Translate this page into:
Green synthesis of silver nanoparticles by Pisum sativum L. (pea) pod against multidrug resistant foodborne pathogens
⁎Corresponding author. mkalaiyarasingl@gmail.com (M. Kalaiyarasi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The consumption of foods contaminated with various foodborne organisms such as, viruses, fungi and bacteria are recognized as the important sources of foodborne illness in animals and humans. Multidrug resistant foodborne bacteria increased morbidity, mortality rates, and severe economic loss and associated with prolong hospitalization. The development of natural and novel antibacterial agent is much needed as there is an increasing concern on multidrug resistant bacteria. This study aimed to synthesize silver nanoparticles using aqeous Pisum sativum L. (pea) pod extract and to characterize the nanoparticles. It was further used on various drug resistant foodborne bacterial pathogens and antimicrobial activity was determined. The synergistic antibacterial activity, antioxidant activity and biocompatibility were also assessed. Green synthesized nanoparticles applied in this study were synthesized using aqueous P. sativum L. (pea) pod extract with particle size 30 nm. The green synthesized silver nanoparticles showed antimicrobial activity against Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 700802, Streptococcus gordonii ATCC 49818, Staphylococcus aureus (MRSA) ATCC 4330 and Staphylococcus aureus ATCC 25923. Among the pathogenic bacterial strains, silver nanoparticles were highly active against S. aureus ATCC 25923 than other bacterial strains (22 ± 2 mm). The antibacterial activity varied between 15 ± 2 mm and 22 ± 2 mm diameter zone of inhibition. The MIC value of the nanoparticles ranged from 6.25 µg/mL to 75 µg/mL and MBC value ranged from 50 µg/mL to 150 µg/mL. The green synthesized nanoparticles showed antioxidant potential. Normal fibroblast cell lines (L-929) was used to test the cytotoxic activity and the increased IC50 value (750.5 ± 5.5 μg/mL) indicated good biological compatibility. The present findings revealed that green synthesized silver nanoparticles exhibited potent activity against various food borne pathogenic bacteria. These nanoparticles might be well developed as novel antibacterial substances for the treatment of food borne multidrug resistant bacterial infection.
Keywords
Nanomaterials
Green synthesis
Antibiotic
Bactericidal
Drug resistant
1 Introduction
Green synthesis is a suitable, rapid and highly acceptable method for the biosynthesis of various metal nanoparticles. In recent years, various plant parts such as bark, stem, fruit, seed and leaf extracts were used effectively for the biosynthesis of metal nanoparticles (Mittal et al., 2013). Among various metal nanoparticles, silver nanoparticles were widely used due to their anti-proliferative, anti-fungal and anti-bacterial activity (Nayak et al., 2015). Silver nanoparticles are widely used in medicines, cosmetics, biofertilizers, seed and food preservation and in food packing (Dipankar and Murugan, 2012). Apart from these applications, these particles are widely used in the field of micro-electronics, catalysis, diagnostics, and bio-molecular detection (Mohanta et al., 2016b). There are several methods used to synthesize silver nanoparticles, including chemical and physical methods, viz, microwave-assisted process, sono-chemical, radiation assisted, thermal decomposition of silver compounds, chemical and photochemical reactions in reverse micelles, and green chemistry techniques. The green synthesis based nanoparticles has several advantages over the physical and chemical method as it is eco-friendly, cost effective and very simple to manage commercial-scale processes without using much toxic chemicals, elevated temperature, high pressure and applying excessive energy (Song and Kim, 2009).
There are various reports on green synthesis of silver nanoparticles using plant materials. Moreover, there is need to evaluate most reliable and simple method for environmentally clean route to biosynthesize, commercially viable, economically stable silver nanoparticles to synthesize by using various natural sources, mainly plant based materials (Chung et al., 2016). In recent years, the application of plant based products and in the biosynthesis of silver nanoparticles has been investigated (Arunachalam et al., 2013; Valsalam et al., 2019). Various medicinal plants are used as the traditional medicine for the preparation of nutritional and healthcare product. Green synthesis of nanoparticles using various biological agents including, plant extracts, algae, fungi, and by-products of these sources has attached much more attention in recent times. Moreover, the application of feed wastes from the legumes in the green synthesis of nanoparticles has not been well documented. The application of food wastes to green synthesize nanoparticles would be an environmentally friendly, low-cost and highly effective to recycle the nutrients for the production of pharmaceuticals and biomedicines. P. sativum L. (pea) is edible and the pod is considered as waste and used for the preparation of feed for animals. The pea pod and peel contain bioactive compounds such as, flavonoids, and phenols and it has been widely used as traditional medicines to treat number of diseases and disorders (Hadrich et al., 2014). Pea contains bioactive compounds responsible for antidiabetic, antioxidant and antimicrobial properties. However, peas are widely used to treat diabetes in China, India and other Asian countries. In this study, investigation was carried out to synthesize silver nanoparticles using aqueous extract of pod of pea and to analyze bioactive properties.
2 Materials and methods
2.1 Plant extract
The half matured P. sativum L. pod was collected from the agricultural field and the seeds were removed from the pod. The empty pod was used for the green synthesis of silver nanoparticles. The pod was cut into small pieces and finely ground mechanically. About 1 g of pod powder was mixed in 250 mL Erlenmeyer flask containing 100 mL double distilled water. The Erlenmeyer flask was boiled for 10 min using a water bath and stirred continuously. The water extract of pod was cooled and filtered using Whatman’s filter paper number 1 and stored at 4 °C. It was used as the reducing agent for the green synthesis of silver nanoparticles.
2.2 Green synthesis of silver nanoparticles
Green synthesis of nanoparticles was performed using the aqueous extract of P. sativum L. pod. Initially 100 mL of 1 mM silver nitrate was prepared and stored in an Erlenmeyer flask. To this Erlenmeyer flask, 10 mL of plant pod extract was added slowly under continuous stirring. Stirring was achieved using a magnetic stirrer and continued this process until the solution turns brown or reddish brown. The ratio of aqueous extract and silver nitrate solution was maintained at 1:10 ratio and the size and shape of the nanoparticles were maintained appropriately (Valsalam et al., 2019; Anand et al., 2017).
2.3 Characterization of silver nanoparticles
The green synthesized nanoparticles were initially analyzed using UV–Visible spectrophotometer. Scanning electron microscopy (SEM) analysis was performed to determine the size of the nanoparticles. X-ray powder diffraction analysis is one of the standard methods to characterize nanoparticles. SEM and XRD characterization were performed as suggested previously by Ramamurthy et al. (2013). The changes of colour reaction was monitored continuously and checked for every four hours. The morphological characteristics of nanoparticles were analyzed using SEM. Sample was dried in an oven at 60 °C and powdered using a mortar and pestle and the uniformed sample size was used for the determination of XRD (Thomas et al., 2019; Gomathi et al., 2020; Jayaprakash et al., 2017). The diameter of silver nanoparticles was analyzed using Scherer equation as suggested previously by Yousefzadi et al. (2014).
2.4 Bacterial inoculums preparation
The pathogenic bacteria such as, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 700802, Streptococcus gordonii ATCC 49818, Staphylococcus aureus (MRSA) ATCC 4330 and Staphylococcus aureus ATCC 25923 were cultured in nutrient broth (Himedia, Mumbai, India) medium and incubated for 18 h at 37 ± 1 °C. The growth was monitored using UV–visible spectrophotometer at 600 nm and the optical density of the culture was maintained as 1.0 and used for further studies (Zhang et al., 2020).
2.5 Antimicrobial activity of silver nanoparticles
Disc diffusion method was used for the determination of antimicrobial activity of the silver nanoparticles. The green synthesized nanoparticles were dissolved in dimethyl sulfoxide (DMSO, 5%) and sonicated the extract for 10 min at room temperature (28 ± 2 °C). Each disc was loaded with 50 µg silver nanoparticles and used for antibacterial assay. The inoculum cultured in nutrient broth medium was diluted individually and the cell density of the inoculum was approximately 1 × 105 colony forming unit/mL (CFU/mL) and the zone of inhibition was assayed in mm (Gandhi et al., 2021).
2.6 Minimum inhibitory concentration and minimum bactericidal concentration determination
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the sample was determined by broth dilution method as described previously in the literature (Kubo et al., 2004). Silver nanoparticles were diluted at various concentrations (100–1.25%). Initially, silver nanoparticles were serially diluted in nutrient broth containing nanoparticles and continuously the dilution was performed until the dilution reached 1.25%. To the control vial, only nutrient broth was applied. This experiment was performed for all the selected bacterial isolates. The bacterial strains were inoculated and the least concentration which inhibited the bacterial growth at very least doses was considered as MIC. Then the sample was spread on nutrient agar plants and incubated for 37 °C for 24 h. and the CFU value was determined. The values were expressed as μg/mL nanoparticles for MIC and MBC (Kalaiyarasi et al., 2020; Al-Dhabi et al., 2020).
2.7 Synergistic activity of silver nanoparticles
Synergistic activity of green synthesized nanoparticles was analyzed as described previously. Briefly, rifampicin was used as the standard drug and it was diluted with Millipore sterile distilled water. The standard antibiotic, rifampicin was mixed with green synthesized nanoparticles and antibacterial activity was assayed by standard method (Naqvi et al., 2013). The tested bacterial pathogens (P. aeruginosa ATCC 27853, E. coli ATCC 25922, E. faecalis 700802, S. gordonii ATCC 49818, S. aureus (MRSA) ATCC 4330 and S. aureus ATCC 25923) were cultured in newly prepared nutrient broth medium and the standard antibiotic was mixed with nanoparticles. The antibiotic disc was prepared and placed on bacterial culture plates. Each disc contains 5 μg standard and 10 μg silver nanoparticles and the culture was incubated for 24 h and synergistic antimicrobial potential was observed (Malar et al., 2020; Zhang et al., 2020; Al-Ansari et al., 2020).
2.8 Antioxidant activity of green synthesized silver nanoparticles
2.8.1 DPPH scavenging activity
The DPPH free radical scavenging activity of nanoparticles assay was carried out as suggested earlier. The nanoparticles were diluted at various concentrations (10–50 µg/mL) and ascorbic acid was used as the external standard. The absorbance of the reaction was performed at 517 nm against control blank and the percentage scavenging activity was calculated (Ezhilarasi et al., 2020).
2.8.2 ABTS radical scavenging activity
The ABTS radical scavenging activity of nanoparticles was performed as suggested earlier. Nanoparticles were diluted appropriately (10– 0 µg/mL) and ascorbic acid was used as the external standard. The absorbance of the sample was analyzed at 750 nm against control blank and the percentage radical scavenging potential was obtained (Ezhilarasi et al., 2020).
2.8.3 Reducing power assay
The reducing potential of nanoparticles was analyzed as described earlier. Nanoparticles were diluted appropriately (10–50 µg/mL) and the absorbance of the sample was read at 700 nm against the reagent the control blank and the result was expressed as percentage reducing power (Ezhilarasi et al., 2020).
2.8.4 Nitric oxide scavenging activity
Nitric oxide (NO) scavenging assay was performed as described previously. Nanoparticles were diluted appropriately (10–50 µg/mL) and the optical density of the reaction mixture was read at 546 nm and the percentage scavenging activity was determined (Ezhilarasi et al., 2020).
2.8.5 Biocompatibility of silver nanoparticles
The normal fibroblast (L-929) cells were used for the determination of biocompatibility by analyzing the viability of the cells. The culture flask was seeded with Dulbecco’s Modified Eagle’s Medium (DMEM), M-199 medium, fetal bovine serum (10%) and incubated for 24 h at 37 °C with 5% CO2. After 24 h incubation, the adhered mammalian cells were trypsinized (5 min) and the individual cells were separated by centrifugation (1000 rpm, 5 min). The cells were further placed in 96-well microtiter plate with approximately 5000 cells/well and incubated for 12 h to achieve a monolayer with ∼80 to 90% confluence (Song and Kim, 2009). Silver nanoparticles reduced ATP content of the cell and involved in the mitochondrial damage and increased the generation of reactive oxygen species (ROS) and the increase of ROS was based on nanoparticles concentrations. In this study nanoparticles were added at various concentrations ranging from 200 to 1200 μg/mL. To assay the viability of L-929 cells, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution was added and incubated for 5–6 h. After reaction, MTT reagent was completely discarded and DMSO (250 µL) was added to the wells and incubated for 20 min under dark condition. The developed formazan was determined at 595 nm using a microtiter plate reader (Al-Ansari et al., 2021; Wu et al., 2020).
3 Results
3.1 Green synthesis of silver nanoparticles
The pea pod was used for the green synthesis of silver nanoparticles and 1 mM silver nitrate solution was used initially. The colour change was observed and the synthesis of nanoparticles was observed. The colour of the solution was turned dark brown after 2 h of incubation. The colour intensity was improved further and no colour change was observed after 4 h of treatment. The development of silver nanoparticles was observed using various characterization methods.
3.2 Characterization of nanoparticles
The green synthesized nanoparticles were tested and the absorption spectra were monitored continuously to confirm nanoparticles in the sample. The absorbance spectrum indicated the development of silver nanoparticles. The UV-spectrum of the sample was monitored continuously for 36 h and no visible colour change in the nanoparticles after 24 h incubation. The morphology of the green synthesized nanoparticles was further characterized using HRTEM. The synthesized nanoparticles were approximately 30 nm and observed uniform sized particle (Fig. 1). SAED analysis of green synthesized nanoparticles was described in Fig. 2. Fig. 3 shows EDX spectrum and it revealed the chemical composition and purity of the silver nanoparticles. The percentage silver metal observed in this study was more appropriate. The other elements such as Cu, Mg, and Na were detected at lower percentages.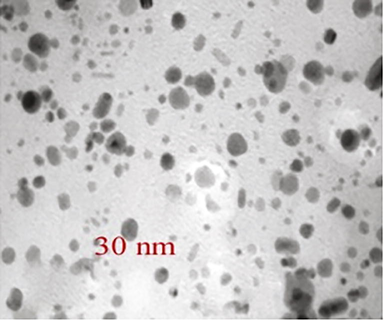
HRTEM analysis of green synthesized silver nanoparticles.
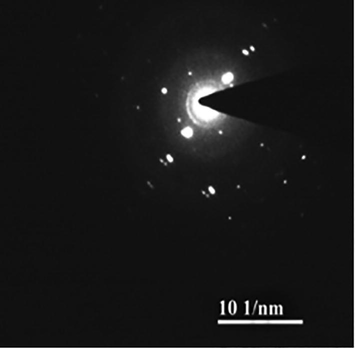
Selected area for electron diffraction (SAED) analysis of green synthesized nanoparticles.
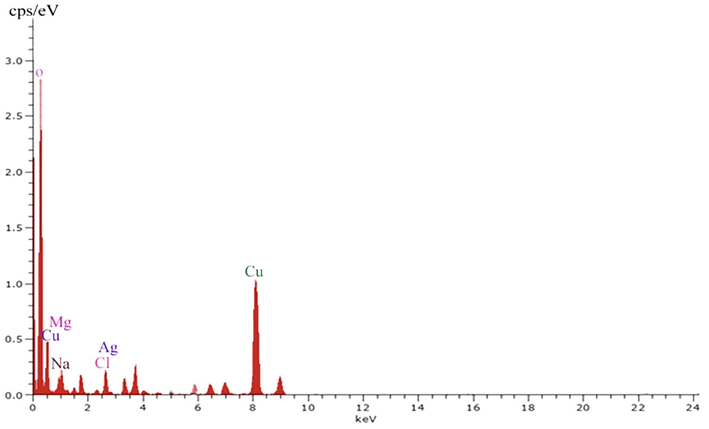
EDX analysis of green synthesized silver nanoparticles.
3.3 Antibacterial activity of intestinal pathogens
The human intestinal pathogens such as, P. aeruginosa ATCC 27853, E. coli ATCC 25922, E. faecalis 700802, S. gordonii ATCC 49818, S. aureus (MRSA) ATCC 4330 and S. aureus ATCC 25923 were used for antibacterial assay and the pea pod synthesized nanoparticles showed activity against these tested bacterial pathogens. The antibacterial activity of silver nanoparticles was described in the Table 1. Among the pathogenic bacterial strains, silver nanoparticles were highly active against S. aureus ATCC 25923 (22 ± 2 mm) than other bacterial strains. The antibacterial activity varied between 15 ± 2 mm and 22 ± 2 mm zone of inhibition. The MIC value ranged from 6.25 µg/mL to 75 µg/mL and MBC value ranged between 50 µg/mL and 150 µg/mL.
Bacteria
Zone of inhibition (mm)
MIC (µg/mL)
MBC (µg/mL)
P. aeruginosa ATCC 27853
15 ± 2
50
100
E. coli ATCC 25922
17 ± 1
50
150
E. faecalis 700802
15 ± 3
75
125
S. gordonii ATCC 49818
18 ± 2
12.5
75
S. aureus (MRSA) ATCC 4330
17 ± 1
12.5
75
S. aureus ATCC 25923
22 ± 2
6.25
50
3.4 Synergistic activity of silver nanoparticles
Synergistic activity of green synthesized nanoparticles was analyzed with rifampicin. The zone of inhibition was 18 ± 2 mm against P. aeruginosa ATCC 27,853 and silver nanoparticles treated antibiotic increased zone of inhibition (21 ± 2 mm). Synergistic activity was observed against S. aureus ATCC 25923 (24 ± 2 mm). Moreover, supplementation of nanoparticles with antibiotics did not improve antimicrobial activity and the result was described in Table 2. Both rifampicin and AgNO3 incorporated disc showed synergistic antibacterial activity against drug resistant bacteria.
Zone of inhibition (mm)
Bacteria
Rifampicin
AgNO3 + Rifampicin
P. aeruginosa ATCC 27853
18 ± 2
21 ± 2
E. coli ATCC 25922
13 ± 1
12 ± 1
E. faecalis 700802
15 ± 2
17 ± 3
S. gordonii ATCC 49818
17 ± 1
19 ± 2
S. aureus (MRSA) ATCC 4330
19 ± 3
21 ± 1
S. aureus ATCC 25923
21 ± 1
24 ± 2
3.5 Antioxidant activity
The antioxidant properties of the silver nanoparticles were analyzed and the result was described in Fig. 4. The DPPH activity of green synthesized nanoparticles was described in Fig. 4a and showed antioxidant activity of 68 ± 3.1% at 50 µg/mL concentration. Nanoparticles showed increased NO activity at 50 µg/mL concentration (86 ± 2.8%) (Fig. 4b). The ABTS activity of nanoparticles was described in Fig. 4c and attained 68 ± 2.7% activities at 50 µg/mL. The reducing activity was 27 ± 21% at 10 µg/mL concentrations and it increased at 50 µg/mL (74 ± 1.5%) (Fig. 4d).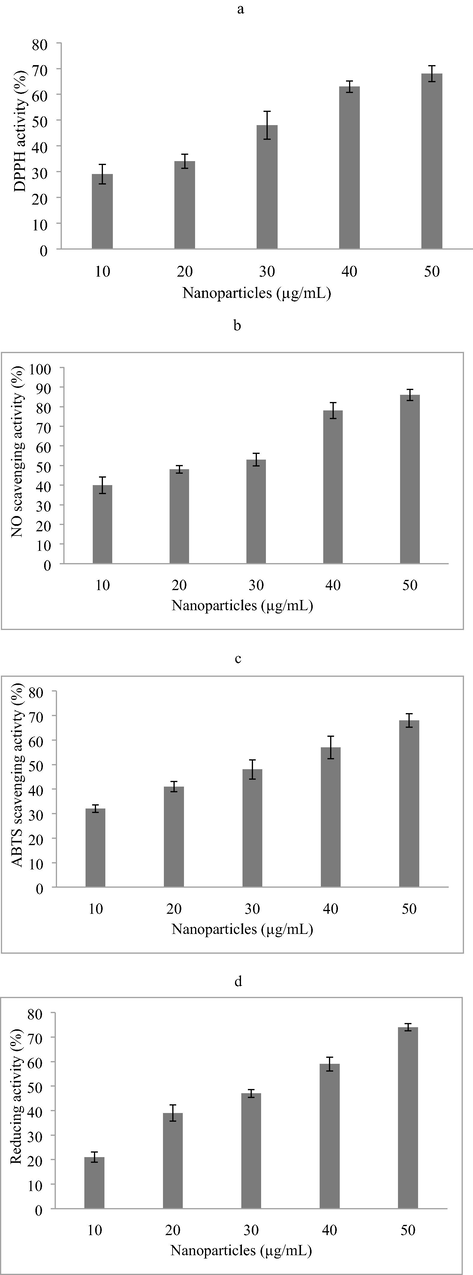
Antioxidant activity of nanoparticles (A) DPPH radical scavenging activity, (b) Nitric oxide scavenging activity; (c) ABTS radical scavenging activity, (d) Reducing power activity.
3.6 Biocompatibility of green synthesized nanoparticles
Biocompatibility analysis is widely used to analyze the possible application in biomedicine and drug use or food additives. Normal fibroblast cell lines (L-929) was used to test the cytotoxic activity using nanoparticles. The toxicity of silver nanoparticles is one of the important concerns on cell lines which significantly influenced on biological uses. At lower concentrations of silver nanoparticles, no inhibitory effect was observed. Moreover, the cell viability declined at increasing concentration of nanoparticles and the result was depicted in Fig. 5. The IC50 value of the green synthesized nanoparticles showed 750.5 ± 5.5 μg/mL which indicated maximum biological compatibility (Fig. 5). The pod extract did not induced toxicity against L-929 cell lines and indicated potential application in medicine and food.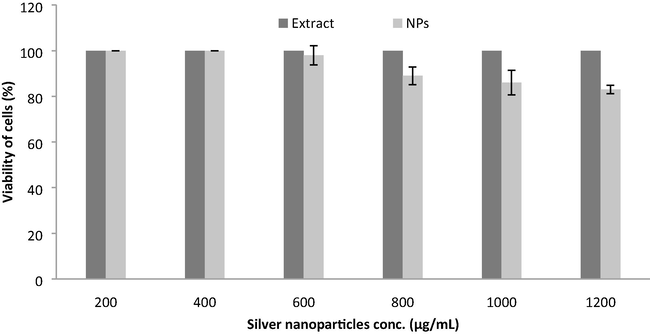
Biocompatibility analysis of pod extract and green synthesized nanoparticles on L929 fibroblast cell lines. Pod extract was considered as control. Error bar indicates standard deviation.
4 Discussion
Medicinal plants are widely used to treat various diseases and disorders. The secondary metabolites produced by the medicinal plants have therapeutic applications. The bioactive potential of medicinal plants varied based on extraction methods, plant parts, growth phases, climatic condition and seasons. The consumption of vegetables has been earlier proven to minimize the risk of various diseases and disorders. P. sativum L. is widely used as astringent, laxative, refrigerant, appetizer, and also used in treating phlegm, acne, diabetes, skin and intestinal inflammation (Yusuf et al., 2007; Angels and Joan, 2007). Hypoglycemic, antimicrobial and antioxidant activities have been observed from this plant. Interestingly, the pod contains potential antihyperglycemic activity (Taha et al., 2011). Petiole and tendril showed the presence of chemical compounds such as, p-coumaric esters, quercetin-3-triglucoside, and kaempferol-3-triglucoside. Plant mediated nanoparticles synthesis is highly efficient than microbial biosynthesis of nanoparticles. Plants have various phytochemicals, including polyphenols that can effectively act as stabilizing agent in the green synthesis of nanoparticles. In this study, pea pod was used as the reducing agent for the green synthesis of nanoparticles. Unlike chemical methods, plant based green synthesis of nanoparticles is economical and eco-friendly. Also, nanoparticles synthesized using plant sources have been highly stable than fungi and microbes (Gomathi et al., 2020).
Phytochemicals are widely used to prepare silver nanoparticles and it generally requires current up to date knowledge on the pharmacological properties (Valsalam et al., 2019). The leaf extract of A. indica has been used previously for the biosynthesis of nanoparticles and spherical shaped nanoparticles were observed. The phytochemical components involved in reducing and capping nanoparticles. Green synthesized nanoparticles have been showed antimicrobial activity against various drug resistant bacterial pathogens (Ahmed et al., 2016). The unrestricted use of commercial antibiotics induced the development of drug resistance among bacteria. This drug resistance may be the result of defence mechanism by bacteria due to horizontal gene transfer. The enzymes synthesized by the bacteria degrade or inactivate the administered drugs (Poole, 2002). Silver is widely used as antimicrobial agents and the synthesized nanoparticles were effective against various diseases. In this study, the green synthesized nanoparticles were effective against P. aeruginosa ATCC 27853, E. coli ATCC 25922, E. faecalis 700802, S. gordonii ATCC 49818, S. aureus (MRSA) ATCC 4330 and S. aureus ATCC 25923. Likewise, antibiotics incorporated with nanoparticles improved synergistic activity. The pattern of antimicrobial activity varied from previous results indicated varying shape, size and strains used for antimicrobial testing. Rifampicin was used to test the synergistic activity with silver nanoparticles and results revealed improved zone of inhibition (18 ± 2 mm against P. aeruginosa ATCC 27853) and silver nanoparticle treated antibiotic increased zone of inhibition (21 ± 2 mm). The nanoparticles showed DPPH scavenging, reducing power, ABTS radical scavenging, and nitric oxide scavenging activities. The antioxidant potential of nanoparticles has been previously described (Thomas et al., 2019).
The green synthesized silver nanoparticles have been showed antibacterial activity against multi-drug bacterial strains (Mani et al., 2021). Likewise, the green synthesized zinc oxide nanoparticles prepared using Tabernaemontana divaricata showed improved antimicrobial potential (Raja et al., 2018). Anand et al. (2020) have been characterized nickel oxide nanoparticles and reported antimicrobial activities against drug resistant bacterial strains. Silver nanoparticles have been synthesized using Moringa oleifera flower (Bindhu et al., 2020), rizhome extract of Curcuma longa and Zingiber officinale (Venkatadri et al., 2020), floral extract of Chrysanthemum indicum L. (Arokiyaraj et al., 2015), M. oleifera (Surendra et al., 2016), Acorus calamus extract (Arasu et al., 2019), and these nanoparticles showed antioxidant, antimicrobial and dye degrading properties. Prunus dulcis (Almond Gum) extract has been used for the green synthesis of ZnO nanoparticles and showed potential activity against antibiotic resistant bacteria (Anand et al., 2019). NiO nanoparticles green synthesized using Aegle marmelos leaf extract have been showed antibacterial activity (Ezhilarasi et al., 2018). The green synthesized nanoparticles showed less toxicity to cell lines. In this study, we analyzed the biological properties of nanoparticles in human fibroblast cell lines (L929). The cell viability was not declined significantly than control cell lines.
5 Conclusions
The green synthesized silver nanoparticles showed potent activity against the foodborne bacterial pathogens. Nanoparticles have the potential to kill both Gram-negative and Gram-positive bacteria. Hence, the green synthesized nanoparticles could be formulated as novel antibacterial agent against both multi-drug resistant Gram-positive and Gram-negative bacteria. Silver nanoparticles may lead to develop various products including food packing materials, coating in medical devices and to develop antimicrobial drugs.
Acknowledgements
The authors extend their appreciation to the Researchers supporting project number (RSP-2021/185), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
We have no conflict of interest for publishing this paper.
References
- Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci.. 2016;9(1):1-7.
- [Google Scholar]
- Insecticidal, antimicrobial and antioxidant activities of essential oil from Lavandula latifolia L. and its deterrent effects on Euphoria leucographa. Ind. Crop. Prod.. 2021;170:113740.
- [CrossRef] [Google Scholar]
- Optimization of medium components for the production of antimicrobial and anticancer secondary metabolites from Streptomyces sp. AS11 isolated from the marine environment. J. King Saud Univ. Sci.. 2020;32(3):1993-1998.
- [Google Scholar]
- Probiotic and antioxidant potential of Lactobacillus reuteriLR12 and Lactobacillus lactisll10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms. 2020;8(10):1461.
- [Google Scholar]
- Bio-synthesis of silver nanoparticles using agroforestry residue and their catalytic degradation for sustainable waste management. J. Clust. Sci.. 2017;28(4):2279-2291.
- [Google Scholar]
- Green synthesis of ZnO nanoparticle using Prunus dulcis (Almond Gum) for antimicrobial and supercapacitor applications. Surface Interface.. 2019;17:100376.
- [CrossRef] [Google Scholar]
- Structural and optical properties of nickel oxide nanoparticles: Investigation of antimicrobial applications. Surface Interface.. 2020;18:100460.
- [CrossRef] [Google Scholar]
- Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian peninsula): plants used in veterinary medicine. J. Ethnopharmacol.. 2007;110(1):130-147.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B: Biol.. 2019;190:154-162.
- [Google Scholar]
- Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.—potential for malaria vector control. Environ. Sci. Poll. Res.. 2015;22(13):9759-9765.
- [Google Scholar]
- One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int. J. Nanomed.. 2013;8:1307-1315.
- [Google Scholar]
- Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B: Biol.. 2020;205:111836.
- [Google Scholar]
- Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res. Lett.. 2016;11:40.
- [Google Scholar]
- The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces. 2012;98:112-119.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochm. Photobiol. B: Biol.. 2018;180:39-50.
- [Google Scholar]
- Green synthesis of nickel oxide nanoparticles using Solanum trilobatum extract for cytotoxicity, antibacterial and photocatalytic studies. Surface. Interface.. 2020;20:100553.
- [CrossRef] [Google Scholar]
- Annealing dependent synthesis of cyto-compatible nano-silver/calcium hydroxyapatite composite for antimicrobial activities. Arab. J. Chem.. 2021;14(11):103404.
- [CrossRef] [Google Scholar]
- Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug. Deliv. Sci. Technol.. 2020;55:101376.
- [Google Scholar]
- Valorization of the peel of pea: Pisum sativum by evaluation of its antioxidant and antimicrobial activities. J. Oleo. Sci.. 2014;63(11):1177-1183.
- [Google Scholar]
- Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterialstudies. J. Photochm. Photobiol. B: Biol.. 2017;169:178-185.
- [Google Scholar]
- Enhanced production antibiotics using green gram husk medium by Streptomyces sp. SD1 using response surface methodology. J. King Saud Univ. Sci.. 2020;32(3):2134-2141.
- [Google Scholar]
- Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J. Agric. Food Chem.. 2004;52(11):3329-3332.
- [Google Scholar]
- In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saud. J. Biol. Sci.. 2020;27(2):682-688.
- [Google Scholar]
- Systematic green synthesis of silver oxide nanoparticles for antimicrobial activity. Environ. Res.. 2021;202:111627.
- [CrossRef] [Google Scholar]
- Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv.. 2013;31(2):346-356.
- [Google Scholar]
- Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma applanatum (Pers.) pat. from similipal biosphere reserve. Odisha, India. IET Nanobiotechnol.. 2016;10(4):184-189.
- [Google Scholar]
- Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed.. 2013;8:3187-3195.
- [Google Scholar]
- Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci.. 2015;457:329-338.
- [Google Scholar]
- Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol.. 2002;92:55S-64S.
- [Google Scholar]
- Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochm. Photobiol. B: Biol.. 2018;181:53-58.
- [Google Scholar]
- I.The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloid. Surf. B Biointer.. 2013;102:808-815.
- [Google Scholar]
- Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng.. 2009;32:79-84.
- [Google Scholar]
- RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol. B: Biol.. 2016;162:550-557.
- [Google Scholar]
- The pericarp of Pisum sativum L. (Fabaceae) as a biologically active waste product. Planta Med.. 2011;77(12):PJ22.
- [Google Scholar]
- Antioxidant and photocatalytic activity of aqueous leaf extract mediated green synthesis of silver nanoparticles using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol.. 2019;19(5):2640-2648.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B: Biol.. 2019;191:65-74.
- [Google Scholar]
- Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saud J. Biol. Sci.. 2020;27(11):2980-2986.
- [Google Scholar]
- Characterization of biofilm formed by multidrug resistant Pseudomonas aeruginosa DC-17 isolated from dental caries. Saud. J. Biol. Sci.. 2020;27(11):2955-2960.
- [Google Scholar]
- The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen) J. Agardh. Mater Lett.. 2014;137:1-4.
- [CrossRef] [Google Scholar]
- Some tribal medicinal plants of Chittagong hill tracts, Bangladesh. Ban. J. Plant Taxon.. 2007;14(2):117-128.
- [CrossRef] [Google Scholar]
- Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saud. J. Biol. Sci.. 2020;27(12):3505-3513.
- [Google Scholar]







