Translate this page into:
GP13, an Arthrospira platensis cysteine desulfurase-derived peptide, suppresses oxidative stress and reduces apoptosis in human leucocytes and zebrafish (Danio rerio) embryo via attenuated caspase-3 expression
⁎Corresponding authors at: Department of Medical Research, Medical College Hospital & Research Centre, SRM Institute of Science & Technology, Kattankulathur, 603 203 Chennai, Tamil Nadu, India (K.M. Karuppiah); SRM Research Institute, SRM Institute of Science and Technology, Kattankulathur 603 203, Chennai, Tamil Nadu, India (J. Arockiaraj). mkarupiya@gmail.com (Kanchana M. Karuppiah), jesuaraj@hotmail.com (Jesu Arockiaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Arthrospira platensis (Ap) has several advantageous adaptive mechanisms including its efficient response to elevated oxidative stress (OS). This study intends to identify the antioxidant effect, in vitro and in vivo, of a novel peptide (GP13), Ap-derived cysteine-desulfurase (CDS) peptide, for antioxidant role and the associated mechanism(s) by which the GP13 elicits cytoprotection under elevated OS.
Using bioinformatics tools, a short sequence of amino acids GP13 with a predicted antioxidant effect has been identified from the cysteine sulfurase domain of ApCDS between 243 and 616 amino acids. The synthesized GP13 peptide expressed significant antioxidant activity against free-radicals at various concentrations (10–80 µM), as established through the DPPH, ABTS, SARS, and HRS assays.
Besides, GP13 demonstrated no cytotoxicity on Vero cells and human leucocytes. In H2O2-induced human leucocytes, the peptide exhibited maximum ROS scavenging activity at a concentration of 80 µM. Reduced apoptotic body formation was identified using Hoechst 33,342 staining in GP13-exposed Vero cells. In vivo results revealed that pre-treated zebrafish larvae with GP13 (10–80 µM) ameliorated the H2O2-induced oxidative damage. Further, GP13 inhibited the H2O2-induced caspase-3-dependent apoptotic response at 96 h post fertilized (hpf) zebrafish larvae.
Together, results demonstrated that in GP13 treated (80 µM) zebrafish there was a reduction in the expression of lipid peroxidation level; and an increase in antioxidant-enzymes. Therefore, it is possible that GP13 peptide derived from ApCDS has in vitro and in vivo antioxidant activity and that GP13 should be further investigated for potential therapeutic utility in OS-mediated complications.
Keywords
Antioxidant activity
Cysteine desulfurase
Arthrospira platensis
Oxidative damage
Caspase-3
Zebrafish
1 Introduction
Reactive species of oxygen (ROS) are the by-product molecules formed during the metabolism of aerobic cells (Upadhyay et al., 2021). These essential molecules, regulate the key physiological events such as homeostasis and signaling. However, excessive ROS results in overt OS, which is a fundamental mechanism in several pathologies, including cancer, metabolic syndrome, cardiovascular disease and neurodegeneration (Ismail et al., 2017). Antioxidants play an important role in neutralizing free-radicals formed in the natural system as end-products of normal biochemical reactions. Excessive free-radicals-mediated OS damages DNA, lipids, and proteins. Hence, antioxidant peptides are recognized as food-additives to normalize OS in the humans towards optimal lipid and protein oxidation. Immunologically significant protein-derived peptides receive attention due to their unusual mode of action and high efficiency (Raju et al., 2020). Peptide chains with sulfur-containing amino acids (cysteine and methionine) exhibit antioxidant properties. Different physicochemical properties such as molecular weight, hydrophobic amino acids (Leucine, Glycine, Phenylalanine, Valine, Tryptophan, Lysine, Histidine, and Proline), and imidazole chain at the C and N terminal have antioxidant capacity (Kumaresan et al., 2018). In antioxidant reactions, these peptides act on lipid-peroxidation inhibitors and transfer chelators by donating hydrogen, metal ions, or scavengers of free-radicals. Thus, hydrogen donor peptides serve as antioxidants in free-radical scavenging systems.
Arthrospira platensis (spirulina), a photosynthetic algae with natural antioxidant capacity, is a whole food or a dietary supplement that provides multi-organ protection against chemical-induced toxicity (Eleiwa et al., 2018). Previously we have demonstrated that sulfur stress in spirulina triggers differential gene expression, which is associated with antioxidant activity. Sulfur plays a crucial role in biological systems and is present in numerous peptides, proteins, and low molecular weight compounds. Cysteine desulfurase (CDS) is a pyridoxal 5′-phosphate (PLP)-dependent homodimeric enzyme that catalyzes the conversion of L-cysteine to L-alanine and sulfane sulfur through the production of a protein-bound cysteine persulfide intermediate on a conserved cysteine residue (Heis et al., 2011). In H2O2-exposed cyanobacteria, overexpression of CDS reduced ROS formation, which shows stress tolerance of CDS against oxidizing agents. This study has evaluated the in vitro and in vivo effects of an ApCDS derived-GP13 peptide for antioxidant role and the associated mechanisms through which GP13 elicits cytoprotection against OS.
2 Materials and methods
2.1 Spirulina culture and challenge study
Spirulina, obtained from the S.R.M Lake (12.825527° N 80.039606° E, Tamil Nadu, India), was cultured in a modified Zarrouk’s medium at 30 °C (pH 9.5) at 12/12-h light/dark cycle. Species was identified using 16s rRNA sequencing. Under optimum culture condition, spirulina was subjected to a stress, caused by sulfur-deprivation in the culture media (Kumaresan et al., 2017). Growth rate was monitored on alternative days until day-20 using a spectrophotometer (SHIMADZU). Chlorophyll absorption was estimated at 655 nm.
2.2 ApCDS gene expression study
Spirulina was harvested during the sulfur-deprivation periods (0, 5, 10, 15, and 20th days) and flash frozen in LN2. Using TRIzol, RNA was isolated as per the manufacturer’s protocol (Life Technologies, Rockville, USA). Isolated RNA was used for cDNA synthesis. ApCDS expression was analysed using cDNA as a template. Following ApCDS primers were used; Forward: GGACAAGCACTCAACCAAGA; Reverse: GGACGCACAGTTACCTCTAAAC. For internal control, 16s rRNA was used; whose primer details are: Forward: GCTTTCCCGACTTGTGGATTA; Reverse: CCCTACCTTGTCCAACAATACC. For the assay, SYBR Green Master Mix (Roche Diagnostics) was utilized. Spirulina culture, which was not deprived of sulfur, served as control. Results were obtained in triplicates.
2.3 ApCDS cDNA sequence analysis
Blast2go program identified the full-length sequence of ApCDS cDNA from our previously established spirulina transcriptome. The identified ApCDS cDNA sequence was submitted to EMBL. Expasy translate tool was used to check the ApCDS protein parameters. Physio-chemical properties of cDNA and protein (molecular weight, isoelectric point, open reading frame, and hydrophobicity) were analyzed. Phylogenetic tree, multiple sequence analysis, 2D and 3D structures were predicted using multiple online bioinformatics tools.
2.4 GP13 peptide analysis
Using in silico analysis GP13 peptide 1GIAVRAGHHCAQP13 was identified from the CDS domain of ApCDS. Expasy Prot-Param tool (https://web.expasy.org/protparam) predicted that GP13 has antioxidant property, based on its hydrophobicity, molecular weight, arrangement of amino acids, and evolutionary conserved sequence. Helical Wheel Projection (http://www.bioinformatics.nl/cgi-bin/emboss/pepwheel) identified the hydrophobicity and helical structure of GP13. According to the bioinformatics output, GP13 was synthesized, HPLC-purified, and its sequence was evaluated (MALDI-TOF MS analysis) at Zhengzhou Peptides Pharmaceutical Technology Co. Ltd, China. Peptide stock of 1 mM in PBS was utilized for the assays.
2.5 In vitro ROS scavenging activity of GP13
To evaluate the peptide’s antioxidant potential, antioxidant assays were performed using a UV–Vis spectrophotometer (UV 1800, SHIMADZU, Japan). Trolox was used for positive control.
2.5.1 DPPH assay
DPPH assay was performed with slight modification (Liao et al., 2014). Different concentrations (10–80 μM) of GP13 or trolox were reacted with DPPH (100 µM). Reactions were incubated for 30 min at room temperature (RT) in dark. After incubation, readings were measured at 517 nm.
2.5.2 ABTS assay
ABTS assay was performed to determine the free-radical scavenging capacity of GP13 with slight variation (Sannasimuthu et al., 2019). Assay was performed by dissolving ABTS (7 mM) in K₂S₂O₈ (2.45 mM). Reaction mix was diluted using 0.2 M PBS (pH 7.4) at 30 °C to obtain an absorbance was taken at 734 nm. Then, 5 µL of GP13 was added to the ABTS solution. Reaction mix was incubated at 30 °C for 1 h, and absorbance was measured at 734 nm.
2.5.3 Hydroxyl radical scavenging
Hydroxyl radical scavenging activity of GP13 was performed with slight modification (Sarkar et al., 2020a). A mixture containing 1,10-phenanthroline (5 mM), 30 µL of EDTA and 30 µL of FeSO4 was mixed with sodium phosphate buffer. Different peptide concentrations were added to the mixture and incubated for 60 min at 37 °C. Absorbance was measured at 536 nm.
2.5.4 Superoxide radical scavenging
Superoxide radicals were produced in a PMS-NADH system via NADH oxidation. Assay was performed by reducing NBT to purple color formazan (Sannasimuthu et al., 2018). PBS (20 mM), NBT (50 μM), PMS (15 μM), NADH (73 μM), along with GP13 (10–80 μM), were mixed to a volume of 200 µL. Then the reaction mixture was incubated for 5 min at RT. Spectroscopic absorbance was measured against the blank at 562 nm.
2.6 Hemolytic assay
Human blood was collected from a healthy volunteer (Ethical Approval No. CDRI/IEC/2014/A1). Whole blood was centrifuged at 1000 rpm for 10 min at 4 °C to remove plasma; blood cells were washed thrice in PBS. Leucocytes were then isolated from blood cells (Arasu et al., 2017). Cells, exposed to different peptide concentrations, were incubated at RT for 1 h. After incubation, samples were centrifuged for 5 min at 4000 rpm. Then, the supernatant was diluted with PBS, and the absorbance was measured at 560 nm.
2.7 MTT assay
To analyze whether or not GP13 influences cell viability, MTT assay in Vero cells (obtained from The King Institute of Preventive Medicine and Research, Guindy, Chennai, India) was performed (Mosmann, 1983). Cells were plated in a 96 well-plate (1X105 cells/well) and grown till 80 percent confluence. Different concentrations of GP13 (10–80 μM) were used for a 24 h challenge on the cells and the reading was taken at 570 nm using a microplate reader (LIZA).
2.8 Intracellular ROS on leucocytes
Leucocytes were seeded in 6-well plates and incubated with DCFH-DA (10 µM) and H2O2 (1 mM) for 30 min at 37 °C, followed by the peptide treatment at different concentrations (10–80 μM). Cells not exposed to H2O2 served as control. Quantity of fluorescent intensity was measured by Image J software (Sannasimuthu et al., 2018).
2.9 H2O2-induced cell apoptosis
The efficacy of GP13 on H2O2-stimulated apoptosis in Vero cells were evaluated as described (Kang et al., 2013). Cells were treated with different peptide concentrations, with or without H2O2 (1 mM) for 6 h. Cells not exposed to H2O2 served as control. Treated cells were then stained with the cell permeable Hoechst-33342. After 10 min incubation, cells were observed under a fluorescent microscope.
2.10 Lipid-peroxidation assay
For the MDA assay (Ohkawa et al., 1979), peptide treated cells were incubated with 1% thiobarbituric acid, 0.37% sodium dodecyl sulphate, and 6.8% acetic acid for 2 h at 95 °C. Reaction mixture was cooled at RT, then N-butanol and pyridine were added at 15:1 ratio and vortexed. Reaction mix was centrifuged at 3000 rpm for 15 min, and readings were measured at 532 nm.
2.11 Zebrafish maintenance and embryo collection
Four months-old male and female zebrafish (Wild type-AB) were taken from a commercial market at Kolathur, Chennai, Tamil Nadu. They were left undisturbed in the laboratory for two weeks, for acclimatization. Then, they were processed for breeding with a breeding set of 1-female: 2-male ratio. Embryos were collected in a petridish containing E3 media, where healthy and dead embryos were separated. Stable embryos at 6hpf were chosen for further analysis by examining them under a compound microscope.
2.12 GP13 peptide toxicity analysis
In a 6-well plate about 3-4hpf, embryos (16/well) were exposed to different peptide concentrations (10–80 μM) in E3 medium. Embryos which were subjected to OS using 1 mM H2O2 served as positive control. Developmental parameters were observed every day at an interval of 24 h, upto 96hpf.
2.13 Antioxidant-enzyme assay
Antioxidant-enzyme activity was assessed using 96hpf-embryos. Zebrafish larvae in E3 medium were exposed to GP13 in Tris buffer with 1 mM H2O2 for 2 days. Samples were then homogenized and separated by centrifugation at 2500 rpm for 10 min at 4 °C. Supernatant was utilized for the assay.
Superoxide dismutase (SOD) activity was performed to examine the enzyme's tendency to resist superoxide anion-induced epinephrine autoxidation. The embryo supernatant was incubated for 3 min with a mixture containing epinephrine (5 mg/mL), bovine catalase (0.4U/mL) and NaHCO3 buffer. Absorbance was measured at 480 nm·H2O2 disappearance was represented as U/mL (Hamdi et al., 2011).
Phosphate buffer containing 200 μM H2O2 was applied to an experimental cuvette. Catalase (CAT) enzyme activity was determined using a mixture of the supernatant and peptide after 1 min incubation; this reaction mixture was read at 240 nm. Absorbance could decrease when the H2O2 gets degraded due to CAT (Hadwan, 2018).
In lipid-peroxidation assay, MDA concentration in the supernatant of zebrafish larvae was measured (Adeyemi et al., 2015). Thiobarbituric acid (TBA) was added to the supernatant and incubated for 10 min in a water bath at 95 °C, and was read at 532 nm.
2.14 DCFH-DA analysis in zebrafish embryo
Untreated control and GP13 treated (10–80 μM) 96hpf-embryos were collected in 6-well plates containing E3 medium. To induce OS, treated embryos were incubated for 1 h with H2O2 and then washed with PBS. ROS intensity was examined using the larvae stained with DCFH-DA (10 μM) under Leica fluorescent microscope and the florescent photographs were recorded. Software Image J measured the intensity of the fluorescence.
2.15 Detection of activated caspase-3 expressions to analyze apoptosis
Zebrafish larvae (96 hpf) were collected in a 1.5 mL tube and rinsed twice with 1 mL of 1x PBST. Embryos were fixed using para-formaldehyde (4%) and incubated at 4 °C, overnight. Methanol was used to permeabilize the larvae; after permeabilization, 1 mL PDT (1x PBST, 0.3% Triton-X, 1% DMSO) was applied. After 30 min exposure, PDT was discarded, then blocking buffer was applied. Rabbit anti-activated caspase-3 antibody was used for incubation, to block the process, preceded by the incubation of the secondary antibody (anti-Rabbit, Dylight 488, Rockl and antibodies & assays, USA), which continued for 8 h (Sorrells et al., 2013). Finally, the larvae were washed and observed under a fluorescent microscope.
2.16 Real-time PCR study
After the exposure experiments, the 96hpf zebrafish larvae (n = 40) were homogenised, and total RNA was extracted using TRIZOL. mRNA was converted to cDNA and real-time-PCR experiment was conducted (KAPA BIOSYSTEM kit). After normalising with β-actin, the ΔΔct value was determined, and the fold change was evaluated using the 2-ΔΔt technique. Primer sequences utilized are described elsewhere (Issac et al., 2021).
2.17 Statistics
All the results were taken in triplicates indicating Mean ± SD . Statistical analysis was done Bonferroni post-hoc test and Tukey’s multiple comparison test in Graph Pad Prism (ver. 5.0) were performed.
3 Results
3.1 ApCDS sequence analysis
Length of the ApCDS cDNA sequence was 1878 bp, with the protein containing 625 amino acids and molecular mass of 68.120 kDa. Encoded protein contains 69 negatively charged residues (Asp + Glu) and 48 positively charged residues (Arg + Lys) with an isoelectric point of 5.52. Instability index of 39.47 indicates ApCDS as a stable protein (E-Suppl. Table 1). ApCDS polypeptide contains cysteine desulfurase (SufS) domain at 243–616, forming the biggest part that belongs to the pyridoxal phosphate dependent aspartate aminotransferase superfamily. The SufS domain has aminotransferase class-V domain at 244–612, the chemical binding site at T312-T313; I316; H334; D417; S419-Q420; T434; H436-K437 and active site at K437 (E-Suppl. Fig. 1). Multiple sequence analysis shows similarity with Helianthus annus (49.12%), Coffea arabica (48.61%), Cucurbita maxima (48.1%), and Momordica charantia (47.6%) (E-Suppl. Fig. 2). Clustal Omega Program revealed the homology of the sequences, in which the CDS functional regions were strongly conserved; while the semi-conserved regions in CDS had molecular potential.
In the secondary structure, Prabi-Gerland tool revealed the presence of 30.88% alpha-helix, 12.64% of extended strand and 56.48% of random coil. Based on the C-score (-2.59), RMSD value 14.3 ± 3.8 Å and TM score 0.41 ± 0.14, the best structure of ApCDS 3D was obtained from I-TASSER tool. The Ramachandran Plot revealed that 71.9% of amino acid residues are in the favourable region, 14.1% in allowed region and outlier region (14%) of ApCDS. Report shows that the c-value of the best model is −3.29, RMSD 11.3 ± 4.8 Å and TM score 0.35 ± 0.12, which is similar to our selected model (Das and Roychoudhury, 2014).
3.2 Characterisation of GP13 peptide
A peptide sequence 573GIAVRAGHHCAQP585 named GP13 has been identified based on the highly conserved regions using bioinformatics analysis from ApCDS (E-Suppl. Fig. 2). HPLC analysis identified that the peptide has 95.9% purity.
3.3 ApCDS gene expression analysis
ApCDS mRNA expression was analyzed at various time intervals. On 10th, 15th, and 20th days (Fig. 1), mRNA levels were significantly increased. However, the expression on the day-15 was maximum than the other days.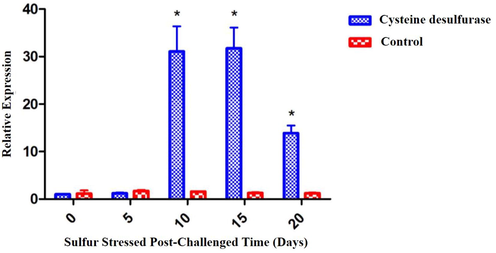
Gene expression patterns of ApCDS by qRT-PCR. Data are expressed as a relative fold calculated against the internal control 16 s rRNA at different intervals of time (0, 5, 10, 15, and 20 days). Values are shown as mean ± standard deviation (SD) of three replicates. The asterisk (*) represents p < 0.05 compared to their respective controls by one-way ANOVA followed by Bonferroni post-hoc test using Graph Pad Prism 5.0.
3.4 In vitro antioxidant activity of GP13
Free-radical scavenging activity of GP13 was significantly higher (72%) at 80 µM and lower (49%) at 10 µM than Trolox (96.5% at 80 µM and 84% at 10 µM) (E-Suppl. Fig. 4A).
In the ABTS radical scavenging assay, GP13 showed significantly (p < 0.05) higher activity at 80 µM (91%) with a gradual decrease (69%) at 10 µM.
Hydroxyl scavenging activity of GP13 was significantly increased (72.3%) at 80 μM and reduced (48.1%) at 10 μM. At the same time, Trolox exhibited significantly (p < 0.05) higher activity (89.1% at 80 µM) as provided in E-Suppl. Fig. 4C.
Results demonstrate that GP13 at 80 µM has significantly (p < 0.05) higher superoxide anion radical scavenging activity (85.2%), almost similar to positive control, Trolox (94.3% at 80 µM) that is nearly similar to GP13 peptide activity (E-Suppl. Fig. 4D).
3.5 Haemolytic assay and intracellular ROS on leucocytes
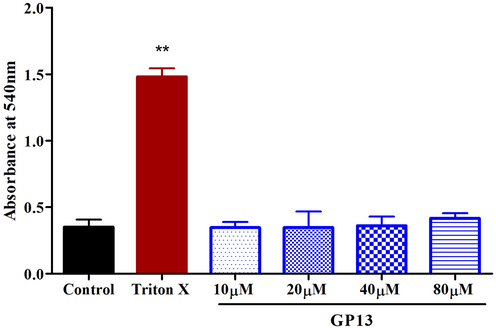
GP13 showed no hemolytic effect even at the highest concentration (80 µM). (Fig. 2). The H2O2-treated larvae showed an increase in fluorescence intensity of upto 99% in the zebrafish larvae, which indicates the oxidatively stressed condition (Fig. 3). In the GP13-treated group (80 µM), there was a decrease in fluorescence intensity to 39%.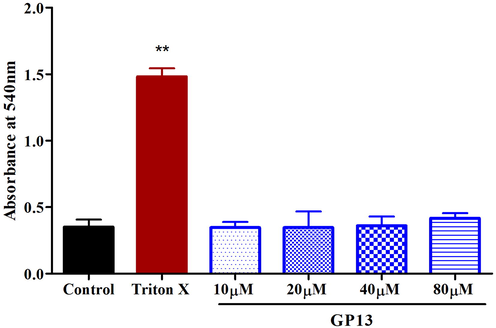
Hemolytic assay was performed on human whole blood with Triton X (0.01%) as positive control and PBS as control. Values are shown in mean ± SD of three replicates. The asterisk (*) represents the significance (p < 0.05) of peptide compared with control measured by one-way ANOVA followed by Bonferroni post-hoc test using Graph Pad Prism 5.0.
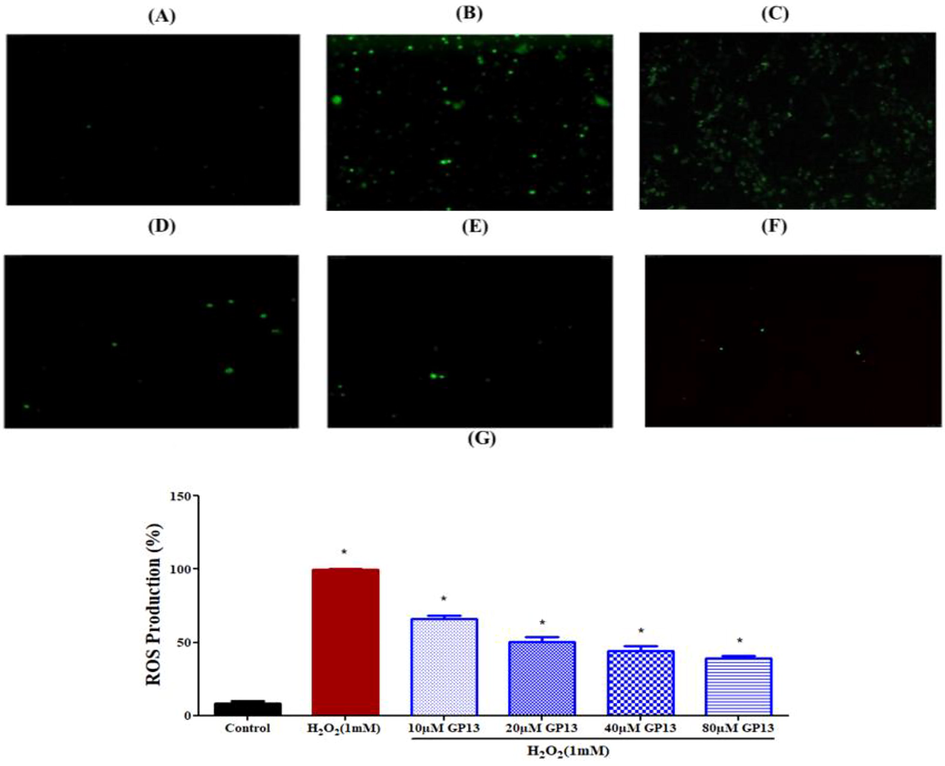
The Reduction of intracellular ROS production in human leucocytes treated by different concentration of GP13 and stained using DCFH-DA dye presented in microscopic images (magnification = 10X and scale bar = 100 µm). (A) Control (untreated cells), (B) H2O2 treated cells (C) H2O2 (1 mM) + 10 µM GP13, (D) H2O2 (1 mM) + 20 µM GP13, (E) H2O2 (1 mM) + 40 µM GP13, (F) H2O2 (1 mM) + 80 µM GP13, and (G) Quantitative analysis analysis of ROS production at different concentration of GP13 peptide treatment. Values were presented as mean ± SD (n = 3). The asterisk (*) represents the significance at p < 0.05 between the GP13 peptide treatment and control groups.
Cell viability test in Vero cells. MTT assay was performed on vero cells with GP13 peptide at different concentration (10–80 μM) where PBS treatment as control. Triton X (0.01%) was used as a positive control. Bar diagram showed the cell viability on treatment with different concentration of peptide measured in Microplate Reader (n = 3). Data were expressed as mean ± SD.
3.6 Cell viability, apoptosis, and lipid-peroxidation assay on Vero cells
GP13 had no cytotoxic effect at all the concentrations used (10–80 µM) (Fig. 4). Hoechst dye identifies cell death through the presence of apoptotic cells. Results reveal the brightly stained condensed nuclei in H2O2-induced Vero cells (99%) than the control (untreated cells). Although this H2O2-induced effect was countered by GP13 exposure at different concentrations (10–80 µM), the maximum reduction in apoptosis was observed in 80 µM (32%) (Fig. 5). The protective effect of GP13 against H2O2-induced lipid-peroxidation in Vero cells is shown in Fig. 6. Maximum reduction in MDA concentration was shown in 80 µM of GP13.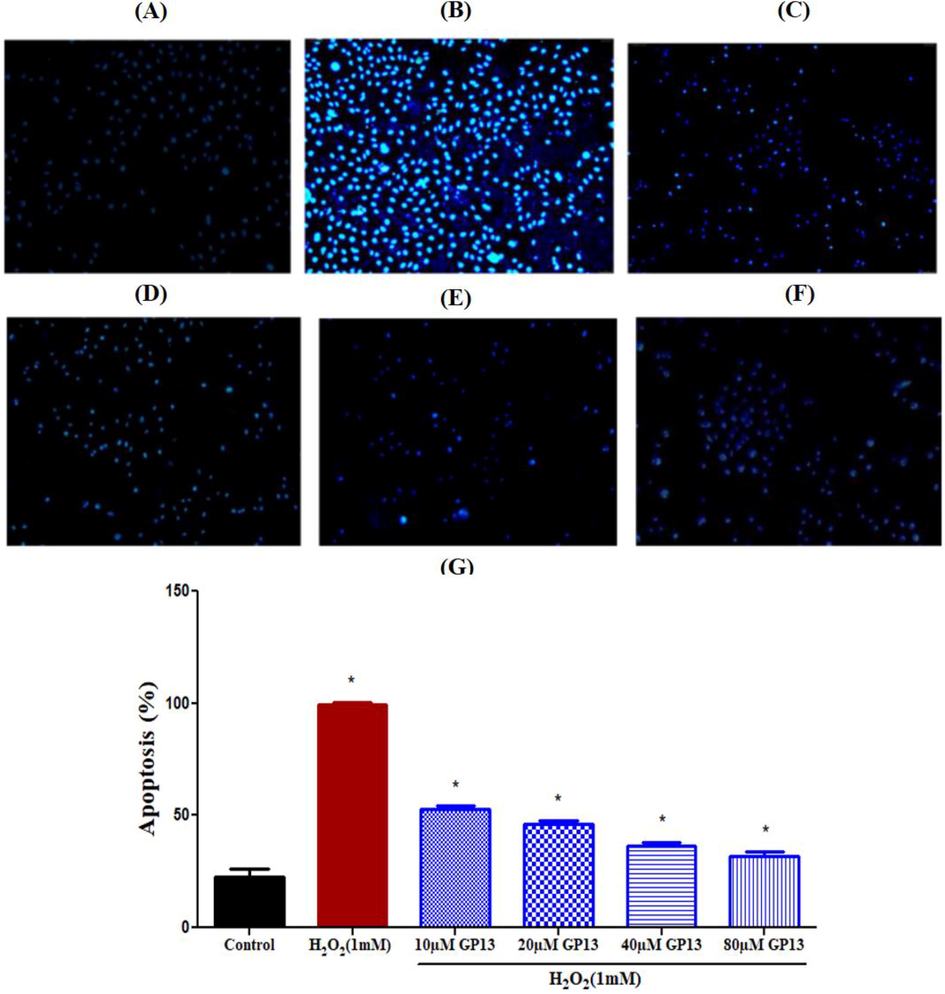
Effect of GP13 peptide on H2O2 induced stress against apoptosis in Vero cells. The apoptotic body formation was observed under fluorescent microscope after Hoechst 33342 staining (A) Control (untreated cells), (B) H2O2 (1 mM), (C) H2O2 (1 mM) + 10 µM GP13, (D) H2O2 (1 mM) + 20 µM GP13, (E) H2O2 (1 mM) + 40 µM GP13 and (F) H2O2 (1 mM) + 80 µM GP13. (G) Apoptosis level was measured using Image J software. The experiments were conducted in triplicates. The asterisk (*) represents the significance at p < 0.05 between treatment and control by one-way ANOVA followed by Bonferroni post-hoc test using Graph Pad Prism 5.0.
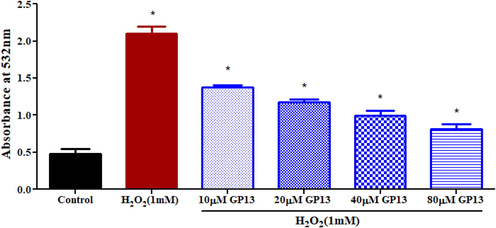
Effect of GP13 on lipid peroxidation level in oxidative stressed Vero cells. Different concentrations of GP13 treated along with H2O2 (1 mM) showed a reduction in lipid level·H2O2 treated cells as a positive control. Values are shown in mean ± SD of three replicates. The asterisk (*) represents the significance (p < 0.05) between peptide treated and control (untreated cells), H2O2 measured using one-way ANOVA followed by Bonferroni post-hoc test using Graph Pad Prism 5.0.
3.7 Toxicity study in zebrafish embryo
GP13 peptide treated zebrafish larvae at a various concentrations (10–80 µM) did not show any adverse effect in the morphology and survival rate of the larvae, whereas the H2O2 (1 mM) treated larvae showed mortality and malformations (Fig. 7 & E-Suppl. Fig. 5). Heart rate was evaluated at 72hpf to identify the cardiotoxicity of GP13. There was a reduction in heart rate in the H2O2-exposed group, while there was no significant change in the heartbeat even at the maximum concentration (80 µM) of GP13 (E-Suppl. Fig. 5).
Representative photomicrographs of morphological malformation were observed during the exposure period. Control with E3 medium treatment and GP13 peptide at different concentrations showed no developmental toxicity, whereas the H2O2 (1 mM) treatment group was observed with malformations such as yolk sac edema (YSE).
3.8 Antioxidant-enzyme activity in zebrafish larvae
Antioxidant-enzyme activities (SOD and CAT) of GP13 was estimated in the H2O2-stressed larvae supernatant. SOD (9U/mL) and CAT (4U/mL) activities were remarkably decreased in the H2O2 treated group. Meanwhile, the GP13 peptide at 80 μM showed a higher level of SOD (34U/mL) and CAT (22U/mL) activity than the control.
The H2O2 induced group found to have increased lipid-peroxidation levels (30 nmol/mL) than the control group (E-Suppl. Fig. 6C). Parallely, there was a decrease in lipid-peroxidation levels in the GP13-exposed group. A higher level of lipid-peroxidation inhibition was observed in 80 µM (14 nmol/mL). Overall, results show that GP13 normalizes the harmful effect of free-radicals by enhancing the antioxidant-enzymes.
3.9 ROS scavenging effect of GP13 on zebrafish embryo
The ability of GP13 to scavenge ROS in H2O2-treated zebrafish larvae was evaluated through the observed changes in the degree of fluorescence intensity. Mean fluorescence intensity was found to be significantly (p < 0.01) higher in the H2O2-induced group than the control. However, the group which was pre-treated with GP13 showed a reduction in the mean fluorescent intensity (Fig. 8).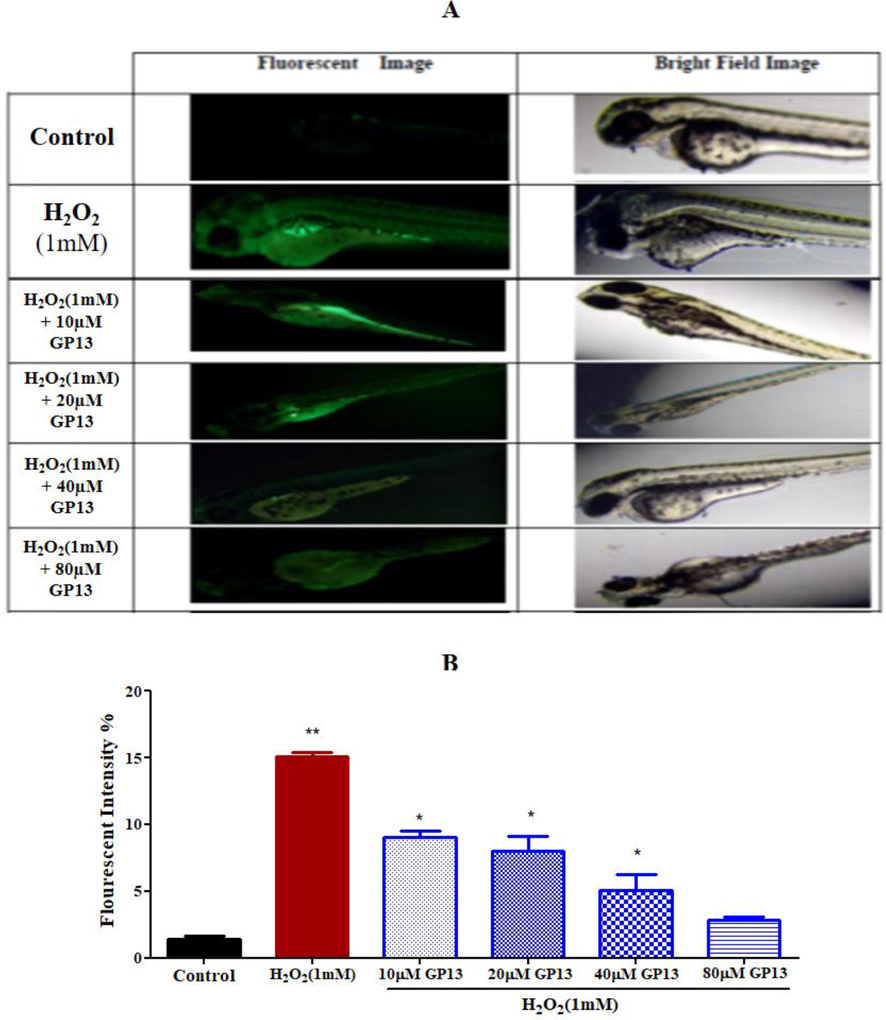
Production of reactive oxygen species (Intracellular ROS levels) measured using DCFH-DA dye in 96 hpf zebrafish larvae. (A) Fluorescent images represent the exposure against various concentration of GP13 along with H2O2 (1 mM) (B) Fluorescent intensity measured using Image J software presented in graphical representation. H2O2 was used as a positive control. The values were presented in mean (n = 3). The single asterisk (*) represents the significance at p < 0.05 between the GP13 exposed and control (without treatment) by one-way ANOVA and Bonferroni post Hoc test in Graph Pad 5.0.
3.10 Caspase-3- dependent apoptosis on zebrafish embryo and antioxidant gene expression study
At 80 μM, GP13 demonstrated better activity with no toxicity effect. Thus 40 μM was selected for further analyses. Changes in activated caspase expression was evaluated in the presence or absence of H2O2 and GP13 using the whole mount immunofluorescence method. The intensity was higher in the H2O2-treated embryos than the control due to an increase in the number of apoptotic cells. However, this H2O2-mediated effect was ameliorated in the combined challenge of GP13 (80 μM) + H2O2 (Fig. 9). It is well-known that apoptosis is mediated by caspases, which exist in all cells as pro-caspases (inactive form). Antioxidant gene expression under H2O2-induced stress showed decreased mRNA expression in GPx, GSH and GCS than the GP13 pre-treated groups (Fig. 10).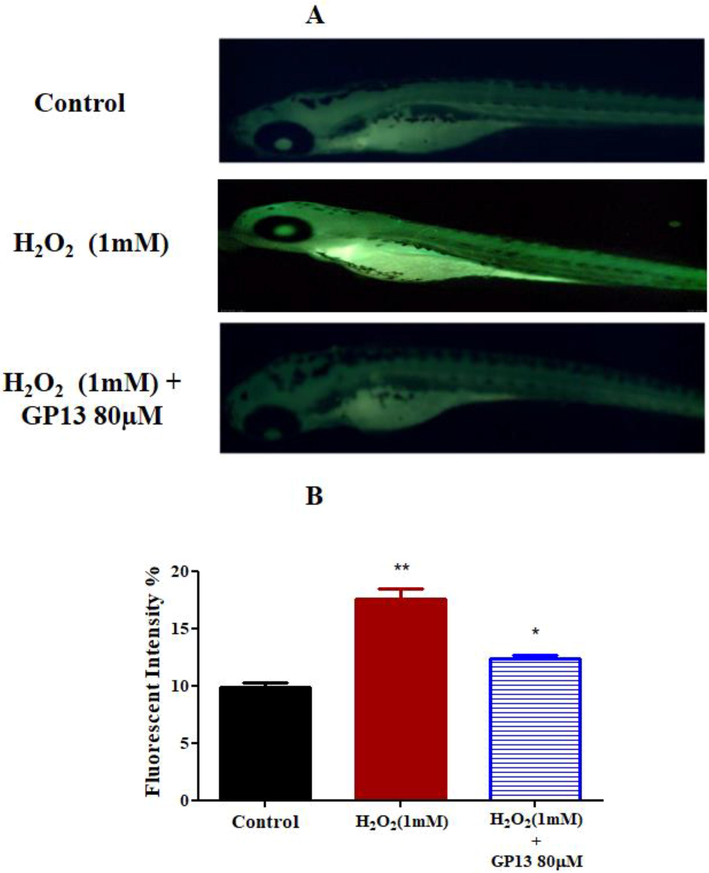
Effect of GP13 on caspase 3 expression in zebrafish larvae (A) Fluorescence photomicrographs of zebrafish larvae (96 hpf) showing the active caspase 3 expressions. (B) Quantitative bar diagram of active caspase 3 expressions in 96 hpf zebrafish larvae calculated using Image J software. The untreated zebrafish larvae were used as the control. Experiments are performed in triplicate and expressed as mean ± SD. *p < 0.05 and *p < 0.01 indicating the fluorescence intensity significant level between GP13 treated and control.
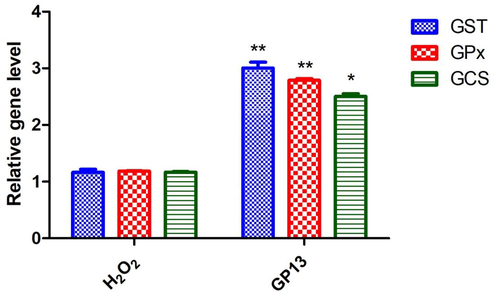
Antioxidant gene expression analysis by qRT-PCR assay. Experiments performed to confirm the antioxidant gene glutathione-S-transferase (GST), glutathione peroxidase (GPx) and gamma-glutamylcysteine synthetase (GCS) levels in the zebrafish embryo after treatment with 80 µM of GP13 peptide and then H2O2. Relative gene levels in the treated or un-treated controls. The asterix indicates the significant difference (*p < 0.05 and **p < 0.01) due to Bonferroni post-hoc test performed in Graph Pad Prism 5.0.
4 Discussion
Arthrospira platensis contains several antioxidants, which enhances the activities of SOD and CAT, prevents lipid-peroxidation and DNA damage, and scavenges free-radicals (Wu et al., 2016). CDSs, which provide sulphur for iron-sulfur cluster formation, are found in all phyla, including cyanobacteria. Studies show that CDS overexpression protects cyanobacteria from OS (Banerjee et al., 2017). In our study, CDS overexpression occurred on day-15 due to sulfur stress-induction, indicating CDS’s protective effect against stress. Hence, investigations were performed on the antioxidant role of CDS.
Full length cDNA sequence of CDS was identified from established A. platensis transcriptome database using genome sequencing technology, and stored at EMBL as accession-ID LT838803. Conserved and semi-conserved domain analysis of ApCDS showed potential functional property. The phylogenetic analysis was developed into three clusters of Cyanobacteria, Proteobacteria, and Plantae (E-Suppl. Fig. 3) to analyze the evolutionary interaction of the ApCDS protein sequence with other species in managing abiotic and biotic stress (Sarkar et al., 2020b). Principally, the involvement of stress mechanism might be responsible for the change in cluster pattern, which is an evolutionary process. Although the in-silico peptide analysis revealed the presence of hydrophobic and hydrophilic residues in the chain, GP13 is more hydrophobic (46.15%) in nature. Studies have reported that the peptide that exhibits hydrophobicity can scavenge free-radicals due to the presence of a major proton donating imidazole ring (Raju et al., 2021); both, acidic- (glutamine) and basic- amino acids (arginine and glycine) are involved in antioxidant activity (Yang et al., 2020); the short chain of amino acids and low molecular weight may contribute to antioxidant role (Ma et al., 2021) and among the sulfur-containing amino acids, only cysteine has a thiol group, which is involved in the oxidation–reduction reaction and exhibits antioxidant properties (the capacity to chelate metal and free-radical scavenging) (Kim et al., 2020). In support, we have identified that GP13 peptide has Ala (23%), Arg (7.69%), Cys (7.7%), Glu (7.7%), Gly (15.3%), His (15.4%), Pro (7.7%) and Val (7.7%) which are responsible for the radical scavenging and antioxidant properties.
In vitro assays show that GP13′s free-radical scavenging activity is concentration-dependent. Report shows that cysteine is the most active amino acid in ABTS because of the presence of histidine, tryptophan, and tyrosine (Alam et al., 2013) and that proline is an efficient hydroxyl ion scavenger. In GP13, presence of cysteine (7.7%) and histidine (15.4%) could contribute to the peptide’s free-radical scavenging activity (E-Suppl. Fig. 4B). In this context, proline in GP13 suggests its contribution to the antioxidant activity.
Studies show that superoxide anion radical damages cells by compromising DNA and cell membrane (Xie et al., 2008); and the presence of leucine and glycine in a Caesin derived peptide contributed to high scavenging activity against superoxide anionic radicals (Ohkawa et al., 1979). In GP13, presence of glycine (15.3%) could be the reason for its super anionic radical scavenging activity.
Cytotoxicity is a major criteria in predicting the toxic hemolysis of a compound (Evans et al., 2013). For a peptide to be a therapeutic agent, it should be non-toxic. In this regard, GP13, as an antioxidant, can be a safe and efficient therapeutic molecule because GP13 shows protectivity against H2O2-induced OS. Studies demonstrate that excessive ROS causes deleterious cellular effects and that restoration of physiological ROS facilitates normal cellular functions (Gapeyev et al., 2017). Results from our study show that GP13 enhanced the intracellular ROS scavenging property in leucocytes. Also, the cytoprotective role of GP13 in H2O2-induced cells was observed in Vero Cells.
Toxicity studies of GP13 on zebrafish did not show any malformations and is safe even at higher concentrations. Further, H2O2-induced free-radical scavenging effect revealed the potential scavenging effect of GP13 against oxidative damage in zebrafish in a dose-dependent manner. Possibly, presence of cysteine and histidine in GP13 could be the reason for the ROS scavenging activity (Fig. 8A and 8B). Besides, the total SOD and CAT activity of GP13 against the H2O2 stressed zebrafish larvae found to be reduced (E-Suppl. Fig. 6A & 6B). In support, a study shows that these enzymes could eliminate excess free-radicals and help the conversion of reactive toxic components to non-toxic elements and protect the cellular organelles from oxidative damage (Issac et al., 2021).
In the H2O2-challenged zebrafish embryos, caspase-3 expression was elevated. Caspase-3, the executioner caspase and a downstream-effector in apoptosis, coordinates cellular structures' destruction (DNA fragmentation or cytoskeletal protein degradation) (Redza-Dutordoir and Averill-Bates, 2016), which is a hallmark cellular event in apoptosis. While oxidants can activate caspase-3, intracellular GSH concentration regulates caspase-3 activity by reducing the accessibility of caspase-3 for proteolytic cleavage, resulting in resistance to apoptosis. As discussed previously, GP13 exposure in embryos restored normal antioxidant-enzyme activities, which could be a possible mechanism by which GP13 elicits protective effect under elevated OS-mediated cellular damage. Glutathione-related enzymes (glutathione-peroxidase, glutathione-S-transferase, and gamma-glutamylcysteine-synthetase) control the antioxidant defence system (Cheng et al., 2017). An estimate of the antioxidant-enzymes in our investigation revealed that GP13 is a potent activator of antioxidant genes.
Overall, GP13, a safe cytoprotective molecule, influences the redox system by augmenting the antioxidant enzyme activities and scavenging free-radicals to restore the balance in oxidant: antioxidant ratio. However, further investigation is needed to determine the peptide's activity in immune challenge tests.
5 Conclusion
Under the sulfur deprived conditions, the overexpression of ApCDS on day 15 indicated the involvement of oxidative stress reduction in cyanobacteria. Further, bioinformatic analysis predicted a short peptide GP13 to have functional property. Cell free and Cell-based assays on leucocytes and Vero cells revealed the potential role of GP13 in the antioxidant system. Pre-treated zebrafish embryo at 24–96 h showed no developmental toxicity and found safer in in-vivo model. GP13 was found to scavenge the H2O2-induced OS by regulating caspase-3 expression and increasing the antioxidant enzymes. Therefore, GP13 have a cytoprotective impact by scavenging free radicals and restoring normal intracellular antioxidant activity in a dose dependent manner.
Author contribution
PS, AG, SVR – performed the experiments, wrote the manuscript.
AF, AAAO, ORA, HAEA, FA – contributed reagents/materials, analysed the data.
KMK, JA – conceived and designed the study, analysed the data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Teratogenicity, genotoxicity and oxidative stress in zebrafish embryos (Danio rerio) co-exposed to arsenic and atrazine. Comp. Biochem. Physiol. Part – C Toxicol. Pharmacol.. 2015;172-173:7-12.
- [CrossRef] [Google Scholar]
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J.. 2013;21(2):143-152.
- [CrossRef] [Google Scholar]
- Bacterial membrane binding and pore formation abilities of carbohydrate recognition domain of fish lectin. Dev. Comp. Immunol.. 2017;67:202-212.
- [CrossRef] [Google Scholar]
- Changes of oxidative stress, glutathione, and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS ONE. 2017;12(1):e0170016.
- [CrossRef] [Google Scholar]
- Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci.. 2014;2:53.
- [CrossRef] [Google Scholar]
- Antioxidant activity of Spirulina platensis alleviates doxorubicin-induced oxidative stress and reprotoxicity in male rats. Orient. Pharm. Exp. Med.. 2018;18(2):87-95.
- [CrossRef] [Google Scholar]
- Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp.. 2013;73:e50166
- [CrossRef] [Google Scholar]
- The protection of DNA in blood leukocytes from damaging action of ultraviolet radiation using the “Useful Sun” strategy. Biophys. Russian Fed.. 2017;62(3):444-449.
- [CrossRef] [Google Scholar]
- Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem.. 2018;19:7.
- [CrossRef] [Google Scholar]
- Protective effect of the octadecaneuropeptide on hydrogen peroxide-induced oxidative stress and cell death in cultured rat astrocytes. J. Neurochem.. 2011;118:416-428.
- [CrossRef] [Google Scholar]
- Differential expression of cysteine desulfurases in soybean. BMC Plant Biol.. 2011;11:166.
- [CrossRef] [Google Scholar]
- Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Tradit. Complement. Med.. 2017;7(4):452-465.
- [CrossRef] [Google Scholar]
- Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3 – dependent apoptotic response in zebrafish larvae National Centre for Cell Science. Fish Physiol. Biochem.. 2021;47(2):293-311.
- [Google Scholar]
- Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem.. 2013;138(2-3):950-955.
- [CrossRef] [Google Scholar]
- In vitro antioxidant actions of sulfur-containing amino acids. Arab. J. Chem.. 2020;13(1):1678-1684.
- [CrossRef] [Google Scholar]
- Transcriptome changes of blue-green algae, Arthrospira sp. in response to sulfate stress. Algal Res.. 2017;23:96-103.
- [CrossRef] [Google Scholar]
- Molecular insight into the metabolic activities of a protein-rich micro alga, Arthrospira platensis by de novo transcriptome analysis. Mol. Biol. Rep.. 2018;45(5):829-838.
- [CrossRef] [Google Scholar]
- Recrystallization of dihydromyricetin from Ampelopsis grossedentata and its anti-oxidant activity evaluation. Rejuvenation Res.. 2014;17(5):422-429.
- [CrossRef] [Google Scholar]
- Molecular basis of function and the unusual antioxidant activity of a cyanobacterial cysteine desulfurase. Biochem. J.. 2017;474:2435-2447.
- [CrossRef] [Google Scholar]
- Inhibitory effect of low-molecular-weight peptides (0–3 kDa) from Spirulina platensis on H2O2-induced oxidative damage in L02 human liver cells. Bioresour. Bioprocess.. 2021;8:1-9.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods.. 1983;65(1-2):55-63.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [CrossRef] [Google Scholar]
- Raju, S. V., Mukherjee, A., Sarkar, P., Issac, P.K., Lite, C., Paray, B.A., Al-Sadoon, M.K., Al-Mfarij, A.R., Arockiaraj, J., 2021. RM12 similar to substance P from tachykinin of freshwater murrel Channa striatus influence intracellular ROS in vitro fish erythrocytes and developmental toxicity and antioxidant enzymes in vivo zebrafish embryo. Fish Physiol. Biochem. 2021 474 47, 1073–1085. https://doi.org/10.1007/S10695-021-00950-9.
- Piscidin, fish antimicrobial peptide: structure, classification, properties, mechanism, gene regulation and therapeutical importance. Int. J. Pept. Res. Ther.. 2021;27(1):91-107.
- [CrossRef] [Google Scholar]
- Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta - Mol. Cell Res.. 2016;1863(12):2977-2992.
- [CrossRef] [Google Scholar]
- Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic. Biol. Med.. 2019;135:198-209.
- [CrossRef] [Google Scholar]
- Radical scavenging property of a novel peptide derived from C-terminal SOD domain of superoxide dismutase enzyme in Arthrospira platensis. Algal Res.. 2018;35:519-529.
- [CrossRef] [Google Scholar]
- TL15 of Arthrospira platensis sulfite reductase scavenges free radicals demonstrated in oxidant induced larval zebrafish (Danio rerio) model. Int. J. Biol. Macromol.. 2021;166:641-653.
- [CrossRef] [Google Scholar]
- Antioxidant molecular mechanism of adenosyl homocysteinase from cyanobacteria and its wound healing process in fibroblast cells. Mol. Biol. Rep.. 2020;47(3):1821-1834.
- [CrossRef] [Google Scholar]
- Analysis of apoptosis in zebrafish embryos by whole-mount immunofluorescence to detect activated caspase 3. J. Vis. Exp.. 2013;82:e51060
- [CrossRef] [Google Scholar]
- Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere. 2021;267:129216.
- [CrossRef] [Google Scholar]
- Wu, Q., Liu, L., Miron, A., Klímová, B., Wan, D., Kuča, K., 2016. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016 908 90, 1817–1840. https://doi.org/10.1007/S00204-016-1744-5.
- Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem.. 2008;111(2):370-376.
- [CrossRef] [Google Scholar]
- Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem.. 2020;319:1-14.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101665.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







