Translate this page into:
Green synthesis, characterization of silver nanoparticles using aqueous leaf extracts of Solanum melongena and in vitro evaluation of antibacterial, pesticidal and anticancer activity in human MDA-MB-231 breast cancer cell lines
⁎Corresponding authors. karthika_zoo@avinuty.ac.in (Karthika Pushparaj), arun47biotech@gmail.com (Arun Meyyazhagan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present study validated the green synthesis and characterization of AgNPs using the leaf extracts of Solanum melongena (SM-AgNPs). The efficiency of the phytocompounds in the leaf extract in reducing Ag+ to Ag0 ions was studied. The synthesized SM-AgNPs were characterized by spectroscopic methods and standard methods were adopted for the antioxidant activity, HPTLC, cytotoxicity and antimicrobial assays and pesticidal activity as well. The synthesised nanoparticle was measured to be 20.54 nm which was confirmed by the characterization. The total phenolic content was 5.98 ± 0. GAE/g which indicated potent antioxidant activity. HPTLC profile showed thirteen peaks corresponding to the phenolic compounds with Rf values ranging from 0.05 to 0.95. The average particle size and zeta potential was 75.14 nm-19.8 mV respectively. Cytotoxicity assay of the synthesized AgNP’s showed viability percentage of 50.23 at 100 mg/mL. Treatment of MDA-MB-231 cell lines with the SM-AgNPs induced early apoptosis in a greater number of cells. The synthesizedAgNPs were effective against the bacterial isolates Escherichia coli, Pseudomonas aeruginosa, Shigella flexneri, Proteus vulgaris, Klebsiella pneumoniae and Staphylococcus aureus. High mortality of Bemisiatabaci was observed in the100 µg/mL of the SM-AgNPs treated concentrations. The study demonstrated the efficiency of SM-AgNPs against cancer cell lines, microbial isolates and insect pests.
Keywords
Solanum melongena
Leaves
Silver nanoparticles
Cytotoxicity
Anti-bacterial
Pesticidal
1 Introduction
The association between nanomaterials and mankind naturally developed before several hundreds of years and has deep roots in Indian Traditional System of Medicine (Pan et al., 2014). Nano biotechnologies have transformed a wide array of applications into state- of –art technologies which play a promising role in the food sector (Pushparaj et al., 2022), bio-medical applications and diagnostics (Balu et al., 2021), bio-sensors, artificial implants and integrated pest management (Bamal et al. 2021). Amongst the wide variety of nanomaterials, gold and silver and other polymeric nanoparticles were the commonest choice for the research works which aimed at targeted drug delivery and biocompatibility studies (Yeung et al., 2020). This implied the necessity and thirst of alternative tools which are in parallel devoid of side effects were the need of the hour to counteract the environmental problems. Plethora of literature has evidenced that the plants have the richest source of potential secondary metabolites like phenols, alkaloids, tannins, flavonoids which exhibit the active inhibition properties against pathogens. Therefore, the integration of these sources along with the nano-carrier materials like silver will undoubtedly provide targeted and full-fledged action (Rahman et al., 2021; Ramadan et al., 2022). The plant-mediated synthesis of nanoparticles occupies the apex among various other methods because of ease in availability of plants, safe, and non-toxic in mainly the presence of array of plant metabolites aid quick reduction of silver ions. The silver nanoparticles (AgNPs) are most preferred due to excellent biocompatibility. The present study involved the green synthesis AgNPs using aqueous leaf extract of Solanum melongena. L. The plant is a native of India and is widely studied for its therapeutic properties due to abundant impactful bioactive chemicals like phenolics, caffeic acid, quinic acid, cinnamic acid and chlorogenic acid, flavonoids such as nasunin and quercetin and bioflavonoid glycoside named solanoflavonein (Helmja et al., 2009; El-Beeh et al., 2022). Therefore, based on the above reports the present study utilized the S. melongena leaf extract for the green synthesis of AgNPs (SM-AgNPs). Further, the study aimed to characterize and analyse the effectiveness of the green synthesized SM-AgNPs for anti-cancer, anti-bacterial and pesticidal properties.
2 Materials and methods
2.1 Green synthesis of AgNPs
Fresh leaves of SM were collected from Mettupalayam, Tamilnadu, South India. The dried leaves (10 g) were pulverized and sieved to fine powder, mixed with 100 mL of double distilled water, boiled for 30 min and filtered. Concentration of the silver nitrate solution was optimized, 1–5 mL of SM extract was added to 1–5 mM AgNO3 solution. Reaction time for the reduction varied from 0 to 5 mins and the absorbance was noted. Obvious colour change indicated the formation of AgNPs (Fig. 1A) and confirmed by using UV–Visible spectroscopy.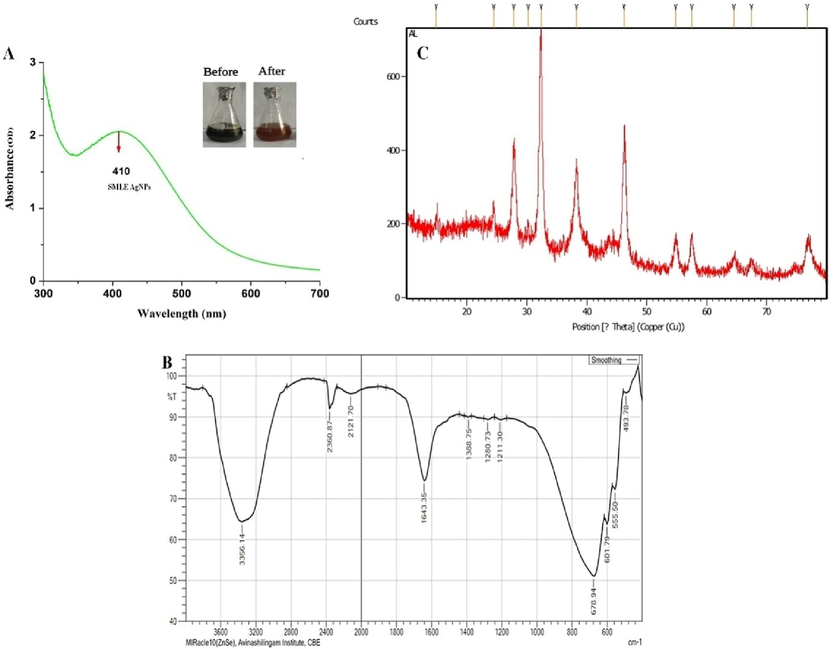
UV–visible spectrum (A); the inner figure shows the notable colour change after synthesis of AgNP, FT-IR spectra (B) and XRD pattern of green synthesized SM-AgNPs (C).
2.2 Characterization of SM-AgNPs
Validation of optical property of the synthesized silver nanoparticles was measured by absorbance at 200–800 nm using UV–visible spectroscopy. The FT-IR (Shimadzu, FTIR–8400S) analysis was done and bands were probed in the fingerprint region between 4000 and 500 cm-1. Further, dried powder of SM-AgNPs were fabricated on glass surface and XRD spectra was documented using X-ray diffractometer (X’ Pert Pro PANalytical’s) at fixed parameters. The size, surface morphology of SM-AgNPs was characterized by using FEI QUANTA 250EDAX and FEI TECHNAI SPRIT, Netherlands with magnification of 20X −30,000X, spatial resolution of 50–100 nm. Double side carbon tape was fabricated with SM-AgNPs (0.5 mg) and mounted on 8 mm di-aluminium stub and observed at different magnification and the images were captured. Dynamic light scattering (DLS) was done to estimate the particle size dispersal of SM-AgNPs (Nanopartica, SZ100-Horiba).
2.3 Phytochemical screening, estimation of phenols and HPTLC profile
Qualitative screening of the plant secondary metabolites was performed using the standard methods (Harborne, 1973). Total phenolic contents were determined by the slightly modified Folin-Ciocalteu method described by Wolfe et al. (2003). HPTLC was carried out following the method of Syed et al. (2013).
2.4 Antioxidant activity by DPPH assay
The antioxidant activity, 1, 1-diphenyl-2-picrylhydrazil (DPPH) radical scavenging assay was adopted by the modified method of McDonald et al. (2001). The results were calculated in percentage of radical scavenging activity using butylatedhydroxytoluene (BHT) as standard.
2.5 Cell viability assay
The extent of cytotoxicity of the synthesized sample in the MD-MBA-23 cancer cell lines were determined by the MTT dye reduction assay (Igarashi and Miyazawa, 2001). About 100 μL of treated cells were incubated with 50 μL of MTT at 37 °C for 3 h. After 10-15 min, added 200 μL of PBS to samples, MTT was aspirated and removed. 200 μL of acid-propanol was added and kept undisturbed overnight in dark. The absorbance was noted at 650 nm in a microtitre plate reader (Bio Rad U.S.A). The optical density of the control was fixed, and the percent viability of the treated cells was calculated by standard methods.
2.6 Flow cytometry/cell cycle analysis
The MD MBA 23 cells (5x105 cells /well) were plated in 6-well micro plates. Cells were thoroughly washed. After treatment with IC50 concentration of SM-AgNPs, cells were thoroughly washed twice and suspended in 300 μL of PBS, and later fixed with 4 mL of ice-cold 70% ethanol. Cells were stained with Propidium iodide (PI) and post centrifugation, the cells were washed again with PBS. The pellets were suspended in 1 mL of PI/Triton X-100 staining solution (0.1% Triton X-100 in PBS, 0.2 mg/ml RNase A, and10mg/ml PI) and incubated for 30 min at room temperature. The stained cells were analyzed using Mo-Flo flow cytometer (DakoCytomation, Glostrup) to calculate the percentage of cells in the different phases of the cell cycle.
2.7 Single cell gel electrophoresis (SCGE) /comet assay
Quantification of the DNA damage in treated cell lines was evaluated by comet assay (Bowman et al., 2014). Marking were noted for presence of the “tail”, 100 cells/ slide were scored and the frequency of DNA damage was evaluated for the number of cells bearing the comet.
2.8 Antibacterial activity of SM-AgNPs
Agar well diffusion technique was adopted for using selected bacterial isolates Escherichiacoli (MTCC 443), Pseudomonasaeruginosa (MTCC 741), Shigella flexneri (MTCC 1457), Proteusvulgaris (MTCC 426), Klebsiella pneumoniae (MTCC 109) and Staphylococcus aureus (MTCC 96), obtained the stock bacterial pathogens from the Department of Microbiology, Periyar University, India. The inoculum was swabbed on sterile Muller-Hinton agar (MHA) loaded petriplates. Two wells were bored onto the above agar plates and 20 μL of the SM-AgNPs and chloramphenicol (positive control) were added separately. They were incubated at 37◦C for 24 h and the inhibition zone formed around the well was noted (Bauer et al., 1966).
2.9 Pesticidal analysis
Adults of Bemisia tabaci were collected from the citrus crops in the fields of Mettupalayam and transported to laboratory. About 500 adult flies were kept in a cage and fed with the leaves of guava. Optimum laboratory conditions were maintained with the temperature of 25 ± 2.5 °C, relative humidity of 65 ± 5% and a photoperiod of 14 N:7D in the chamber. The adults were subjected to bioassay experiments. The adults were treated with 2.5, 5, 10, 15, 20, 25, 50 and 100 µg/mL concentrations of synthesized SM-AgNPs and the mortality was noted.
2.10 Statistical analysis
Two-way ANOVA was performed to test the efficiency of the dose, where the interaction of the factors was F (14, 96) = 99.75, the comparison were found to be highly significant at p < 0.001. All the data were presented as the mean ± standard error of three experiments. All the tests were designed using GraphPad Prism 9.0.
3 Results
Reduction of silver ions into AgNPs by SM extract was confirmed by the visual change of colour from dark yellow to reddish brown. The absorbance peak at 441 nm further validated the formation of AgNPs shown in Fig. 1A. The brown color of the solution indicated the completion of the reduction reaction which is due to the consequence of increased concentration of silver nitrate. The FT-IR spectrum of green synthesised SM-AgNPs exposed prominent peaks at 3356.1, 2360, 2121 and 1643 cm-1 which signified the occurrence of carboxyl, hydroxyl and amine groups in the progression of synthesis shown in Fig. 1B. The XRD patterns of the SM-AgNPs are shown in Fig. 1C. The XRD spectrum ensured the formation of nanocrystals, which were confirmed by the 2θ values of 38.14°, 53.05°, 66.04°, and 77.17° corresponding to 111, 200, 300, and 311 planes for silver, respectively. The EDS analysis of the SM-AgNPs confirmed the presence of silver element in the sample. The EDS spectrum showed a significant peak at 3.00 keV (Fig. 2) which is the characteristic absorption of the metallic silver due to SPR. The EDS spectrum provided the quantitative and qualitative composition of elements like C, O, Cl, K. Ag, K and Ca the composition of Ag+ions was maximum up to 40.88%. The morphological structure of SM-AgNPs was characterized using FE-SEM the particles were observed to be granular, irregular and spherical in shape as shown in Fig. 2. The size distribution histogram of DLS indicated that the particle corresponded 20.54 nm. The DLS histogram of green synthesized SM-AgNPs are shown in Fig. 3 and the size of SM-AgNPs ranged from 20 to 120 nm. The average particle size distribution of SM-AgNPs was 75.14 nm. The zeta potential of the SM-AgNPs had a prominent peak at − 19.8 mV.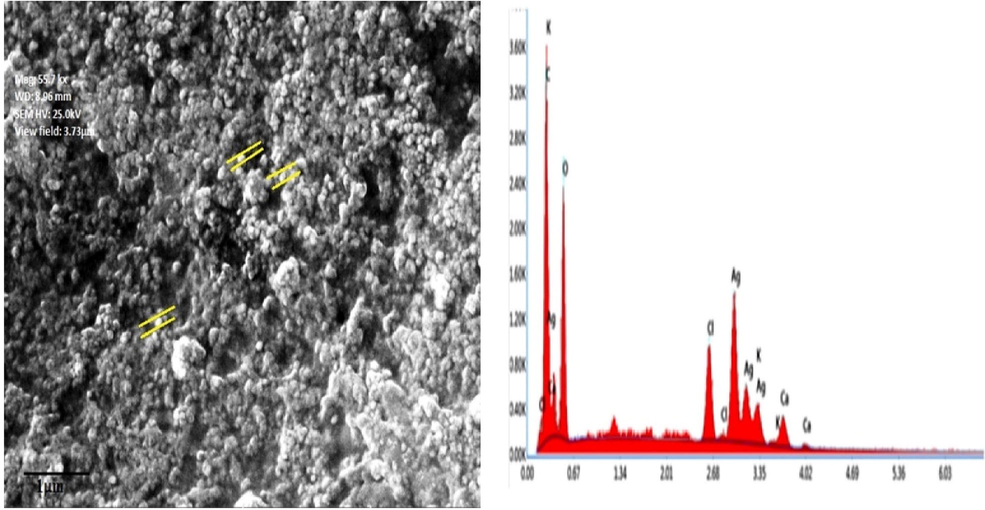
FESEM micrographs and EDS analysis of synthesized SM-AgNPs.
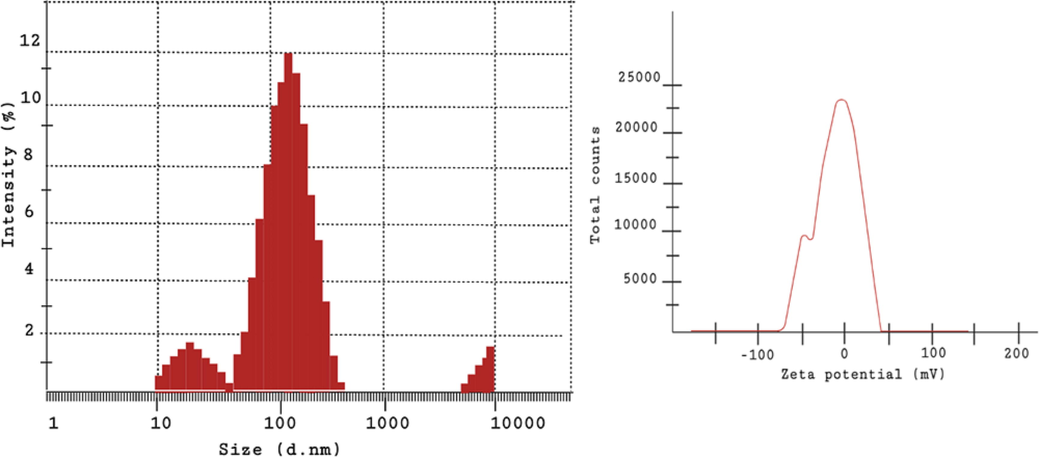
DLS and the zeta potential depicting the size distribution image of the SM-AgNPs.
The SM-AgNPs showed that the presence of alkaloids, phenol, anthocyanin, flavanoids and saponin. The total phenol content of the synthesized AgNPs was 5.98 ± 0.19 mg gallic acid equivalent/g samples (GAE/g).The HPTLC chromatogram (Fig. 4) showed the presence of bands corresponding to phenolic compounds and peaks in densitogram showed thirteen peaks of phenolic compounds with the respective of Rf values ranging from 0.05 to 0.95 (Supplementary material Table 1). Nine of the peaks corresponded to known compounds, the Rf value of the quercetin standard (0.51) closely matched with one of the phenol compound (Rf = 0.50, phenol 4) present in the SM-AgNPs.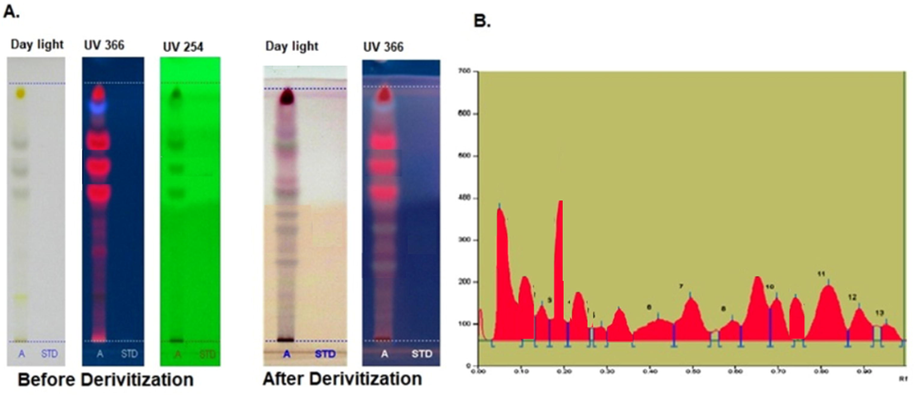
The phenol profile of green synthesized SM-AgNPs using HPTLC analysis (A) Peak densitogram of phenolic compounds in the sample (B).
The presence of scavenging free radicals was confirmed by the color change from purple to yellow which exhibited the antioxidant activity. The antioxidant potential of SM-AgNPs was evaluated by DPPH radical scavenging assay having IC50 38.04 μg/mL. The synthesized SM-AgNPs was evaluated for the anti-cancerous activity on the breast cancer cell line MD MBA 23. The numbers of viable cells were found to decrease with the increasing concentration of the drug. However, the IC50 was achieved for 100 mg/mL with the viability percentage of 50.23 shown in Fig. 5A. From the cell line photographs (Fig. 5B), it was evidenced that the death was due to the shrinkage of cells and the disruption of cellular components.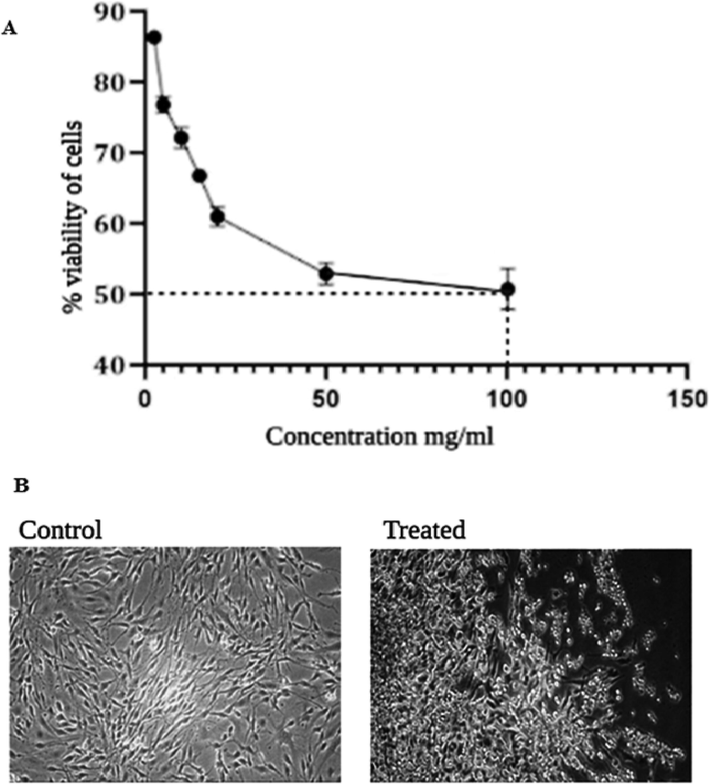
Cytotoxicity assessment in MDA MB-231 cell line exposed to 100 mg/ml concentration of SM-AgNPs for 24 h using MTT assay (A). Alterations in the morphology of MDA MB 231 cell line due to the cytotoxic effect SM-AgNPs (B).
The Annexin-V/FITC-PI assay identified that larger percentage of viable cells groups are present in untreated, conversely a large number of treated cells were in early apoptotic stage and late apoptotic stage (Fig. 6), while a few in necrotic stages. Therefore, it is noted that SM-AgNPs caused a significant rise in the percentage of AnnexinV/FITC positive cells (upper right quadrant) indicating late apoptosis. In the comet assay, the SM-AgNPs pre-treated cells showed significant damage in the DNA and the tail formation was evidenced due to the fragmentation of the nucleic acid material (Fig. 7). On contrary, no such changes such as DNA in tail, tail length and olive tail moment were seen in the control cells. The SM-AgNPs exhibited potential antibacterial activity by forming zones of inhibition (ZOI) ranging from 14.5 to 27.2 mm (Fig. 8). The gram negative bacterial isolates like E. coli P. aeroginosa, S. flexneri, P. vulgaris and K. pneumoniae showed high sensitivity as the penetration of SM-AgNPs was easy due to less rigidity of cell wall, while on the other hand the S. aureus (Gram positive) demonstrated lowest ZOI of 14.5 mm. The pesticidal activity of the SM-AgNPs reported high mortality at 100 µg/mL. Two-way ANOVA was performed to test the efficiency of the dose, where the interaction of the factors was F (14, 96) = 99.75, the comparisons were found to be highly significant at p < 0.001. Notable morphological changes were observed in the control and the treated groups shown in Fig. 9.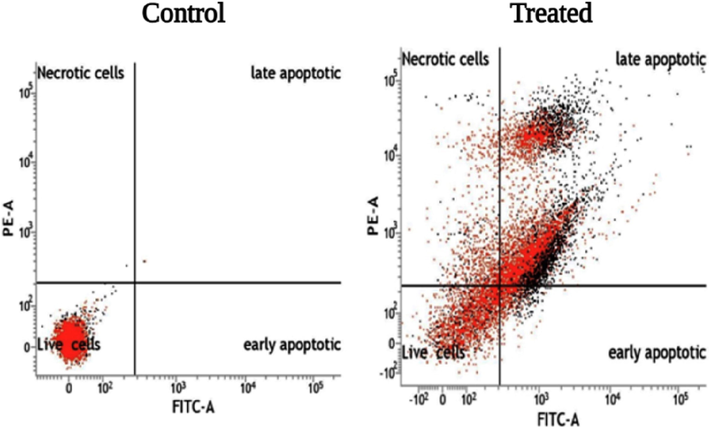
Flow cytometry graph showing the apoptosis of cells Control (A) Treated with 100 mg/mL of green synthesized SM-AgNPs (B).
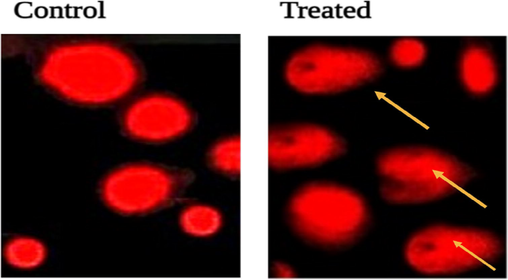
Visualization of DNA damage induced by the green synthesized SM-AgNPs on MDA-MB-231 cell lines in 24 hrs Control (A) and Treated (B).
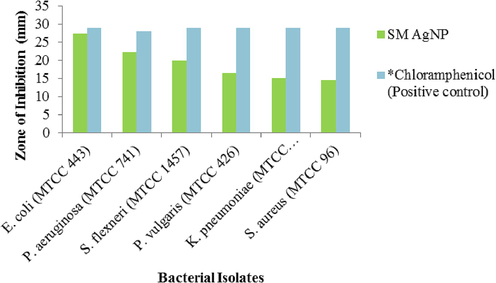
Zone of inhibition of SM-AgNPs against selected bacterial isolates. The values are expressed in mean of performed triplicates. *Standard antibiotic.
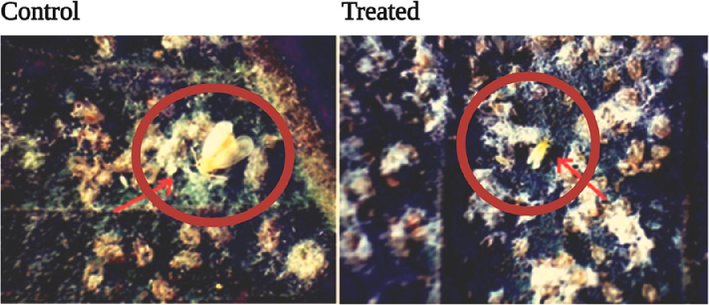
The morphological changes observed in the control and the treated sample of B. tabacciis shown. In control, the colonies are viable with the populations comprising of eggs, larvae, pupae and adults (A). While in the treated (100 µg/mL), the eggs and the pupae are dried and non– viable and the adult fly showed distinct morality with distortion and shrinkage of body (B).
4 Discussion
In the present study, the color change from green to brown as well as the absorption band at 441 nm in UV-spectra indicated the formation of silver nanoparticle and completion of reaction. The UV–Vis spectra of SM-AgNPs with different plant extracts of B. vulgaris, B. nigra, C. bursa-pastoris, L. angustifolia and O. vulgare showed maximum absorbance between 412 and 426 nm (Salayová et al., 2021). Similarly, the AgNPs synthesised using flower extracts of A. esculentus showed an absorbance peak at 430 nm in 24 h which confirmed the synthesis of AgNPs (Devanesan and Al Salhi, 2021). Determination of the functional groups and the efficient capping and stabilization by the phytocompounds on the synthesised AgNPs were characterized by FT-IR spectroscopy. The AgNPs of Phlomis leaf extract the fingerprint region of the spectra showed strong bands at 3419, 2927, 1625 and 1383 cm−1 which related to –OH, alkane C–H, C = O, C = C and C–H stretching, apart from these C–N and aromatic rings were observed at 1069 and 832 cm−1 (Allafchian et al.,2016). In another study, the AgNPs were synthesised using the fruit extracts of Conocarpuslancifolius, the gradual shift of the peaks towards a higher wavelength was noticed after the completion of biogeneic synthesis of AgNPs. Since, the plants are rich in phenolics, there is an obvious shift in the peaks corresponding to the phenolic group from the region of 1250–1270 cm−1 to 1485–1620 cm−1 (Oves et al., 2022). The biomolecules have a strong affinity to the metal ions such that they form pleated layer over the NPs acting as capping agents to prevent agglomeration and augmenting the stability. The XRD patterns confirm the lattice plane diffraction patterns of the formed AgNPs. The dried powder of AgNPs from Ceropegia bulbosa Roxb root tuber extract showed distinct peaks at 38°, 44°, 64°, and 77° which corresponded to the lattice planes of (1 1 1), (2 0 0), (2 2 0), and (3 1 1) respectively (Vetrivel et al., 2019). The characteristic absorption of metallic silver due to SPR and provide the qualitative and quantitative composition of elements. The characteristic peak at 3 keV corresponded to silver ions while the presence of other small peaks Cl, Ca, O, C and S in the EDS spectrum confirmed the incidence of biomolecules of leaf extract on the surface of the synthesized AgNPs. The FE-SEM unveiled the surface morphology, size and distribution of the SM-AgNPs and the size distribution histogram of DLS indicated that the particle corresponded 20.54 nm. Likewise, the FE-SEM reported the structure of nanoparticles to be spherical, hexagonal, and irregular in shape and had size ranging from 30 to 80 nm (Khodashenas and Ghorbani, 2019). The SM leaf extract was rich in phenols which catalysed the potential synthesis SM-AgNPs and further improved capping and stabilization efficiency. The antioxidant properties of phenolic compounds such as flavonoids are due to ROS scavenging mechanisms, chelation of metal ions and inhibition of enzymes pathways of free radicals release. The HPTLC analysis showed the presence of nine sharp and prominent peaks which related to known compounds, with the Rf distance between 0.05 and 0.95, however another study on the HPTLC analysis reported the range between 0 and 1, this suggested that, the potential role of solvent in eluting all possible phytocompound in the extracts.
The results showed that SM-AgNPs exhibited strong cytotoxic activity against the MDA MB cell lines. The AgNPs synthesised using Acanthospermum austral exhibited efficient cytotoxic potential against peripheral blood mononuclear cells (PBMCs), however the plant extract was not cytotoxic even at higher concentrations, while the cell death was due to the toxicity due to silver ions. In the similar study by Moshfegh et al (2019), the anti-cancerous activity of AgNPs of Polysiphonia alga showed best inhibitory activity at100 µg/mL. Generally the plant metabolites show proliferative effect and modulate the immune response. In other words, it can be understood that, meagre quantity of silver in the form of silver ions might be toxic than the same quantity which is in the form of phytocompound fabricated NPs (Mussin et al., 2021). Significantly, the non-toxic property of the leaf extracts underlines the context of application in pharmacognosy and in treating other infections. Moreover, another study proved that uncoated AgNPs induced cytotoxicity at comparatively low concentrations suggesting that phyto-fabrication curtails the contact of the active surface area and limits the cellular interaction (Orta‐García et al., 2015). The SM-AgNPs induced early apoptosis in treated groups, this can be anticipated that generally the AgNPs trigger the ROS and induce oxidative cells. The similar fact was reported in a study on AgNPs synthesised using Fagonia indica extracts, wherein notable morphological observations in cell membrane and nuclear condensation were found and further confirmed through gene expression assays in MCF-7 cell lines (Ullah et al., 2020). A comparative study on the cytotoxic effects of AgNPs synthesised using Putranjiva roxburghii seed extract (PRSE) on Human Colon (HCT-116), Pancreatic (PANC-1) and Breast (MDA-MB 231) cancer cell lines reported high cell death in the order of silver nitrate > PRSE_AgNPs > PRSE. The morphological changes were noted through flow cytometry and DNA fragmentation assays. The means of cell death occurred via apoptosis in treated cell lines (Balkrishna et al., 2020). Sathish kumar et al. (2009) proposed that the action of silver ion with oxygen and sulfhydryl (–S–H) groups form R–S–S–R bonds in cell wall, that cause respiration blockage leading to cell death. DNA fragmentation was observed in the treated cell lines which were supported by similar reports. The AgNPs treatment notably increased the tail length and formation of number of tail DNA in the cancer cell lines (Bin-Jumah et al., 2020). Nanoparticles have received much attention in formulating mitigation strategies in plant- pathogens in agriculture (Elekaet al., 2010).A notable morphological change was observed in the control and the treated groups which resulted in mortality of the flies. Rouhani et al., 2012 stated the LC50 value for Ag-Zn NPs was 539.46 mg/mL, effective against the oleander aphid, Aphis nerii. On the perspective of agri-environmental nanotechnology. Martins et al. (2019) reported the application of the carbon nanomaterial to deter the fecundity, digestion, metabolic efficiency and differentially impacted the moth development of S. frugiperda. The SM leaf extract was predominantly rich in phenolic compounds which facilitated in the reduction of Ag+ ions to Ago which substantiated the reducing power of the plant extract and proved to be potential natural reducing agent shown in Fig. 10. The synthesised SM-AgNPs were seamlessly nanosized less the 50 nm which assisted in smooth entry into the bacterial cells through membrane rupture. This lead to inflammation and exudation of cellular components and resulted in cell death. Abundant availability of natural capping and reducing agents augment the potential of the synthesized SM-AgNPs in delivering active biomolecules inside the cancer and bacterial cells and cease their growth.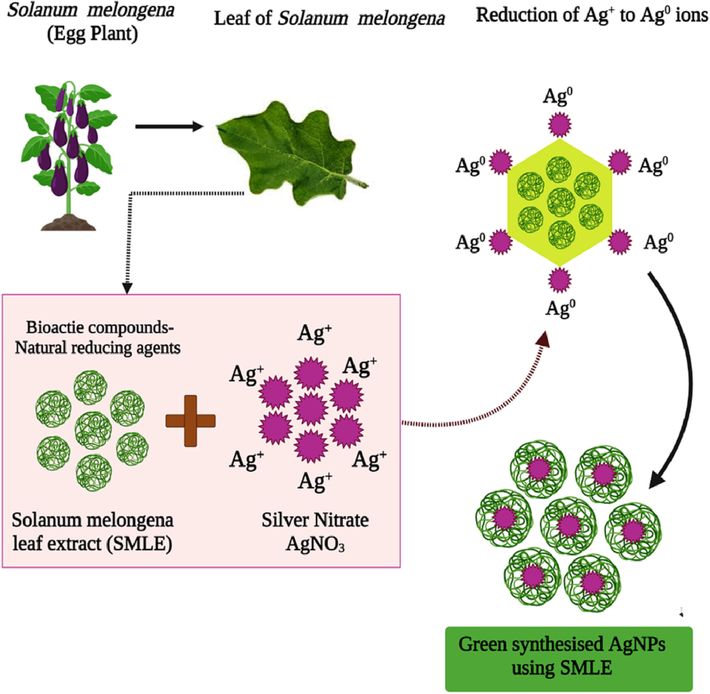
Proposed mechanism of the green synthesis of AgNPs using the natural reducing agents present in the S. melongena leaf extracts.
5 Conclusion
Present study on green synthesis of AgNPs showed that the environmentally benign and renewable source the leaves of Solanum melongena can be used as an effective capping as well as reducing agent for the synthesis of AgNPs. The characterization, cytotoxic, antibacterial and pesticidal efficacies are thoroughly documented and evidenced. The present research work demonstrated the efficiency of SM-AgNPs in anticancer, antibacterial and pesticidal activity at optimum concentrations. The efficacy was due to the appropriate size that favoured the mechanism of action that were observed in in vitro assays. Further, the phenolic content strengthened and fortified the activity in cell lines and bacterial cultures. The foliage of the candidate plant, S. melogena is a real agricultural waste, hence the present study provided cues to utilize a zero-cost raw material into a potentially useful source for synthesising nano-particles. However, the comparative efficacy can be studied by employing other metallic compounds and their potentiality can be further explored in the upcoming years.
Acknowledgement
The authors are thankful to Avinashilingam institute for home science and higher education for women, Tamil Nadu, India for providing lab facilities to carry out the research work. The authors also extend their appreciation to the researchers supporting project number (RSP2023R479) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization and anti-cancerous effect of Putranjivaroxburghii seed extract mediated silver nanoparticles on human colon (HCT-116), pancreatic (PANC-1) and breast (MDA-MB 231) cancer cell lines: a comparative study. Int. J. Nanomed.. 2020;15:573-585.
- [CrossRef] [Google Scholar]
- Comet assay measures of DNA damage are predictive of bladder cancer cell treatment sensitivity in vitro and outcome in vivo. Int. J. Cancer. 2014;134(5):1102-1111.
- [Google Scholar]
- Resilin-mimetics as a smart biomaterial platform for biomedical applications. Nat. Commun.. 2021;12(1):149.
- [CrossRef] [Google Scholar]
- Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: an updated review. Nanomaterials (Basel, Switzerland). 2021;11(8):2086.
- [CrossRef] [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45(4):493-496.
- [Google Scholar]
- Effects of green silver nanoparticles on apoptosis and oxidative stress in normal and cancerous human hepatic cells in vitro. Int. J. Nanomed.. 2020;15:1537-1548.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using the flower extract of abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int. J. Nanomed.. 2021;16:3343-3356.
- [CrossRef] [Google Scholar]
- Novaluron nanoparticles: formation and potential use in controlling agricultural insect pests. Colloids Surf A Physicochem Eng Asp. 2010;372(1–3):66-72.
- [Google Scholar]
- Anti-aging trait of whey protein against brain damage of senile rats. J. Umm Al-Qura Univ. Appl. Sci.. 2022;8:8-20.
- [Google Scholar]
- Harborne J. B. Phenolic compounds. InPhytochemical methods 1973 (pp. 33-88). Springer, Dordrecht.
- Analysis of the stable free radical scavenging capability of artificial polyphenol mixtures and plant extracts by capillary electrophoresis and liquid chromatography-diode array detection-tandem mass spectrometry. J. Chromatogr. A. 2009;1216(12):2417-2423.
- [CrossRef] [Google Scholar]
- The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. BBA. 2001;1530(2–3):162-171.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019 1;12(8):1823–38
- [Google Scholar]
- Biological effects of oxidized carbon nanomaterials (1D versus 2D) on Spodoptera frugiperda: material dimensionality influences on the insect development, performance and nutritional physiology. Chemosphere. 2019;215:766-774.
- [CrossRef] [Google Scholar]
- Biological synthesis of silver nanoparticles by cell-free extract of Polysiphonia algae and their anticancer activity against breast cancer MCF-7 cell lines. Micro & Nano Lett. 2019
- [CrossRef] [Google Scholar]
- Antimicrobial and cytotoxic activity of green synthesis silver nanoparticles targeting skin and soft tissue infectious agents. Sci. Rep.. 2021;11:14566.
- [CrossRef] [Google Scholar]
- Analysis of cytotoxic effects of silver nanoclusters on human peripheral blood mononuclear cells 'in vitro' J. Appl. Toxicol.. 2015;35(10):1189-1199.
- [CrossRef] [Google Scholar]
- Oves, M., Rauf, M. A., Aslam, M., Qari, H. A., Sonbol, H., Ahmad, I., Zaman, G. S., Saeed, M. (2022). Green synthesis of silver nanoparticles by ConocarpusLancifolius plant extract and their antimicrobial and anticancer activities. Saudi journal of biological sciences. 1;29(1):460-71
- Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid. Based Complement. Alternat. Med.. 2014;2014:525340
- [CrossRef] [Google Scholar]
- Nano-from nature to nurture: a comprehensive review on facets, trends, perspectives and sustainability of nanotechnology in the food sector. Energy. 2022;1(240):1227.
- [Google Scholar]
- Biochemical and histopathological alterations induced by subchronic exposure to zinc oxide nanoparticle in male rats and assessment of its genotoxicicty. J. Umm Al-Qura Univ. Appl. Sci.. 2022;8:41-49.
- [Google Scholar]
- Multifunctional therapeutic potential of phytocomplexes and natural extracts for antimicrobial properties. Antibiotics (Basel, Switzerland). 2021;10(9):1076.
- [CrossRef] [Google Scholar]
- Rouhani, Mohammad, Amin Samih, Mohammad, & Kalantari, Salma. (2012). Insecticied effect of silver and zinc nanoparticles against Aphis nerii Boyer of fonscolombe (Hemiptera: Aphididae). Chilean journal of agricultural research, 72(4), 590-594. https://dx.doi.org/10.4067/S0718-58392012000400020
- Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: morphology and antibacterial efficacy. Nanomaterials (Basel, Switzerland). 2021;11(4):1005.
- [CrossRef] [Google Scholar]
- Syed, M. H., Yasmeen, A., Hussain, M. S., Subramanian, N.S. (2013). Preliminary phytochemical screening and HPTLC fingerprinting of leaf extracts of Pisoneaaculeata. Journal of Pharmacognosy and Phytochemistry. 1;2(1)
- Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid. Med. Cell. Longev.. 2020;2020:1215395.
- [CrossRef] [Google Scholar]
- Fabrication and characterization of noble crystalline silver nanoparticles from Ceropegia bulbosa Roxb root tuber extract for antibacterial, larvicidal and histopathology applications. Nanosci. Nanotechnol. Lett.. 2019;11(1):11-21.
- [Google Scholar]
- Antioxidant activity of apple peels. J. Agric. Food Chem.. 2003;51(3):609-614.
- [CrossRef] [Google Scholar]
- Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol.. 2020;1(2):53-63.
- [Google Scholar]
Further reading
- Single cell gel electrophoresis assay (comet assay) for evaluating nanoparticles-induced DNA damage in cells. Methods in molecular biology (Clifton. N.J.). 2012;906:415-422.
- [CrossRef] [Google Scholar]
- Bray, H. G., &thorpe, W. V. (1954). Analysis of phenolic compounds of interest in metabolism. Methods of biochemical analysis, 1, 27–52. https://doi.org/10.1002/9780470110171.ch2
- Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem.. 2016;212:521-527.
- [CrossRef] [Google Scholar]
- Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces. 2009;73(2):332-338.
- [Google Scholar]
- Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv.. 2021;11(5):2804-2837.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102663.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







