161.7 ± 10.51Aa
87.2 ± 6.90Ab
21.1 ± 0.14Ac
186.0 ± 2.04Aa
167.5 ± 2.43Ab
33.5 ± 4.25Ac
0.743 ± 0.301Aa
0.771 ± 0.026Aa
0.327 ± 0.016Ac
Glyc (+0.08 M)
223.0 ± 2.31Ba
38.2 ± 2.42Bb
27.5 ± 6.01Ab
243.9 ± 3.28Ba
92.7 ± 2.40Bb
38.7 ± 7.07Ac
1.555 ± 0.302Ba
0.423 ± 0.009Ab
0.351 ± 0.045Ab
Salin (+200 %)
181.1 ± 0.99Ca
43.4 ± 2.43Bb
17.1 ± 3.24ABc
224.6 ± 2.28Ca
78.1 ± 1.24Cb
71.6 ± 9.63Bb
2.076 ± 0.154Ca
0.759 ± 0.132Ab
1.647 ± 0.327Bc
Glyc + Salin (+600 %)
159.8 ± 3.75 Da
18.3 ± 1.94Cb
7.3 ± 0.38Bc
207.0 ± 4.64 Da
47.7 ± 4.20Db
20.6 ± 2.28Cc
4.936 ± 0.153 Da
0.943 ± 0.015Ab
2.465 ± 0.293Cc
3.5 Lipid accumulation
Lipids are the important fraction of algal biomass for biodiesel production, which is influenced by the nutrients and conditions used in cultivation. Results showed that S. obliquus grown in KC medium showed total lipids of 215.3 mg/g dw at late exponential phase (Table 2). Application of glycerol and salinity individually enhanced the lipid accumulation to 228.6 and 229.3 mg/g dw, respectively. However, the combined effect of Glyc + Salin showed the highest recorded lipid content of 240.4 mg/g dw. Enhancement of lipid accumulation by glycerol was previously attributed to the nature of storage lipids composed of a glycerol backbone (Li et al., 2022). In addition, enhanced lipid accumulation at high salinity is attributed to the modification of intracellular lipid biosynthesis pathways under harsh conditions towards the storage of neutral lipids, rather than structural lipids biosynthesis (Abomohra et al., 2020a; Pancha et al., 2015). Therefore, Glyc + Salin resulted in combined action of glycerol and salinity stress towards lipid accumulation and showed the highest lipid content. LP refers to Lipid productivity. NaCl concentrations (g/L) of the Control, +200 % and + 600 % were 0.47, 1.41, and 2.82, respectively. The same capital letter in the same column for different fatty acid groups at the same treatment, and the same small letter for the same fatty acid group at different treatments represent insignificant differences at P ≤ 0.05 using Tukey test.
Fatty acids
Control
Glyc (+0.08 M)
Salin (+200 %)
Glyc + Salin (+600 %)
Lipids (mg/g dw)
215.3 ± 10.9a
228.6 ± 10.2ab
229.3 ± 9.2b
240.4 ± 11.6c
LP (mg/L.day)
31.86 ± 2.97a
41.43 ± 3.40b
47.40 ± 3.42b
56.00 ± 5.73c
Fatty acids (mg/g dw)
14:0
0.26 ± 0.01
0.42 ± 0.03
7.00 ± 0.93
6.56 ± 0.13
16:0
31.55 ± 2.48
33.05 ± 2.61
40.83 ± 0.49
40.13 ± 3.69
16:1n-10
3.68 ± 0.23
3.59 ± 0.82
7.48 ± 0.33
6.50 ± 0.69
16:1n-7
3.58 ± 0.16
4.45 ± 0.39
5.19 ± 0.57
8.46 ± 0.27
16:2
4.80 ± 0.08
5.32 ± 0.49
4.07 ± 0.80
5.78 ± 0.76
16:3
7.83 ± 0.19
12.22 ± 0.12
6.40 ± 0.62
4.80 ± 0.29
16:4n-3
13.07 ± 0.15
12.16 ± 1.22
10.63 ± 0.39
8.97 ± 0.86
18:0
7.70 ± 0.55
10.76 ± 1.15
14.32 ± 2.37
15.87 ± 0.46
18:1n-9
48.98 ± 3.71
53.10 ± 0.29
44.12 ± 1.81
47.76 ± 3.40
18:2n-6
25.77 ± 0.04
28.06 ± 1.55
24.33 ± 1.83
22.68 ± 0.83
18:3n-3
33.69 ± 2.78
36.73 ± 0.45
31.09 ± 0.04
30.89 ± 2.18
18:3n-6
2.00 ± 0.09
4.67 ± 0.67
1.98 ± 0.00
6.29 ± 0.22
18:4n-3
6.42 ± 0.40
8.66 ± 2.24
4.06 ± 0.38
2.17 ± 0.18
20:0
0.10 ± 0.00
3.14 ± 0.22
2.88 ± 0.41
10.56 ± 0.04
SFAs
39.61 ± 1.91Aa
47.35 ± 1.27Aa
65.03 ± 2.35Ab
73.12 ± 3.39ABb
MUFAs
56.25 ± 3.64Ba
61.14 ± 1.50Aa
56.78 ± 1.57Aa
62.72 ± 4.35Aa
PUFAs
93.57 ± 2.51Cab
107.83 ± 4.18Ba
82.56 ± 2.04Bb
81.59 ± 4.39Bb
Total FAMEs
189.43 ± 8.07 Da
216.32 ± 6.94Cb
204.38 ± 5.95Cab
217.43 ± 12.13Cb
Lipid productivity is a crucial parameter linked to the synergistic action of biomass production, lipid content, and required incubation period on the overall volumetric lipid production. The control culture showed lipid productivity of 31.86 mg/L.day (Table 2). Nevertheless, all treatments showed significant improvement in lipid productivity compared to the control. However, combined action of Glyc + Salin showed the uppermost measured lipid productivity of 56.0 mg/L.day, representing 75.8 % higher than the control. This higher lipid productivity in the Glyc + Salin treatment is attributed to the enhanced growth rate coupled with lipid accumulation in S. obliquus.
3.6 Fames profile
The growth conditions and the composition of the growth medium considerably affect the fatty acid profile of microalgae (Leong et al., 2022). Cells grown in typical KC medium showed FAMEs yield of 189.4 mg/g dw (Table 2). However, adding 0.08 M glycerol or increase of salinity to + 200 % positively influenced total FAMEs recovery, reaching 216.3 and 204.4 mg/g dw, respectively. Application of Glyc + Salin showed insignificant difference in FAMEs recovery in comparison with the individual treatments, while increased by 14.8 % higher than the control. As lipid productivity of Glyc + Salin showed 75.8 % higher than that of the control, it is favorable for FAMEs recovery from Glyc + Salin due to enhanced FAMEs productivity. Fatty acid profile revealed the existence of 14 fatty acids in all studied samples within a carbon range of C14-C20. The main fatty acids across all measured samples were 18:1n-9, 16:0, and 18:3n-3, which agrees with previous studies (Abomohra et al., 2020a). However, it was different from the dominant fatty acids recorded in microalgal consortia cultivated in wastewater; including C16:0, followed by C18:1, C18:2 and C18:3, comprising about 80–93 % of total fatty acids (Leong et al., 2020). In the control, SFAs, MUFAs, and PUFAs represented 20.9 %, 29.7 %, and 49.4 %, respectively (Table 3). NaCl concentrations (g/L) of the Control, +200 % and + 600 % were 0.47, 1.41, and 2.82, respectively.
Fatty acids
Control
Glyc (+0.08 M)
Salin (+200 %)
Glyc + Salin (+600 %)
14:0
0.14
0.19
3.44
3.02
16:0
16.64
15.26
19.99
18.43
16:1n-10
1.95
1.65
3.66
2.98
16:1n-7
1.89
2.06
2.55
3.89
16:2
2.54
2.47
1.99
2.65
16:3
4.13
5.65
3.14
2.22
16:4n-3
6.90
5.61
5.21
4.12
18:0
4.08
4.99
6.99
7.32
18:1n-9
25.83
24.56
21.58
21.95
18:2n-6
13.62
12.97
11.89
10.44
18:3n-3
17.76
16.99
15.22
14.20
18:3n-6
1.06
2.17
0.97
2.90
18:4n-3
3.40
3.99
1.98
1.01
20:0
0.05
1.45
1.41
4.87
SFAs
20.91
21.89
31.82
33.64
MUFAs
29.67
28.27
27.78
28.83
PUFAs
49.42
49.84
40.40
37.53
The main characteristics of biodiesel, in the form of FAMEs, were estimated and compared with the recommended values by international standards (Table 4). Generally, kinematic viscosity, cetane number, and specific gravity complied with the recommended values by the international standards in all treatments. Higher biodiesel cetane number refers to enhanced ignition quality, which results in more rapid and efficient combustion in diesel engine (Tien Thanh et al., 2022). The present study showed that cetane number of the control sample was 51.40, which meet the American standard (≥47) but at the marginal accepted value of the European standard (≥51). Combined application in Glyc + Salin enhanced the cetane numbers to 53.98, which is advantageous for biodiesel characteristics. In addition, FAMEs from the control and Glyc samples showed iodine values of 140.69 and 140.59 g I2 per 100 g, respectively, exceeding the values recommended by the European standard. Conversely, both Salin and the combined Glyc + Salin maintained values within the acceptable range, 118.54 and 111.96 g I2 per 100 g, respectively (Table 4). This confirms the Glyc + Salin efficacy in promoting the yield and characteristics of the biodiesel. In addition, recent research confirmed the enhanced economic feasibility and environmental sustainability of recycling byproducts from biodiesel production to enhance biomass and bioenergy recovery from microalgae (Xu et al., 2019). Overall, the suggested approach highlights the potential of integrating Glyc + Salin treatments with byproduct recycling strategies to optimize microalgal biodiesel production process, ultimately contributing to more sustainable and economically viable biofuel industries. NaCl concentrations (g/L) of the Control, +200 % and + 600 % were 0.47, 1.41, and 2.82, respectively.
Characteristics
Control
Glyc (+0.08 M)
Salin (+200 %)
Glyc + Salin (+600 %)
US
Europe
ADU
1.72
1.72
1.42
1.33
−
−
Kinematic viscosity
4.12
4.12
4.31
4.36
1.9–6.0
3.5–5.0
Specific gravity
0.88
0.88
0.88
0.88
0.85–0.9
−
Cloud point
−2.99
−2.97
0.99
2.17
−
−
Cetane number
51.40
51.41
53.39
53.98
Min 47
51–120
Iodine value
140.69
140.59
118.54
111.96
−
Max 120
HHV
41.56
41.56
41.04
40.88
−
−
Reference
This study
ASTM D6751-08
EN 14,214
4 Conclusions
The present work confirmed that 0.08 M glycerol supplementation is optimal for improved biomass productivity, while higher concentrations showed growth retardation. Salinity up to + 400 % NaCl positively influenced the growth. Interestingly, combination of 0.08 M glycerol at + 600 % NaCl (Glyc + Salin) demonstrated enhanced growth, nutrient removal, and lipid production. Glyc + Salin proved the effectiveness in mitigating the inhibitory effects of high salinity. The lipid content and productivity were also significantly increased, with favorable fatty acid profiles meeting biodiesel standards. Further exploration and optimization of these conditions in outdoor large-scale using seawater could validate the stability of the alga at the suggested conditions and participate in the development of eco-friendly and economically-viable strategies in microalgal biodiesel industry.
Disclosure of funding
Authors would like to thank the University where the work was done for funding the present work and providing resources.
CRediT authorship contribution statement
Adel W. Almutairi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Screening of marine microalgae isolated from the hypersaline Bardawil lagoon for biodiesel feedstock. Renew. Energy. 2017;101:1266-1272.
- [Google Scholar]
- Effect of lipid-free microalgal biomass and waste glycerol on growth and lipid production of Scenedesmus obliquus: Innovative waste recycling for extraordinary lipid production. Bioresour. Technol.. 2018;249:992-999.
- [Google Scholar]
- Enhancement of biodiesel yield from a halophilic green microalga isolated under extreme hypersaline conditions through stepwise salinity adaptation strategy. Bioresour. Technol. 2020:123462.
- [Google Scholar]
- Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci.. 2020;81:100868
- [CrossRef] [Google Scholar]
- Recent advances in micro-/nanoplastic (MNPs) removal by microalgae and possible integrated routes of energy recovery. Microorg.. 2022;10:2400.
- [CrossRef] [Google Scholar]
- Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: Screening studies towards biodiesel production. J. Appl. Phycol.. 2013;25:931-936.
- [CrossRef] [Google Scholar]
- Enhancement of lipid extraction for improved biodiesel recovery from the biodiesel promising microalga Scenedesmus obliquus. Energy Convers. Manag.. 2016;108:23-29.
- [Google Scholar]
- Microalgal biomass production as a sustainable feedstock for biodiesel: current status and perspectives. Renew. Sustain. Energy Rev.. 2016;64:596-606.
- [Google Scholar]
- Enhancement of biodiesel yield and characteristics through in-situ solvo-thermal co-transesterification of wet microalgae with spent coffee grounds. Bioresour. Technol. 2020:124640.
- [Google Scholar]
- Full utilization of marine microalgal hydrothermal liquefaction liquid products through a closed-loop route: towards enhanced bio-oil production and zero-waste approach. 3 Biotech. 2022;12:209.
- [CrossRef] [Google Scholar]
- Evaluation of halophilic microalgae isolated from Rabigh Red Sea coastal area for biodiesel production: Screening and biochemical studies. Saudi J. Biol. Sci.. 2022;29:103339
- [CrossRef] [Google Scholar]
- Valorization of lipidic food waste for enhanced biodiesel recovery through two-step conversion: A novel microalgae-integrated approach. Bioresour. Technol.. 2021;342:125966
- [CrossRef] [Google Scholar]
- Assessing the potential of green CdO2 nano-catalyst for the synthesis of biodiesel using non-edible seed oil of Malabar Ebony. Fuel. 2023;333:126492
- [CrossRef] [Google Scholar]
- New microalgae media formulated with completely recycled phosphorus originating from agricultural sidestreams. J. Appl. Phycol.. 2023;1:1-16.
- [CrossRef] [Google Scholar]
- A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol.. 1959;37:911-917.
- [CrossRef] [Google Scholar]
- Cultivation of Chlorella vulgaris using sequential-flow bubble column photobioreactor: A stress-inducing strategy for lipid accumulation and carbon dioxide fixation. J. CO2 Util.. 2020;41:101226
- [CrossRef] [Google Scholar]
- Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production. World J. Microbiol. Biotechnol.. 2013;29
- [CrossRef] [Google Scholar]
- Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol.. 2018;1911(19):731-745.
- [CrossRef] [Google Scholar]
- Lipid-rich particles of processed food waste for microalgae harvest through lipid-enriched floating biomat formation. Bioresour. Technol.. 2024;394:130251
- [CrossRef] [Google Scholar]
- Enhancement of lipid production of chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl. Biochem. Biotechnol.. 2016;180:1043-1055.
- [CrossRef] [Google Scholar]
- Physiologische und biochemische Beiträge zur Taxonomie der Gattung Chlorella. Arch. Mikrobiol.. 1970;70:211-216.
- [Google Scholar]
- Krzemińska, I., Szymańska, M., Ciempiel, W., Piasecka, A., 2023. Auxin supplementation under nitrogen limitation enhanced oleic acid and MUFA content in Eustigmatos calaminaris biomass with potential for biodiesel production. Sci. Reports 131(13), 1–11. Doi: 10.1038/s41598-023-27778-y.
- Leong, W.H., Kiatkittipong, K., Kiatkittipong, W., Cheng, Y.W., Lam, M.K., Shamsuddin, R., Mohamad, M., Lim, J.W., 2020. Comparative performances of microalgal-bacterial co-cultivation to bioremediate synthetic and municipal wastewaters whilst producing biodiesel sustainably. Process 8, 1427. Doi: 10.3390/PR8111427.
- Novel sequential flow baffled microalgal-bacterial photobioreactor for enhancing nitrogen assimilation into microalgal biomass whilst bioremediating nutrient-rich wastewater simultaneously. J. Hazard. Mater.. 2021;409:124455
- [CrossRef] [Google Scholar]
- Dual nutrient heterogeneity modes in a continuous flow photobioreactor for optimum nitrogen assimilation to produce microalgal biodiesel. Renew. Energy. 2022;184:443-451.
- [CrossRef] [Google Scholar]
- Enhanced waste glycerol recycling by yeast for efficient biodiesel production: Towards waste biorefinery. Biomass Bioenergy. 2022;159:106410
- [CrossRef] [Google Scholar]
- Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot.. 2007;99:1161.
- [CrossRef] [Google Scholar]
- Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol.. 2015;189:341-348.
- [CrossRef] [Google Scholar]
- Producing docosahexaenoic acid (DHA)-rich algae from biodiesel-derived crude glycerol: Effects of impurities on DHA production and algal biomass composition. J. Agric. Food Chem.. 2008;56:3933-3939.
- [CrossRef] [Google Scholar]
- Shetty, P., Gitau, M.M., Maróti, G., 2019. Salinity stress responses and adaptation mechanisms in eukaryotic green microalgae. Cells 8, 1657. Doi: 10.3390/CELLS8121657.
- Flocculation of Chlorella vulgaris by shell waste-derived bioflocculants for biodiesel production: Process optimization, characterization and kinetic studies. Sci. Total Environ.. 2020;702:134995
- [CrossRef] [Google Scholar]
- Fundamental understanding of in-situ transesterification of microalgae biomass to biodiesel: A critical review. Energy Convers. Manag.. 2022;270:116212
- [CrossRef] [Google Scholar]
- Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: A sequential integrated route for enhanced energy recovery. Energy Convers. Manag.. 2019;197:111907
- [CrossRef] [Google Scholar]
- Yang, F., Hanna, M.A., Sun, R., 2012. Value-added uses for crude glycerol--a byproduct of biodiesel production. Biotechnol. Biofuels 51(5), 1–10. Doi: 10.1186/1754-6834-5-13.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103258.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







