Translate this page into:
Growth performance, digestive enzyme activity and immune response of Macrobrachium rosenbergii fed with probiotic Clostridium butyricum incorporated diets
⁎Corresponding author. Fax: +880 41 731244. sarower@yahoo.com (Md. Golam Sarower)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
To determine antagonistic effect of Clostridium butyricum against Vibrio harveyi and its probiotic effect on growth performance, digestibility and immune response of fresh water prawn, Macrobrachium rosenbergii juveniles were examined following feeding with C. butyricum incorporated feed for 60 days. Significant reduction of V. harveyi growth was found at 8 hr and onward in in-vitro and at 10 days and onward in in-vivo challenge test. After rearing prawn with the bacteria in feed treatment for 60 days, body weight and growth rate of prawns was significantly higher (p < 0.05) than in control. Digestive protease and amylase activities in the gastrointestinal tract were also significantly (p < 0.05) higher than in controls. Immune response was a little high in treatment group, although it was not significant (p > 0.05) compared to control group. This study revealed that probiotic, C. butyricum incorporated diets were found to be beneficial for M. rosenbergii culture in terms of hindering the growth of pathogenic bacteria and increasing the growth, protease and amylase activities of prawn. Results from this study will be helpful to improve fresh water prawn farming.
Keywords
Probiotic
Clostridium butyricum
Pathogenic
Antagonistic effect
Growth
Immunity
1 Introduction
Aquaculture has become the fastest-growing food-producing sector and is contributing significantly to global food supply, food security and national economic development over the last three decades. Macrobrachium rosenbergii is a species of aquaculture importance because of its several biological characteristics such as rapid growth, high fecundity, wide range of salinity and temperature tolerance (Johnson, 1982; New, 1995; Roustaian et al., 2001; Roychoudhury and Mukherjee, 2013). Fresh water prawn is one of the most important cultured species in Bangladesh. In the year of 2014–15, the production of prawn and shrimp was about 44278 MT that comprises 6.2% of the total fish production (National Fish week, 2016). It has a high commercial value in international market due to delicious healthy choice of food for human consumption. This is the second largest export industry after readymade garments, generating $504 million USD annually (National Fish week, 2016). But one of the major constraints behind the development of prawn aquaculture is their high mortality due to the outbreaks of different viral, fungal and bacterial diseases especially vibriosis caused by different Vibrio spp. including V. harveyi, V. parahaemolyticus, V. alginolyticus, V. anguillarum, V. vulnificus, V. splendidus that cause a devastating economic loss worldwide (Shruti and Soumya, 2012). Although a number of chemotherapeutic agents including antibiotics have played a major role in combating many diseases of cultured aquatic organisms, their undesirable consequences such as persistence of antibiotic residues in farm and development of antibiotic-resistant bacteria discourage the use of these chemotherapeutic agents. (Karunasagar et al., 1994; Chaithanya et al., 1999; Jernberg et al., 2010). In addition, use of excessive antibiotics in pond has an adverse effect on the aquatic environment. Many drugs used in the culture ponds to control diseases, may be taken up by and accumulated in the polyculture animals after resolving in the water, thus affecting the seafood safety (Biao and Kaijin, 2007).
In recent times, the use of probiotics has become a popular alternative to antibiotics for improving and maintaining a healthy environment that leads to more environment-friendly aquaculture practices (Gatesoupe, 1999). Because prawn possesses a non-specific immune response, probiotic treatments may provide a broader spectrum and greater non-specific disease protection through competitive exclusion and immune modulation (Rengpipat et al., 2000; Nayak, 2010). However in aquaculture, probiotics can be administered either as a food supplement or as an additive to water and have been shown to be effective in a wide range of species for the promotion of growth enhanced nutrition, immunity and survival rate (Moriarty, 1998; Venkat et al., 2004; Wang, 2007).
Clostridium butyricum, a mesophilic endospore-forming gram-positive bacteria is a suitable probiotic supplement in fish feed owing to its capacity to produce butyric acid as well as lactic acid and survive in the media of low pH and relatively high bile concentrations (Kong et al., 2011). It is widely being used as a probiotic for humans and animals in East Asian countries, such as Japan, Korea and China (Kamiya et al., 1997). Although a number of exogenous and commercial probiotics have shown to be effective in prawn and shrimp culture (Rengpipat et al., 2000; Balcazar et al., 2007), no hitherto report has been published yet on the effectiveness of endogenous probiotics in prawn culture. However, relatively less attention has been paid toward the selection and development of probiotic bacteria for the culture of M. rosenbergii. In addition to these, expensive commercial probiotics make prawn farmer difficult to its access for application in prawn culture. Therefore, the aim of the present research was to isolate probiotic from the native microflora associated with M. rosenbergii and to evaluate its probiotic potential. One bacterial strain such as C. butyricum was studied in terms of its antibacterial activity to pathogens in a culture system and ability to enhance the growth, digestibility and immunity of the juveniles of M. rosenbergii.
2 Methods and material
2.1 Bacterial species isolation and characterization by PCR
Probiotic bacteria (C. butyricum) and Pathogenic bacteria (Vibrio harveyi), isolated from the intestinal tract of M. rosenbergii were subjected to characterize by PCR. Characterized isolates of C. butyricum and V. harveyi were kept as stocks and served as potential probiotic bacteria and pathogenic bacteria, respectively.
2.1.1 Isolation and characterization of pathogenic bacteria
V. harveyi was isolated from the intestinal tract of M. rosenbergii. ISO method was followed in this case. 0.1 mL stock solution of intestinal tract was taken and mixed with 0.9 mL alkaline saline peptone water (ASPW) in sterilized test tube. Then the mixture was incubated at 37 °C for 6 h ± 1. This was the first selective enrichment. After that, whole culture obtained in first selective enrichment was transferred into another test tube containing 10 mL ASPW. Then the solution was incubated at 41.5 °C for 18 h ± 1. This was the second selective enrichment. Serial dilution (tenfold) was done and 0.1 mL suitable dilution of each culture was inoculated in thiosulfate citrate bile and sucrose (TCBS) agar plates. The inoculated TCBS agar plates were incubated at 37 °C. After 24 h ± 3 h of incubation, the colonies of different plates were analyzed by PCR for characterizing Vibrio harveyi (ISO/TS 21872-1, 2007). Amplification of 16S rRNA gene specific for Vibrio spp. was performed using a pair of primer Vibr-f: 5′-CGGTGAAATGCGTAGAGAT-3′ and Vibr-r: 5′-TTACTAGCGATTCCGAGTTC-3′ (Tarr et al., 2007). A standard PCR was executed with a 50 μL reaction mixture containing 50 ng of template DNA, Top DNA Polymerase (Bioneer Corporation, Daejeon, Korea) at 0.5 U/μL, 2.0 mM MgCl2, 0.4 μM primers, 200 μM dNTP and 1X buffer as recommended by the manufacturer. PCR parameters were carried out in a thermal cycler (C1000TM, BIO-RAD, USA) as follows: predenaturation at 94 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min. The whole PCR parameters were terminated by a final extension step at 72 °C for 10 min. The PCR products were analyzed by gel electrophoresis in 2% agarose (Bioneer Corporation) containing 0.5 μg/mL ethidiumbromide in TAE buffer. DNA bands were visualized by High Performance UV Transilluminator (UVP, CA, USA) and photographed using the DigiDoc-It 120 gel documentation system (UVP). To compare the size of double stranded DNA from 100 to 2,000 base pairs, 100 bp designed DNA markers were used. The DNA marker consists of 13 double stranded DNA fragments ranging in sizes from 100 to 1,000 (Bioneer, Korea).
2.1.2 Isolation and characterization of probiotic bacteria
In order to isolate C. butyricum from the intestinal tract of M. rosenbergii, a method used by (Lucchini et al., 1998) was followed with some modifications. The stock solution was diluted (tenfold dilution) with peptone water (Hirsch, 1960). 0.1 mL suitable dilution of each stock solution was inoculated in Rainforced Clostridium Medium (RCM) with bacteriological agar and incubated at 37 °C temperature for 48 h under anaerobic condition (By warping each Petri dish with parafilm paper). After incubation the white, glistening, with rhizoid (has thin, branching projections) edge colonies were collected and analyzed by PCR for characterizing C. butyricum.
Amplification of 16S-23S intergenic spacer regions specific for C. butyricum was performed with the set of primers; CB16F: 5′-GGA GAA CCT GCG GCT GGA T-3′ and CB23R:5′-AAA TCT CCG GAT CTC TGG CT-3′ (Nakanishi et al., 2005). PCRs were performed following similar procedure as for 16S rRNA gene of V. harveyi with editing annealing temperature at 58 °C. PCR products were also analyzed through the similar procedure of 16S rRNA gene of V. harveyi.
2.2 In-vitro challenge test for antagonistic effect of C. butyricum
For in-vitro challenge test the method provided by ISO was followed with some modifications. Characterized V. harveyi and C. butyricum were cultured separately in nutrient broth media and centrifuged at 10,000g for 5 min. The supernatants were discarded, and V. harveyi as well as C. butyricum were separately resuspended in peptone water. The V. harveyi suspension was diluted to approximately 104 colony forming units (cfu)/mL and the C. butyricum suspension was diluted to 106 cfu/mL, called test solutions. After that, 0.5 mL of each test solutions was mixed together. Then one mL of the mixer solution was inoculated in Thiosulfate Citrate Bile Salt (TCBS) agar media at subsequent intervals of 4 h up to 12 h. Test solution of V. harveyi without probiotic was also inoculated in TCBS agar media at 0, 4, 8 and 12 h subsequently. All the inoculated TCBS agar plates were incubated at 37 °C for 48 h. Standard plate count was done after incubation.
2.3 In-vivo challenge test for antagonistic effect of C. butyricum
The effect of candidate probiotic should be tested in vivo as well. When the probiotic effect is supposed to be nutritional, the candidate probiotics could be added to the culture of the prawn and their effect on growth and/or survival parameters could be assessed. However, when biological control of the microbiota is desired, representative in vivo challenge tests seem to be the appropriate tool to evaluate the potential effect of the candidate probiotic on the host. In total, sixty M. rosenbergii juveniles purchased from local market of Khulna (2.56 ± 0.41 g in average weight and 5.12 ± 0.29 cm in average total length) were randomly distributed into 6 aquaria (45 × 30 × 30 cm) each containing ten (10) prawn species. The present experiment was set up for 60 days with a completely randomized design in two treatments (Treatment-1 containing juveniles provided probiotic incorporated feed with 2 × 109 cfu/g C. butyricum, each and they were injected with 107 cfu/mL V. harveyi; Treatment-2 or Control containing juveniles provided with fresh commercial feed without any probiotic and they also were injected with 107 cfu/mL V. harveyi) which were triplicated. For preparing the probiotic incorporated feed, the cells of probiotic bacteria were harvested by centrifugation at 3000 × 10 rpm for 10 min. The cells were thoroughly mixed with the commercial scampi feed to obtain a concentration of 2 × 109 cfu/g feed where the cell density of probiotics was adjusted using spectrophotometer. Finally, the mixture was spread out and aseptically dried in an oven for 1–2 h at 60 °C according to previous studies of Rahiman et al. (2010). In case of V. harveyi dose for injection the load 107 cfu/mL also adjusted using spectrophotometer. All M. rosenbergii were fed with 7–11% feed of their body weight for the first month and from the next month 5–6% of body weight at a frequency of twice per day throughout the culture period. From starting day of the culture, every 10 day interval equal number of M. rosenbergii was randomly selected from each replication under both treatments and the load of V. harveyi in their intestine was counted following ISO method (ISO/TS 21872-1, 2007).
2.4 Cultured with C. butyricum administration feed in pond
M. rosenbergii juveniles (2.56 ± 0.41 g in average weight and 5.12 ± 0.29 cm in average total length) were purchased from local market of Khulna and acclimatized to the earthen pond conditions. After acclimatization juveniles were collected and divided into a control group and a probiotic-treated group. They were fed with a commercial diet (Mega feed) consisting of 38% crude protein, 5% crude lipid, 5% ash, 2% fibre and 5% moisture content at a rate of 7–11% of their body weight for the first month and from the next month 5–6% per day at a frequency of twice per day until harvested. The experiment was set up in triplicates, with three control earthen ponds and three probiotic earthen ponds where each pond was 70 m2 in size and 1 m in depth and each containing 50 juveniles. At the end of the experiment, random sampling was done from each pond to understand the effect of probiotic treated feed.
2.5 Determination of growth performance
At the end of the experiment, the growth parameters such as weight gain (WG) and specific growth rate (SGR) of M. rosenbergii from the treatment and control ponds were determined by the following equations (Olvera-Novoa et al., 1990; Ziaei-Nejad et al., 2005) where, t is the culture period, ln W0 is the natural logarithm of the weight of M. rosenbergii at beginning of the experiment, and ln Wt is the natural logarithm of the weight of M. rosenbergii at day t.
2.6 Digestive enzymes assays
M. rosenbergii were collected from experimental ponds, washed with cold distilled water and immediately frozen at −80 °C until enzyme assays were conducted. As with monitoring of bacteria, digestive tract was dissected out and homogenized. Samples were homogenized in 9 volumes of 0.05 M Tris–HCl buffer, pH 7.8, in a Wise Tis Homogenizer in 700 × 10 rpm for five minutes, and were centrifuged at 4800g for 60 min at 4 °C according to Lovett and Felder (1990). The supernatant was used as crude enzyme source. Amylase enzyme activity was assayed followed by the method described in previous literature (Bernfeld, 1955) where one unit of amylase activity was defined as the amount of enzyme required to produce 1 mg maltose in 30 min at 35 °C. Protease enzyme activity was estimated by the method of Anson (1938) where one unit of protease activity was defined as the amount of enzyme required to produce 1 mg tyrosine in 30 min at 37 °C.
2.7 Differential and total haemocyte analysis
Differential haemocyte counts (DHC) was performed adopting the method described by Owens and O’Neill’s (1997). Haemolymph was withdrawn from the ventral sinus of the first abdominal segment into a 1 ml syringe containing equal volume of anti-coagulant, 0.2 mL citrate-EDTA; 0.01 M; tri-sodium citrate 0.03 M; citric acid 0.026 M; NaCl 0.45 M; glucose 0.1 M, adjusted to pH 4.6 with 1 M HC1 (Soderhall and Smith, 1983). Total haemocyte counts (THC) were performed with an improved Neubauer haemocytometer using a modification of the method described by (Bain et al., 2001) for analysis of human blood samples. Counts were conducted with light microscope (Olympus CX-21).
2.8 Data collection and analysis
Collected data were stored, explored and analyzed using Microsoft Excel (Microsoft office, 2007) and Statistical Package for the Social Sciences (SPSS version 16.0; SPSS, Inc., Chicago, IL). Independent sample t-test was applied to address the differences between the treatment and control at 1% significance level using SPSS (version 16.0).
3 Result
3.1 Polymerase chain reaction result
Fragments of 16S rRNA and 16S-23S intergenic spacer region were successfully amplified from three isolates of Vibrio harveyi and C. butyricum collected from biochemical test, respectively (Fig. 1). The specific primers Vibr-f and Vibr-r produced only one genotype (one PCR band) with an approximately 663 bp fragment length from the three Vibrio spp. isolates, analogous to Vibrio spp., as described by Tarr et al. (2007). The specific primers CB16F and CB23R also produced one genotype with approximately 383 bp fragment length from three C. butyricum isolates, analogous to C. butyricum as described by Nakanishi et al. (2005). Therefore, 16S rRNA and 16S-23S intergenic spacer region products revealed that the screened isolates from the prawn intestine were Vibrio spp. and C. butyricum, respectively. These validated isolates were subsequently used for in-vitro challenge test and in-vivo feeding experiment.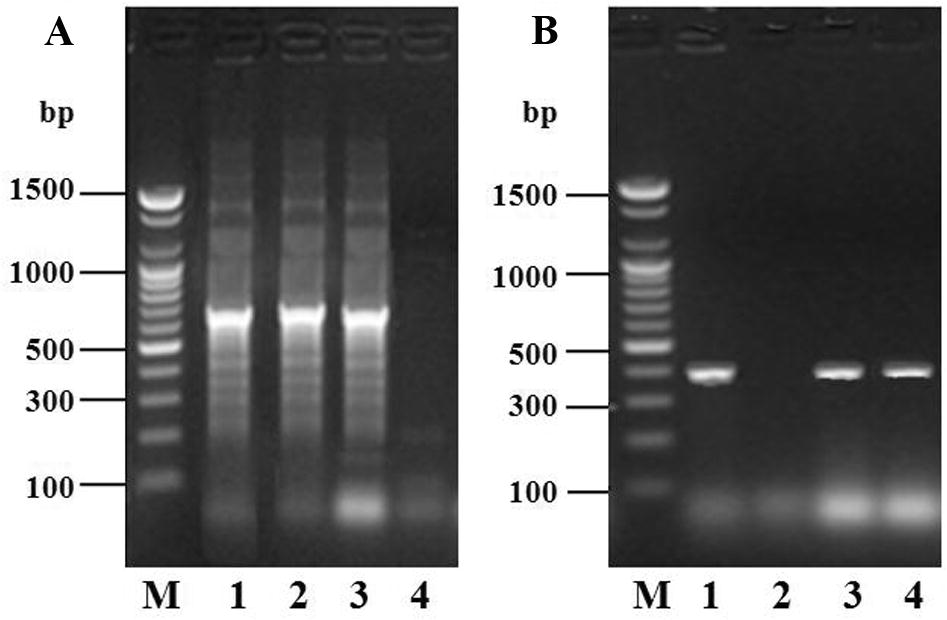
Electrophoretic analysis of PCR-amplified target genes from Vibrio sp. and C. butyricum obtained under optimal conditions of PCR of isolates following biochemical test. Mobilities of the different target genes are indicated on the right. (A) Lane M, 100-bp DNA ladder (size marker); lane 1–4, isolates of Vibrio sp. (B) Lane M, 100-bp DNA ladder (size marker); lane 1–4, isolates of C. butyricum.
3.2 Inhibitory effects of C. butyricum
In-vitro and in-vivo challenge tests were performed to investigate inhibitory effect of C. butyricum on the growth of V. harveyi. In in vitro test the growth of V. harveyi was strongly inhibited by C. butyricum (Table 1). Although, there was no significant (p > 0.05) inhibitory effect of C. butyricum until 8 hours, a significant (p < 0.05) inhibitory effect was observed after 12 hours of incorporation (Table 1). In addition in-vivo test also showed a significant inhibitory effect of probiotics on the pathogenic bacteria (Table 2).
Time (hours)
Control (V. harveyi, CFU/g)
Probiotic (V. harveyi, CFU/g)
0
4.69 × 103 ± 5.39 × 102
4.70 × 103 ± 5.02 × 102
4
2.30 × 104 ± 8.25 × 102
1.34 × 104 ± 7.89 × 102
8
2.36 × 105 ± 2.93 × 102
2.69 × 103 ± 2.72 × 102
12
4.24 × 105 ± 4.92 × 104
5.75 × 103 ± 3.26 × 102⁎
Time (days)
Control (V. harveyi, CFU/g)
Probiotic (V. harveyi, CFU/g)
0
3.25 × 107 ± 7.02 × 102
3.61 × 107 ± 2.11 × 102
10
5.73 × 107 ± 1.73 × 102
2.43 × 107 ± 1.21 × 102
20
2.23 × 108 ± 1.32 × 103
7.53 × 106 ± 3.11 × 102⁎
30
5.32 × 108 ± 1.19 × 103
2.21 × 105 ± 2.37 × 102⁎
40
7.61 × 109 ± 4.90 × 104
1.03 × 105 ± 3.17 × 102⁎
50
8.26 × 109 ± 2.53 × 104
6.32 × 103 ± 1.32 × 101⁎
60
9.79 × 109 ± 7.12 × 104
2.91 × 102 ± 7.02 × 101⁎
3.3 Growth performance
The growth performance parameters include weight gain and specific growth rate of M. rosenbergii after 60 days of feeding with experimental diet. M. rosenbergii fed with probiotic, C. butyricum represented a significantly (p < 0.05) greater specific growth rate than did the control. In addition, significant differences (p < 0.05) were also recorded between treatment and control in case of weight gain. Therefore, the growth parameters revealed that M. rosenbergii fed with probiotic grew significantly (p < 0.05) faster than that of without probiotic (Fig. 2).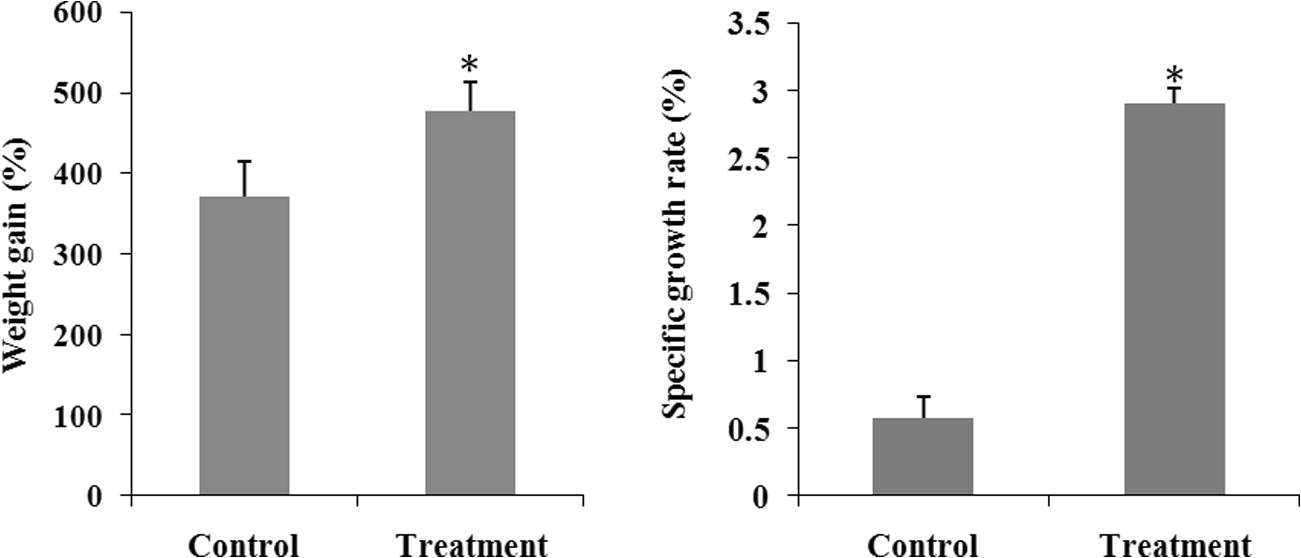
Weight gain and SGR of M. rosenbergii reared with and without C. butyricum probiotic added to feed. Each value is mean ± standard deviation of three individual observations. The significance of differences was calculated by a t-test. *p < 0.05 compared with control.
3.4 Digestive enzyme activity
Different digestive enzyme activity especially protease and amylase were evaluated after 60 days of feeding trial (Fig. 3). Amylase activity in M. rosenbergii treated with C. butyricum were found at a value of 1.22 ± 0.1 mg/mL that was significantly (p < 0.05) higher than the value, 0.7 ± 0.1 mg/mL found in M. rosenbergii which were not treated with C. butyricum. Significantly (p < 0.05) higher protease activity was also found in C .butyricum treated M. rosenbergii (2.73 ± 0.2 mg/mL) than that of feeding without C. butyricum incorporated diet (1.82 ± 0.3 mg/mL). So, the M .rosenbergii fed with C. butyricum treated feed showed significantly (p < 0.05) higher digestive enzyme activity compared to M. rosenbergii fed without C. butyricum (Fig. 3).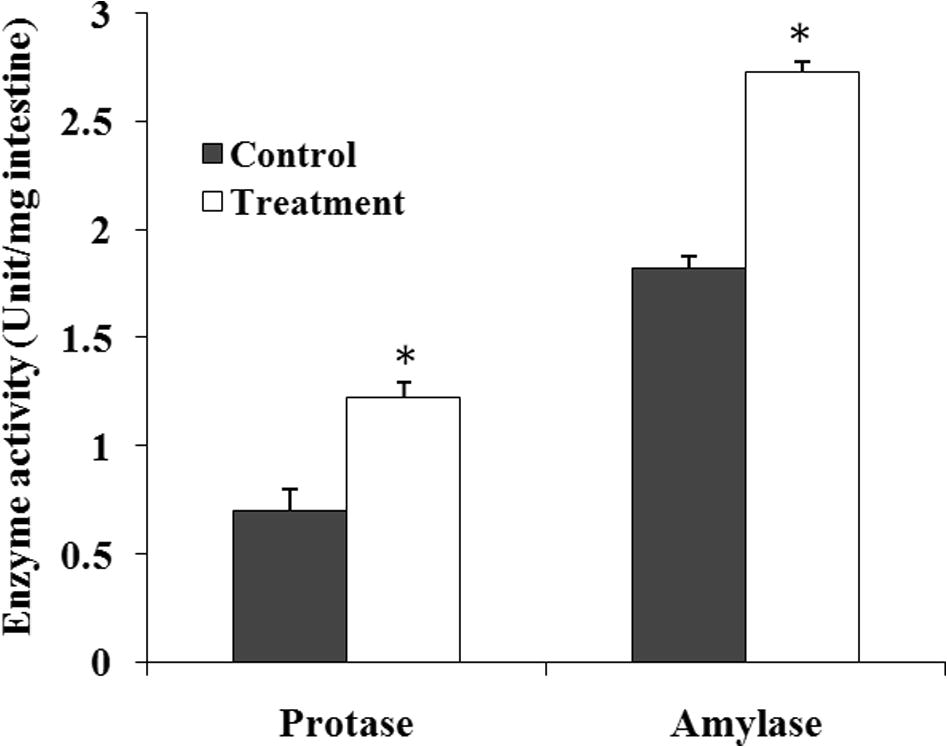
Specific enzyme activity (amylase and protease) in digestive tract of M. rosenbergii reared with and without C. butyricum probiotic added to feed. Each value is mean ± standard deviation of three individual observations. The significance of differences was calculated by a t-test. *p < 0.05 compared with control.
3.5 Immunity assay
Three major groups of haemocytes viz. non-granular haemocyte (NGH), small granular haemocyte (SGH) and large granular (LGH) haemocyte were identified at the end of a 60 day experiment. The total haemocyte counts (THC) were not found significantly higher in treatment group, (5.6 ± 0.67) × 106 mm−3 compared to control group, (5.2 ± 0.73) × 106 mm−3 (Table 3). In control group, NGH represented the highest percentage (75.33 ± 15.93%) followed by SGH (22.24 ± 9.13%) and LGH (2.43 ± 2.2%). In treatment group, the percentages of haemocytes were almost similar to that of control group and no significant difference (P > 0.05) was found among the percentages of different types of haemocytes. The highest percentage of NGH (76.56 ± 17.33%) compared to SGH (21.13 ± 10.32%) and LGH (2.31 ± 1.9%) was also found in the treatment group.
Treatment
1THC (mm−3)
2DHC (%)
3NGH
4SGH
5LGH
Control
(5.2 ± 0.73) × 106
75.33 ± 15.93
22.24 ± 9.13
2.43 ± 2.2
Probiotic
(5.6 ± 0.67) × 106
76.56 ± 17.33
21.13 ± 10.32
2.31 ± 1.9
4 Discussion
Probiotics is very much popular today for its antagonistic effect, disease preventing capacity as well as health promoting properties (Senok et al., 2005). In the present study, the bacterial species, C. butyricum was chosen in order to evaluate its potentiality for controlling pathogenic bacterial load in prawn, which may help to reach a better solution to mitigate viral disease. In-vitro test of the present study revealed that C. butyricum significantly inhibit the growth of V. harveyi. Production of organic acids viz. butyric acid and lactic acid by the fermentation process of C. butyricum results in a lower pH of culture medium that plays an important role in inhibiting the growth of pathogens (Senok et al., 2005; Balcázar et al., 2006; Gao et al., 2013). The in-vivo test demonstrated that probiotic (C. butyricum) treated feed significantly reduced the load of V. harveyi from M. rosenbergii. Probiotics release chemical substances that induce bactericidal or bacteriostatic effect and barrier against the proliferation of opportunistic pathogens (Verschuere et al., 2000). Inhibitory activity, preventive and therapeutic effects of C. butyricum have been found on S. enteritidis and V. parahaemolyticus growth in infected rainbow trout, Oncorhynchus mykiss (Sakai et al., 1995). The results are in agreement with those of C. butyricum (Zhang et al., 2011; Szymanowska-Powalowska et al., 2014; Vieira et al., 2010), which showed significant inhibition of pathogenic bacteria.
The dietary administration of C. butyricum in the present study significantly (P < 0.05) improved the weight gain and SGR due to the beneficial effect of probiotics on the digestive processes of M. rosenbergii. Probiotic strains not only synthesize extracellular enzymes such as proteases, amylases, and lipases but also provide growth factors such as vitamins, fatty acids, and amino acids (Balcázar et al., 2006). Therefore, absorbance of nutrient is more efficient when the feed is supplemented with probiotics (Gibson and Roberfroid, 1995). Rengpipat et al., 2000; Zhang et al., 2011; Yang et al., 2012; Elumalai et al., 2013; Liao et al., 2015 found that C. butyricum treated feed significantly increased the growth of shrimp which is consistent to the present study.
The digestive enzyme activity in crustacean plays a central role in nutritional physiology and may directly or indirectly regulate growth, moulting cycle and complex dietary formulation (Lovett and Felder, 1990; Moullac et al., 1996). The dietary administration of the present study also showed significant (P < 0.05) enhancement of the amylase and protease activity in prawn. This increasing trend of digestive enzyme activity may be caused by the symbiotic behaviour of probiotics. This is because of stimulating a healthier digestive tract microbial ecology and modifies the selection of bacterial enzymes (Yang et al., 2005). Nimrat and Vuthiphandchai, 2011 found that C. butyricum enhance growth but also considerably increase enzyme activity in the shrimp body, which supports the findings of the present study. Kennedy et al. (1998) discussed the similar improvement of protease enzyme activity due to the application of probiotics in the Centropomusun decimal body.
Total haemocyte count (THC) in treatment was not significant compared to the control. It indicates a little bit immunity enhancements in the treatment. Owens and O’Neill (1997) found the similar result and concluded that there was no significant difference between the total cell counts in prawn body fed with probiotic mixed diet and without probiotic diet. Rengpipat et al. (2000) also studied on immunity enhancement of probiotic treated feed and found that the THC didn’t vary significantly between treatment and control group. Saraswati et al. (2013) also investigated the effect of probiotics, C. butyricum on immunity and found higher THC in M. rosenbergii body for probiotic treated feed, although it was not significant. In contrast, Partida-Arangure et al. (2013) observed that Litopenaeus vannamei fed with probiotic incorporated feed revealed significantly (P = 0.04) higher THC than those with normal feed. It is predicted that this deviation happened due to the differences of methodology and species variation. It is mentionable that, classification of crustacean haemocytes remains controversial due to different types of methodology and criteria adopted by different fishery researchers (Hose et al., 1987).
There were no significant differences between the control and treatment in terms of NGH, SGH and LGH (p > 0.05). Hose et al. (1992) studied Sicyonia ingentis and recorded that the NGH comprises 50–60% of the circulating haemocytes, whereas the SGH and LGH represented 30% and 10%, respectively. NGH in Fenneropenaeus indicus varied and was approximately 10–15%, lower value compared to former species and LGC and SGC were 20–25% and 60–65%, respectively (Kakoolaki et al., 2010). The NGH of M. rosenbergii, possesses 70% of haemocytes (Vazquez et al., 1997) that is consistent with the findings of the present study.
5 Conclusion
The present study concluded that the probiotic bacteria, C. butyricum could provide protection against Vibrio spp. infections in M. rosenbergii and will be able to improve the growth. The present study did not find a significant increase in immunity but a slightly increased immunity and improved growth of M. rosenbergii cultured with probiotic mixed feed. The outcomes of the present study will pave the way to mitigate the disease and reap better profit for the M. rosenbergii farmers.
Acknowledgement
The authors would like to thank all the people who helped in the collection of prawn juveniles and pond preparation. The authors gratefully acknowledge the financial support of Bangladesh Fisheries Research Institute (BFRI).
References
- The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol.. 1938;22:79-89.
- [Google Scholar]
- Practical Haematology (nine ed.). Health Sciences Division; 2001. p. :652.
- Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio para haemolyticus. J. Invertebr. Pathol.. 2007;96:147-150.
- [Google Scholar]
- Amylase α and β. In: Methods in Enzymology. New York: Academic Press Inc; 1955. p. :149-158.
- [Google Scholar]
- Shrimp farming in China: operating characteristics, environmental impact and perspectives. Ocean Coast. Manag.. 2007;50:538-550.
- [Google Scholar]
- Efficiency of probiotics (Ecoforce) in the growth and survival of Peneaus monodon. Arthropods. 2013;2:231-236.
- [Google Scholar]
- In vitro protective efficacy of Clostridium butyricum against fish pathogen infections. Indian J. Microbiol.. 2013;53:453-459.
- [Google Scholar]
- Dietary modulation of the human colonic microbiota introducing the concept of probiotics. J. Nutr.. 1995;125:1401-1412.
- [Google Scholar]
- Comparative bacterial activity of blood serum and plasma serum. J. Biol.. 1960;66:391-398.
- [Google Scholar]
- Patterns of hemocyte production and release throughout the molt cycle in the penaeid shrimp Sicyonia ingentis. Biol. Bull.. 1992;183:185-199.
- [Google Scholar]
- ISO/TS 21872–1, 2007. Microbiology of food and animal feeding stuffs — Horizontal method for the detection of potentially enteropathogenic Vibrio spp. — Part 1: Detection of Vibrio parahaemolyticus and Vibrio cholerae.
- Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
- [Google Scholar]
- Diseases of Macrobrachium rosenbergii. In: New M.B., ed. Giant Prawn Farming Developments in Aquaculture and Fisheries Science. Vol 10. Amsterdam: Elsevier; 1982. p. :269-277.
- [Google Scholar]
- Selected morpho-chemical features of haemocytes in farmed shrimp, Fenneropenaeus indicus in Iran. Iran. J. Fish. Sci.. 2010;9:219-232.
- [Google Scholar]
- Bacterioprophylax is using Clostridium butyricum for lethal caecitis by Clostridium difficile. Rev. Med. Microbiol.. 1997;8:S57-S59.
- [Google Scholar]
- Mass mortality of Penaeus monodon larvae due to antibiotic resistant Vibrio harveyi infection. Aquaculture. 1994;128:203-209.
- [Google Scholar]
- Bacterial management strategies for stock enhancement of worm water marine fish: a case study with common snook, Centropomu sundecimalis. Bull. Mar. Sci.. 1998;62:573-588.
- [Google Scholar]
- Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microbiol.. 2011;62:512-517.
- [Google Scholar]
- Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci.. 2015;94:662-667.
- [Google Scholar]
- Ontogenic change in digestive enzyme activity of larval and postlarval white shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae) Biol. Bull.. 1990;178:144-159.
- [Google Scholar]
- Specific detection of a probiotic strain in faecal samples by using multiplex PCR. FEMS Microbiol. Lett.. 1998;158:273-278.
- [Google Scholar]
- Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture. 1998;164:351-358.
- [Google Scholar]
- Adaptation of trypsin, chymotrypsin and alpha-amylase to casein level and protein source in Penaeu svannamei (Crustacea: Decapoda) J. Exp. Mar. Biol. Ecol.. 1996;208:107-125.
- [Google Scholar]
- Rapid species identification and partial strain differentiation of Clostridium butyricum by PCR using 16S–23S rDNA intergenicspacer regions. Microbiol. Immunol.. 2005;49:613-621.
- [Google Scholar]
- Department of Fisheries. Bangladesh: Ministry of Fisheries and Livestock; 2016.
- Probiotics and immunity: a fish perspective. Fish Shellfish Immunol.. 2010;29:2-14.
- [Google Scholar]
- In vitro evaluation of commercial probiotic products used for marine shrimp cultivation in Thailand. Afr. J. Biotechnol.. 2011;10:4643-4650.
- [Google Scholar]
- The use of Alfafa leaf Protein Concentrates as a protein source in diet of Tilapia (Oreochromis mosambicus) Aquaculture. 1990;83:45-58.
- [Google Scholar]
- Use of clinical cell flow cytometer for differential counts of prawn Penaeus monodon haemocytes. Dis. Aquat. Org.. 1997;31:147-153.
- [Google Scholar]
- Effect of inulin and probiotic bacteria on growth, survival, immune response, and prevalence of white spot syndrome virus (WSSV) in Litopenaeus vannamei cultured under laboratory conditions. Afr. J. Biotechnol.. 2013;12:3366-3375.
- [Google Scholar]
- Probiotic Effect of Bacillus NL110 and Vibrio NE17 on the Survival, Growth Performance and Immune Response of Macrobrachium rosenbergii (de Man). Kottayam, India: School of Environment Sciences, Mahatma Gandhi University; 2010.
- Use Carnobacterium sp. as probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss Walbaum) Aquaculture. 2000;185:235-243.
- [Google Scholar]
- Biochemical changes in freshwater prawn Macrobrachium rosenbergii during larval development. J. World Aquacult. Soc.. 2001;32:52-59.
- [Google Scholar]
- Role of algal mixture in Food intake of Macrobrachium rosenbergii during larval development. Indian J. Geo-Mar. Sci.. 2013;42:647-652.
- [Google Scholar]
- Enhancement of resistance to vibriosis in rainbow trout, Oncorhynchus mykiss Walbaum by oral administration Clostridium butyricum. J. Fish Dis.. 1995;18:187-190.
- [Google Scholar]
- Immune response of white shrimp Litopenaeus vannamei that injected with the extract of diatomae Chaetoceros ceratosphorum. J. Basic Appl. Sci. Res.. 2013;3:998-1004.
- [Google Scholar]
- Shruti, C., Soumya, 2012. Vibrio Related Diseases in Aquaculture and Development of Rapid and Accurate Identification Methods, 1National Institute of Oceanography, Regional Centre, Lokhandwala Road, Four Bungalows, Andheri (West), Mumbai, India.
- Induction of degranulation and lysis of haernocytes in the freshwater crayfish, Astacus astacus by components of the prophenol oxidase activating system In vitro. Cell Tissue Res.. 1983;233:295-303.
- [Google Scholar]
- Biotechnological potential of Clostridium butyricum bacteria. Braz. J. Microbiol.. 2014;45:892-901.
- [Google Scholar]
- Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J. Clin. Microbiol.. 2007;45:134-140.
- [Google Scholar]
- Morphology of hemocytes from the freshwater prawn Macrobrachium rosenbergii. J. Morphol.. 1997;234:147-153.
- [Google Scholar]
- Effect of feeding Lactobacillus based probiotics on the gut micro flora, growth and survival of post larvae of Macrobrachium rosenbergii (de Man) Aquacult. Res.. 2004;35:501-507.
- [Google Scholar]
- Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev.. 2000;64:655-671.
- [Google Scholar]
- Effect of probiotic supplemented diet on marine shrimp survival after challenge with Vibrio harveyi. Arq. Bras. Med. Vet. Zootec.. 2010;62:631-638.
- [Google Scholar]
- Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture. 2007;269:259-264.
- [Google Scholar]
- Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and faecal microflora in broiler chickens. Poult. Sci.. 2012;91:2121-2129.
- [Google Scholar]
- Effect of symbiotic on intestinal microflora and digestive enzyme activities in rats. World J. Gastroenterol.. 2005;11:7413-7417.
- [Google Scholar]
- Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr.. 2011;65:329-339.
- [Google Scholar]
- The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture. 2005;252:516-524.
- [Google Scholar]







