Translate this page into:
Ginkgetin alleviates polystyrene microplastics-instigated liver injury in rats through Nrf-2/Keap-1 pathway activation
⁎Corresponding author. nailaraighafoor357@gmail.com (Naila Ghafoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polystyrene microplastics (PS-MPs) are potential environmental toxicants that are reported to instigate oxidative stress (OS) in the liver. Ginkgetin (GK) is a natural biflavonoid with potential therapeutic activities. This experiment was executed to access the putative effect of GK against PS-MPs provoked hepatotoxicity. Four groups were formed from 48 rats including control, PS-MPs (0.01 mg/kg) group, PS-MPs (0.01 mg/kg) + GK (25 mg/kg) co-treated group and GK (25 mg/kg) alone group. The exposure of PS-MPs markedly decreased the expressions of antioxidant genes and Nrf-2, besides escalating Keap-1 expression. It also decreased the activities of antioxidants i.e., glutathione (GSH), glutathione S-transferase (GST), catalase (CAT), glutathione peroxidase (GPx), heme oxygenase-1 (HO-1), superoxide dismutase (SOD), glutathione reductase (GSR), while increasing reactive oxygen species (ROS) and malondialdehyde (MDA) contents. Additionally, a notable escalation in hepatic serum markers i.e., alkaline phosphatase (ALP), alanine transaminase (ALT) and aspartate aminotransferase (AST) level was observed. Furthermore, PS-MPs exposure escalated the levels of inflammatory markers, i.e., tumor necrosis factor-α (TNF-α), interleukin- 6 (IL-6), nuclear factor-kappa B (NF-kB), interleukin-β (IL-1β) level and cyclooxygenase-2 (COX-2) activity. PS-MPs treatment augmented Caspase-3 and Bax expressions and decreased Bcl-2 expression. Nevertheless, GK treatment notably abated PS-MPs prompted liver injuries owing to its hepatoprotective efficacy.
Keywords
Ginkgetin
Hepatic damage
Polystyrene microplastics
Inflammation
Oxidative stress
1 Introduction
Our environment is highly contaminated with different types of pollutants, but the pollution caused by plastics is of utmost concern (Li et al., 2016). When plastic or its products enter into the environment, hydrolytic forces and UV radiation break them into small fragments of less than 5 mm size, these small particles are called microplastics (Suhrhoff and Scholz-Böttcher, 2016). Large polymers of styrene are degraded into tiny particles known as polystyrene microplastics (PS-MPs) (Cox et al., 2019). Cheap prices, high durability and long-term applications have made PS-MPs the first choice of material used in lunch boxes, cleaning supplies, disposable teacups, and various sorts of insulatory materials (Hou et al., 2021). Humans are directly exposed to these microplastics via inhalation, drinking contaminated water and consuming food containing MPs such as table salt, beer, shellfish, honey, and sugar (Romeo et al., 2015). According to a study, the average per capita intake of MPs in the USA is 74,000–121,000 particles (Cox et al., 2019). PS-MPs exert negative impacts on the normal physiological processes of the body which ultimately damage various body organs including liver, stomach, testicular, kidney, brain, and heart (Leal Filho et al., 2019).
Polystyrene microplastics (PS-MPs) could instigate OS that results in lipid peroxidation (LP) and causes oxidative injury in the vital organs including gut, intestines, kidney, testes, and liver (Ijaz et al., 2021; Pan et al., 2021; Goodman et al., 2022). These cellular changes ultimately result in serious metabolic disorders (Deng et al., 2017). Liver is a vital body part that is responsible for the detoxification of harmful substances, at the same time it is highly vulnerable to the damages induced by PS-MPs and its metabolites (Zhao et al., 2020). PS-MPs prompt hazardous damages in humans including disruption in immune responses, induced oxidative stress (OS) and disruptions in IL-6, TNF-α levels as well as malondialdehyde (MDA) content (Dong et al., 2020).
Flavonoids are diverse naturally present polyphenolic entities occurring in plants as secondary metabolites (Panche et al., 2016). These metabolites are widely extracted from vegetables, beverages, cherries, apples, and cloves (Park et al., 2014). A plethora of research showed that these compounds act as strong antioxidant against free radicals (Ishtiaq et al., 2022). Ginkgetin (GK), a flavonoid, is reported in the leaves of Ginkgo biloba. GK is reported to alleviate testicular and renal toxicity due to its antioxidant as well as antiinflammatory activities (Akbar and Ijaz, 2024; Ehsan et al., 2023). Considering the aforementioned therapeutic roles of GK, this study was performed to reveal the remedial potentials of GK on PS-MPs triggered liver damages in albino rats.
2 Materials and methods
2.1 Chemicals
PS-MPs (Purity ≥ 96 %, CAS # 9003–53–6) and GK (Purity 98 %, CAS # 481–46–9) were brought from Merck (US).
2.2 Animals
The experiment was conducted on 48 male albino rats weighing 200 ± 20 g. Animals were confined in animal care facility of University of Agriculture Faisalabad, Pakistan at controlled environmental conditions (Humidity 45 ± 5 %, temperature 23–26 °C, and 12 h. day/dark light cycles). The animals were provided with unrestricted food access with standard pallets along with water. Rats were maintained in line with the animal ethics guidelines of the European Union.
2.3 Experimental design
Forty-eight rats were allocated in 4 groups (n = 12) and kept in steel cages. The dose administrated to rats was as follows: control group, PS-MPs administrated group (0.01 mg/kg), PS-MPs + GK co-treated group (0.01 mg/kg and 25 mg/kg respectively) and GK only administered group (25 mg/kg). These doses were administered according to our previous study (Akbar and Ijaz, 2024). The rats were acclimatized in the laboratory environment for seven days, following that the doses of PS-MPs and GK were administered for 30 days. Upon completion of the treatment, all rats were sedated and decapitated on the last day. Blood was drawn into heparinized tubes. In order to obtain serum, blood was centrifuged for about 15 min at 3000 rpm, which was then kept for further analysis at −20 °C. The liver was excised and preserved at −80 °C for biochemical testing.
2.4 Biochemical profile
Chance and Maehly’s (1955) protocol was executed to assess CAT activity. The methodology of Kakkar et al. (1984) was employed for the analysis of SOD activity. GPx activity was quantified via the methodology of Jollow et al. (1974). GSR activity was appraised with the approach of Carlberg and Mannervik (1975). Habig et al. (1974) strategy was applied to quantify GST activity. The method demonstrated by Sedlak and Lindsay (1968) was applied to ascertain GSH activity, while the HO-1 activity was quantified by examining bilirubin biosynthesis through the technique outlined by Magee et al. (1999). By implementing the approach of Hayashi et al. (2007) the ROS content was assessed. The level of MDA was appraised through the method of Ohkawa et al. (1978).
2.5 qRT-Polymerase Chain Reaction (qRT-PCR)
The expressions of Nrf-2/Keap-1, apoptotic markers (Caspase-3, Bax and Bcl-2) as well as antioxidant genes were appraised by qRT-PCR. The total RNA of the cell was obtained with the help of TRI-zol reagent. RNA was transcribed to produce cDNA via employing Fast Quant reverse transcription kit from Takara located in China. Alterations in the expressions of these parameters were determined by 2-ΔΔCT, considering β-actin as inner control (Ijaz et al., 2022). Table 1 depicted the gene primers.
Gene
Primers 5′ −> 3′
Accession number
Nrf-2
F: ACCTTGAACACAGATTTCGGTG
NM_031789.1
R: TGTGTTCAGTGAAATGCCGGA
Keap-1
F: ACCGAACCTTCAGTTACACACT
NM_057152.1
R: ACCACTTTGTGGGCCATGAA
CAT
F: TGCAGATGTGAAGCGCTTCAA
NM_012520.2
R: TGGGAGTTGTACTGGTCCAGAA
SOD
F: AGGAGAAACTGACAGCTGTGTCT
NM_017051.2
R: AAGATAGTAAGCGTGCTCCCAC
GPx
F: TGCTCATTGAGAATGTCGCGTC
NM_030826.4
R: ACCATTCACCTCGCACTTCTCA
GSR
F: ACCAAGTCCCACATCGAAGTC
NM_053906.2
R: ATCACTGGTTATCCCCAGGCT
GST
F: TCGACATGTATGCAGAAGGAGT
NM_031509.2
R: CTAGGTAAACATCAGCCCTGCT
HO-1
F: AGGCTTTAAGCTGGTGATGGC
NM_012580.2
R: ACGCTTTACGTAGTGCTGTGT
Bax
F: GGCCTTTTTGCTACAGGGTT
NM_017059.2
R: AGCTCCATGTTGTTGTCCAG
Bcl-2
F: ACAACATCGCTCTGTGGAT
NM_016993.1
R: TCAGAGACAGCCAGGAGAA
Caspase-3
F: ATCCATGGAAGCAAGTCGAT
NM_012922.2
R: CCTTTTGCTGTGATCTTCCT
β-actin
F: TACAGCTTCACCACCACAGC
NM_031144
R: GGAACCGCTCATTGCCGATA
2.6 Assay of liver enzymes
The levels of liver serum markers i.e., AST (ab263883), ALP (ab287823) and ALT (ab285264) were analyzed with the help of commercially available ELISA kits purchased from Wiesbaden (Germany) as per the instructor’s guidelines.
2.7 Estimation of inflammatory indices
NF-κB (CSB-E13148r), IL-1β (CSB-E08055r), IL-6 (CSB-E04640r), TNF-α (CSB-E07379r) levels and COX-2 activity (CSB-E13399r) was evaluated via ELISA kit (YL Biotech Co. Ltd., Shanghai, China). The analyses were performed as per the directions of manufacturers.
2.8 Statistical analysis
Results were depicted as Mean ± SEM. One-way ANOVA and Tukey’s test was applied to assess the whole data. The significance level was set at p < 0.05.
3 Results
3.1 Protective role of GK on the expression of Nrf-2/Keap-1
PS-MPs inebriation resulted in a remarkable (p < 0.05) decline in the expression of Nrf-2 and antioxidant genes, whereas upsurging Keap-1 expression in relation to control. Alternatively, GK + PS-MPs co-treatment regulated the expressions of these parameters. Moreover, the rats subjected to GK only, exhibited these expressions approximately nearby to control animals (Figs. 1, 2).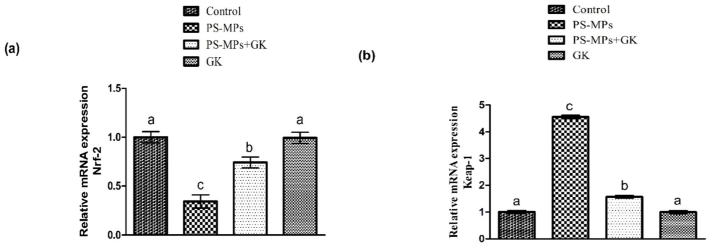
Protective role of GK on (a) Nrf-2, (b) Keap-1 expression. Bars are shown on the basis of mean ± standard error of mean. Different superscripts on bars presenting significant variation.
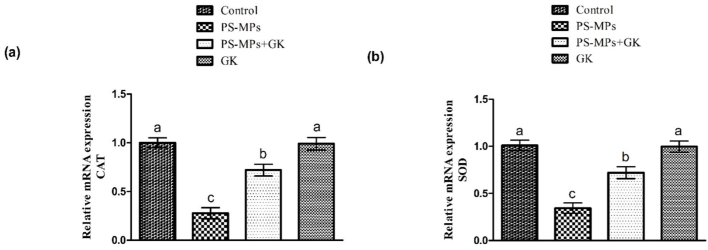
Protective role of GK on (a) CAT, (b) SOD, (c) GPx, (d) GSR, (e) GST along with (f) HO-1 expression. Bars are based on mean ± standard error of mean. Different superscripts on bars presenting significant variation.
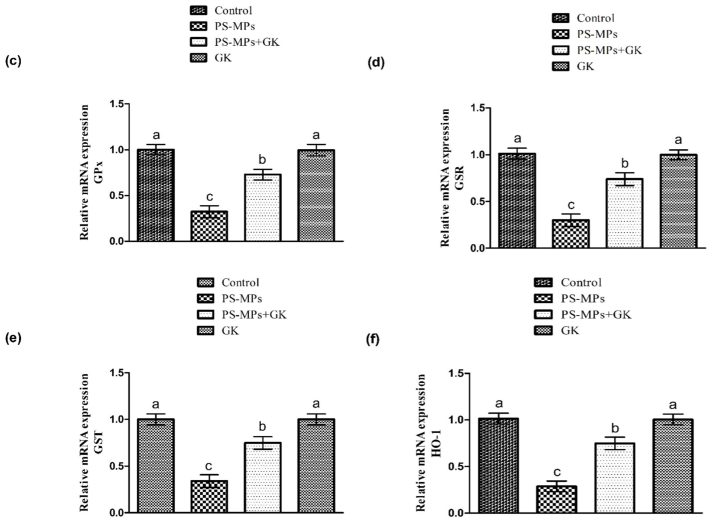
Protective role of GK on (a) CAT, (b) SOD, (c) GPx, (d) GSR, (e) GST along with (f) HO-1 expression. Bars are based on mean ± standard error of mean. Different superscripts on bars presenting significant variation.
3.2 Protective role of GK on biochemical indicators
A substantial (p < 0.05) down-regulation in antioxidants activities, while an escalation in ROS and MDA levels was identified in rats administered with PS-MPs as compared to the control group. Nevertheless, supplementation of GK + PS-MPs substantially augmented the activity of antioxidant enzymes, whereas MDA and ROS contents were significantly decreased relative to PS-MPs treated rats. In addition to this, no marked variations were noted in the level of these biomarkers in GK only supplemented animals, in comparison with the control (Table 2). The values with different superscript are significantly distinct from other groups.
Parameters
GROUPS
Control
PS-MPs
PS-MPs + GK
GK
CAT (Umg−1 protein)
9.59 ± 0.43a
5.03 ± 0.14c
8.41 ± 0.25b
9.62 ± 0.44a
SOD (Umg−1 protein)
7.66 ± 0.24a
4.30 ± 0.15c
6.88 ± 0.16b
7.67 ± 0.33a
GPx (Umg−1 protein)
17.71 ± 0.46a
8.03 ± 0.36c
14.51 ± 0.49b
17.76 ± 0.47a
GSR (nM NADPH oxidized/min/mg tissue)
6.52 ± 0.12a
2.40 ± 0.16c
5.58 ± 0.18b
6.55 ± 0.13a
GST (nM/min/mg protein)
23.47 ± 1.18a
11.22 ± 0.52c
19.89 ± 0.23b
23.55 ± 1.19a
GSH (μM/g tissue)
14.96 ± 0.47a
6.96 ± 0.09c
11.85 ± 0.32b
14.98 ± 0.44a
HO-1(pmoles bilirubin/mg protein/h)
282.06 ± 8.11a
61.30 ± 3.02c
123.05 ± 7.22b
291.16 ± 7.65a
ROS (Umg−1 tissue)
1.21 ± 0.11a
7.99 ± 0.34c
2.36 ± 0.10b
1.19 ± 0.11a
MDA (nmol/mg protein)
0.45 ± 0.12a
3.34 ± 0.16c
1.47 ± 0.14b
0.44 ± 0.13a
3.3 Protective role of GK on liver enzymes
ALT, ALP and AST levels were markedly (p < 0.05) escalated in rats exposed to PS-MPs as compared with the control. While GK + PS-MPs treatment reduced these levels relative to animals subjected to PS-MPs. In addition to this, only GK treated group displayed these levels almost in line with control (Table 3). The values with dissimilar letters are notably distinct from other groups.
Parameters
GROUPS
Control
PS-MPs
PS-MPs + GK
GK
ALT (U/L)
43.41 ± 1.08a
91.41 ± 1.03c
56.97 ± 1.41b
42.77 ± 0.89a
AST (U/L)
82.70 ± 1.31a
173.41 ± 1.95c
95.93 ± 0.89b
81.80 ± 1.19a
ALP (U/L)
118.23 ± 1.88a
347.64 ± 3.93c
189.57 ± 3.06b
116.74 ± 1.59a
3.4 Protective role of GK on apoptotic markers
PS-MPs treatment led to a remarkable (p < 0.05) increment in Caspase-3 and Bax expressions, besides decreased the expression of Bcl-2 as compared with the control group. Nevertheless, GK + PS-MPs supplementation notably (p < 0.05) regulated these expressions relative to PS-MPs administered rats. Additionally, in rats supplemented with GK only, the aforementioned parameters were comparable to the control (Fig. 3).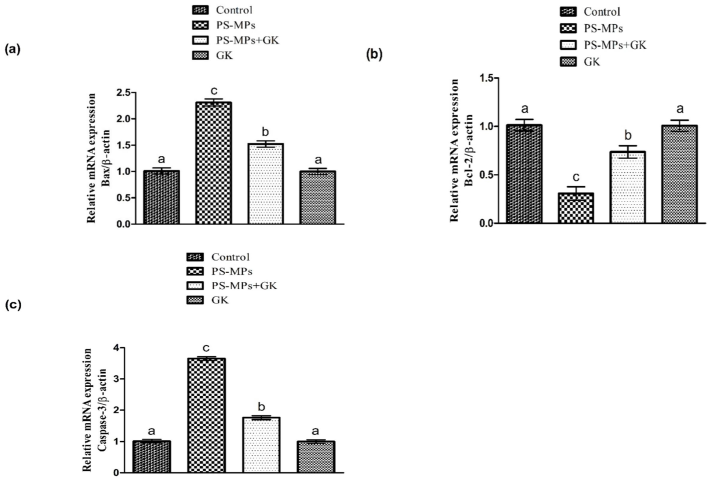
Protective role of GK on (a) Bax, (b) Bcl-2 as well as (c) Caspase-3 expression. Bars are based on mean ± standard error of mean. Different superscripts on bars displaying significant difference.
3.5 Protective role of GK on inflammatory indices
PS-MPs markedly (p < 0.05) elevated the levels of inflammatory markers, as compared to the control. However, in GK + PS-MPs supplemented animals, these levels were noticeably lowered, as compared to the group of rats treated with PS-MPs. However, inflammatory indices levels in GK only animals were presented to be almost similar to the control (Table 4). The values with dissimilar letters are significantly different from other groups.
Parameters
GROUPS
Control
PS-MPs
PS-MPs + GK
GK
NF-κB (ngg−1 tissue)
16.49 ± 0.66a
64.02 ± 1.78c
27.34 ± 1.34b
16.38 ± 0.62a
TNF-α (ngg−1 tissue)
8.55 ± 0.28a
25.80 ± 0.83c
14.40 ± 0.54b
8.53 ± 0.29a
IL-1β (ngg−1 tissue)
24.48 ± 0.49a
85.73 ± 0.97c
33.22 ± 0.77b
24.26 ± 0.32a
IL-6 (ngg−1 tissue)
5.48 ± 0.42a
28.37 ± 0.74c
9.63 ± 0.41b
5.39 ± 0.49a
COX-2 (ngg−1 tissue)
22.97 ± 0.84a
67.93 ± 1.11c
36.83 ± 0.88b
22.91 ± 0.81a
4 Discussion
PS-MPs can aggregate in the hepatocytes and disrupt liver functioning, leading to hepatotoxicity (Sharma et al., 2012). GK, a polyphenolic, dietary flavonoid, extracted from the leaves and nuts of Ginkgo biloba. It has been found that, GK display numerous pharmacological effects i.e., anti-oxidative, neuroprotective, anti-apoptotic, anti-tumor and anti-inflammatory (Baek et al., 2016). Therefore, this investigation was intended to appraise the hepatoprotective role of GK on PS-MPs provoked hepatic damages in male albino rats.
PS-MPs treatment reduced the expresions of antioxidant genes and Nrf-2, while increasing Keap-1 expression. Nrf-2 performs crucial function in the regulation of OS. While Keap-1 acts as the inhibitor of Nrf-2 and controls its stability (Pintard et al., 2004). Nrf-2 separates from Keap-1 during ROS production through some physical modifications. Following the separation, Nrf-2 is relocated to the nucleus, where it engages with small MAF (musculoaponeurotic fibrosarcoma) proteins. Therefore, Nrf-2 has an indispensable part in monitoring the induction of antioxidant genes (Hawkes et al., 2014). Nevertheless, GK administration elevated Nrf-2 as well as anti-oxidant genes expressions, whereas lowering Keap-1 expression. Therefore, it is assumed that GK could modulate the expression of Nrf-2/Keap-1.
The results of our study indicated that PS-MPs administration potentially decreased antioxidants activities i.e., SOD, GSR, GSH, GST, CAT, HO-1 and GPx, while markedly escalating ROS and MDA contents in the liver. These antioxidants are the 1st wall of protection that protects the proteins, lipids and DNA against OS damage via lowering ROS generation (Selamoglu Talas et al., 2009). GSR sustains GSH level by converting glutathione disulfide into GSH. GST plays a critical role in detoxification of toxins to safeguard the cells from damages prompted by oxygen free radicals (Hayes et al., 2005). Hence, this pronounced decrease in the activities of enzymatic antioxidants leads to excessive ROS generation, which weakens the anti-oxidative capacity to fight against free radicals and ultimately prompts OS. MDA is the byproduct of LP, which is indirectly used as a biomarker for the detection of LP and ROS induced cellular damages (Agarwal and Sengupta, 2020). However, the supplementation of GK + PS-MPs potentially augmented the antioxidants activities, while considerably reducing the ROS and MDA concentrations. These attenuative effects of GK are due to its free radical scavenging nature.
PS-MPs intoxication significantly elevated the levels of hepatic biomarkers, including ALP, ALT and AST. The effect of any toxicant on the function of liver can be analyzed by estimating the level of these function enzymes. These aminotransferases are present in hepatic mitochondria and their release indicate hepatic injuries. OS increases the mitochondrial permeability, allowing the leakage of these hepatic markers into blood and thereby elevating their levels that lead to hepatotoxicity (Nagai et al., 2016). Additionally, multiple studies proposed that OS is the major culprit behind the increase in hepatic function markers (Knudsen et al., 2016). However, according to our results, supplementation of GK with PS-MPs remarkably mitigated PS-MPs prompted hepatotoxicity by substantially lowering hepatic enzymes levels owing to its ROS scavenging and hepatoprotective features.
The results of our study showed that PS-MPs administration upsurged Bax and Caspase-3 expressions, besides downregulating Bcl-2. ROS triggered apoptosis and OS are the ultimate causes of hepatotoxicity (Zhang et al., 2003). The overall procedure of apoptosis is controlled by the proteins belonging to Caspase and Bcl-2 families. Bax boosts the apoptosis, besides Bcl-2 performs an antagonistic role to Bax and protects the cells from programmed cell death. The increase in Bax expression and the reduction in Bcl-2 prompt efflux of cyt-C into the cytosol from the membrane of mitochondria. Activation of Caspase-3 is due to increased cyt-C content inside the cytosol. Caspase-3 cleaves the cellular proteins, altering the structural makeup of cells and eventually triggering cell death due to apoptosis (Wang et al., 2013). However, co-treatment of GK + PS-MPs markedly upregulated the Bcl-2 expression, besides reducing the expressions of Caspase-3 and Bax, as a result of its anti-apoptotic potentials.
PS-MPs treatment noticeably elevated the levels of inflammatory markers. Excessive OS leads to the stimulation of inflammation modulator, NF-kB, which further activates the production of IL-6, TNF-α, IL-1β (Janssen-Heininger et al., 2016). The generation of these biomarkers specifies acute inflammatory response and several other anomalies linked with abnormal ROS accumulation. COX-2 is another markers of inflammation and has been reported to instigate inflammation (Kim et al., 2019). Nevertheless, GK supplementation down-regulated these levels due to its anti-inflammatory characteristics. This anti-inflammatory potency of GK might be due to the methoxylation of 5′- or 7′ OH groups and non-methoxylation of 3′ OH groups on A and B rings of GK respectively (Wei et al., 2013).
5 Conclusion
In summary, our investigation demonstrated that PS-MPs administration instigated OS and LP that potently disturbed the levels of oxidative stress markers, liver function enzymes, inflammatory markers along with the expressions of apoptotic markers and induced micromorphological anomalies in the hepatic tissues. Nevertheless, GK administration effectively recovered all these disruptions due to hepatoprotective, anti-inflammatory, antioxidant, as well as anti-apoptotic potential. Due to its therapeutic potential, GK could be integrated into current treatment protocols, providing a novel approach to treat patients suffering from hepatic injury. However, the current study was performed on model animals, therefore, we recommend clinical trials of this compound to check its efficacy on human beings.
CRediT authorship contribution statement
Naila Ghafoor: Writing – original draft, Investigation, Data curation, Conceptualization. Kainat Fatima: Writing – original draft, Methodology, Investigation, Conceptualization. Moazma Batool: Writing – review & editing, Software, Formal analysis, Data curation. Muhammad Imran: Visualization, Validation, Methodology, Data curation. Shaik Althaf Hussain: Writing – original draft, Project administration, Funding acquisition. Usman Atique: Writing – review & editing, Formal analysis, Data curation.
Acknowledgement
The authors would like to acknowledge the funding support by the Researchers Supporting Project number (RSP2024R371), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oxidative stress and its association with male infertility. Male Infertility: Contemp. Clin. Approaches, Androl., ART Antioxidants 2020:57-68.
- [Google Scholar]
- Pharmacotherapeutic potential of ginkgetin against polystyrene microplastics–instigated testicular toxicity in rats: A biochemical, spermatological, and histopathological assessment. Environ. Sci. Pollut. Res.. 2024;31(6):9031-9044.
- [Google Scholar]
- Ginkgetin blocks constitutive STAT3 activation and induces apoptosis through induction of SHP-1 and PTEN tyrosine phosphatases. Phytother. Res.. 2016;30(4):567-576.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250(5475):5480.
- [Google Scholar]
- Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater.. 2020;385:121575
- [Google Scholar]
- Attenuative Effects of Ginkgetin Against Polystyrene Microplastics-Induced Renal Toxicity in Rats. Pak. Vet. J.. 2023;43(4):819-823.
- [Google Scholar]
- Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega. 2022;7(38):34136-34153.
- [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- Regulation of the human thioredoxin gene promoter and its key substrates: a study of functional and putative regulatory elements. Biochim. Biophys. Acta - Gen. Subj.. 2014;1840(1):303-314.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [Google Scholar]
- Reproductive toxicity of polystyrene microplastics: in vivo experimental study on testicular toxicity in mice. J. Hazard Mater.. 2021;405:124028
- [Google Scholar]
- Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male Sprague Dawley rats. Dose-Response. 2021;19(2):15593258211019882
- [Google Scholar]
- Chemoprotective effect of vitexin against cisplatin-induced biochemical, spermatological, steroidogenic, hormonal, apoptotic and histopathological damages in the testes of Sprague-Dawley rats. Saudi Pharm. J.. 2022;30:519-526.
- [Google Scholar]
- Therapeutic effect of oroxylin A against bisphenol A-induced kidney damage in rats: a histological and biochemical study. Pak. Vet. J.. 2022;42(4):11-516.
- [Google Scholar]
- D33 role of oxidative stress in inflammation: Gstp modulates Nf-[kappa] b activation and pro-inflammatory responses in lung epithelial cells. Am. J. Respir. Crit. Care Med.. 2016;193:1.
- [Google Scholar]
- Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacol.. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Anti-inflammatory actions of folate-functionalized bioactive ion-releasing nanoparticles imply drug-free nanotherapy of inflamed tissues. Biomater.. 2019;207:23-38.
- [Google Scholar]
- Correlation between liver cell necrosis and circulating alanine aminotransferase after ischaemia/reperfusion injuries in the rat liver. Int. J. Exp. Pathol.. 2016;97(2):133-138.
- [Google Scholar]
- Plastic debris on Pacific Islands: Ecological and health implications. Sci. Total Environ. 2019:181-187.
- [Google Scholar]
- Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ.. 2016;566:333-349.
- [Google Scholar]
- Magee, C.C., Azuma, H., Knoflach, A., Denton, M.D., Chandraker, A., IYER, S., Sayegh, M., 1999. In vitro and in vivo immunomodulatory effects of RDP1258, a novel synthetic peptide. J. Am. Soc.Nephrol.10(9), 1997-2005.
- Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anti-Cancer Drugs. 2016;27(1):17-23.
- [Google Scholar]
- Reaction of linoleic acid hydroperoxide with thiobarbutiric acid. J. Lipid Res.. 1978;19:1053-1057.
- [Google Scholar]
- Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci. Total Environ.. 2021;781:146753
- [Google Scholar]
- Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol.. 2014;153(3):552-560.
- [Google Scholar]
- Cullin-based ubiquitin ligases: Cul3–BTB complexes join the family. The Embo. J.. 2004;23(8):1681-1687.
- [Google Scholar]
- First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull.. 2015;95(1):358-361.
- [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192-205.
- [Google Scholar]
- Role of synthesized organoselenium compounds on protection of rat erythrocytes from DMBA-induced oxidative stress. Biol. Trace Elemt. Res.. 2009;128:167-175.
- [Google Scholar]
- Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) J. Apoptosis. 2012;17:852-870.
- [Google Scholar]
- Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—A lab experiment. Mar. Pollut. Bull.. 2016;102(1):84-94.
- [Google Scholar]
- The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem. Toxicol.. 2013;58:61-67.
- [Google Scholar]
- Zhang, H., Morisaki, T., Nakahara, C., Matsunaga, H., Sato, N., Nagumo, F., Tadano, J., Katano, M., 2003. PSK-mediated NF-jB inhibition augments docetaxel-induced apoptosis in human pancreatic cancer cells NOR-P1. Oncogene 22 (14), 2088 2096.
- Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ.. 2020;710:136279
- [Google Scholar]







