Genome-wide identification, characterization and expression profiles of heavy metal ATPase 3 (HMA3) in plants
⁎Corresponding author. ahmad.kabir@ru.ac.bd (Ahmad Humayan Kabir)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
HMA (heavy metal associated) is a member of the ATPases protein family involved in metal transport in plants. This study characterizes several HMA3 homologs and infers their molecular functions in different plant species. Arabidopsis AtHMA3 (AT4G30120) was used as a reference to retrieve 11 HMA3 homologs having 97–100% query cover, 535–542 residues, 56,983 to 58,642 (Da) molecular weight, and 5.74 to 8.16 pI value, 29.10 to 33.89 instability index, and 0.222 to 0.380 grand average of hydropathicity. Topological analyses showed 4 transmembrane domains in these HMA3 homologs positioned similarly in terms of cytoplasmic and non-cytoplasmic regions along with ∼22–28% α-helices, ∼22–28% extended strands, and ∼50% random coils. HMA3 protein of Arabidopsis lyrata subsp. lyrata and Eutrema salsugineum are located at chromosome 2, while others are positioned at chromosome 4. All these HMA3 homologs are localized in the plasma membrane sharing a few common biological and molecular functions. Besides, these HMA3 genes contain 8–9 exons in which promoter positions are varied among the homologs. The cis-acting elements of HMA3 genes were projected to be involved with stress response, anaerobic induction, and light-responsive regulation in plants. Three out of five motifs encode E1-E2_ATPase involved in proton-pumping in the plasma membrane. The Arabidopsis thaliana HMA3 protein clustered with Camelina sativa and Capsella rubella show a close phylogenetic relationship. Also, AtHMA3 exhibits a close association with AtHMA3 with MTPA2, ZAT, NRAMP3, IRT2, and NRAMP2 under the local network of AtHMA3 linked to metal transport. Further, AtHMA3 is most potentially expressed during senescence, germinating seed, seedlings, young rosette, bolting, and young flower. In addition, AtHMA3 showed a significant upregulation (>6.0 fold) under Fe-deficiency. These findings may provide essential background to perform wet-lab experiments to understand the role of HMA3 in metal homeostasis.
Keywords
ATPases family
Phylogeny
Conserved motif
Gene interactions
Sequence homology
1 Introduction
Heavy metals are abundant in nature due to natural and anthropogenic causes. Heavy metals are taken up by humans through water and food-based meals and may cause serious health problems. Many of the metals, such as Fe, Cu, Zn, are essential for plants, but they need to be at an optimized level. In contrast, some of the heavy metals (Pb, Cd) are highly toxic to plants hampering photosynthesis, nutrient uptake, and yield in plants (Shanker et al., 2005). However, plants acclimatize different strategies consisting of uptake, sequestration, and chelation to regulate metal homeostasis in withstanding heavy metal toxicity (Tripathi et al., 2012). The association of different metal transporters and their binding capacity play an integral part in the cellular detoxification and maintenance of metals in plants.
The ATPases (P-type adenosine triphosphatases) are the largest superfamily of integral membrane proteins involved in transporting transition metal cations in plants. Eight P-type IB ATPases are encoded in the genome of Arabidopsis thaliana (Baxter et al., 2003). The ATPases are clustered into two groups in plants: monovalent Cu/Ag ion transporting ATPases and divalent Zn/Cd/Co/Pb ion transporting ATPases (Williams et al., 2005). Due to the distinctive N-terminal sequence, ATPases in plants are named HMA (heavy metal associated) protein. In Arabidopsis, HMA1-HMA4 and HMA5-HMA8 proteins are belonging to cluster 1 (Zn/Cd/Co/Pb) and 2 (Cu/Ag) groups (Baxter et al., 2003). In particular, HMA3 proteins participate in heavy metal ion transport and detoxification in plants. In Arabidopsis thaliana, AtHMA3 localized in tonoplast is involved in the vacuolar storage of Cd (Chao et al., 2012). Furthermore, Arabidopsis overexpressed with AtHMA3 showed increased tolerance to Cd, Zn, Pb, and Co, while AtHMA3‐knockout mutant exhibited sensitivity to Cd and Zn (Morel et al., 2009). Similarly, the overexpression of SaHMA3h in tobacco improved the Cd accumulation and tolerance of transgenic plants (Zhang et al., 2016). Further, BjHMA3 is shown to be associated with the varied Cd accumulation in leaves of Brassica rapa (Zhang et al., 2019). Again, OsHMA3 ectopic over-expression resulted in increased Cd tolerance and lower Cd concentration in leaves and grains but increased Cd concentration in rice roots (Ueno et al., 2010).
Protein topology, such as transmembrane helices, domain recognition, binding sites is crucial features for metal binding capacity in plant system. As a result, the identification of metal sites along with the components at transcriptional and Posttranslational regulation eventually determines the functions of a protein in response to metals. One of the identified candidate genes in Arabidopsis halleri was AhHMA3, which is highly similar to HMA3 in Arabidopsis thaliana (AT4G30120) (Becher et al., 2004). HMA3, located in the vacuolar membrane, participates in vacuolar sequestration of Zn, Cd, Co, and Pb in Arabidopsis (Morel et al., 2009). However, the function of HMA3 in hyperaccumulators remains unclear in planta. Although the molecular functions of Arabidopsis HMA3 are relatively well established, the analysis of HMA3 homologs and interactions with other transporters/genes are barely studied.
The characterization of HMA3 possesses the immense potential to combat metal homeostasis in plants. The in silico characterization of HMA was performed in a previous study on Brassica oleracea (Sutkovic et al., 2016) but its validation and interactions among the other species are yet to be done. The in silico characterization of HMA3 homologs may provide in-depth insight into these genes/proteins. In this study, we have searched for HMA3 homologs based on Arabidopsis heavy metal ATPase 3 (AtHMA3) referred to as AT4G30120 across different plant species. The CDS, mRNA, and protein sequences of these HMA3 homologs were taken into computational analysis with advanced bioinformatics software and online-based platforms.
2 Materials and methods
2.1 Retrieval of HMA3 genes/proteins
The AtHMA3 gene, designated AT4G30120 in the Uniprort/Aramene database (protein accession: NP 194741.2 and gene accession: NM 119158.4), was retrieved from NCBI to serve as a homology search reference (protein accession: NP 194741.2 and gene accession: NM 119158.4) (Stephen et al., 1997). The search is narrowed to records with an expect value of 0 to 0. The related FASTA gene and protein sequences were obtained from the NCBI database. For each species, one accession was chosen for analysis throughout the filtering process.
2.2 Analyses of HMA3 genes/proteins
As previously stated, the ProtParam tool (https://web.expasy.org/protparam) was used to examine the physico-chemical characteristics of HMA3 protein sequences (Gasteiger et al., 2005). The ARAMEMNON database (http://aramemnon.uni-koeln.de/) was used to detect chromosomal and exon position. The CELLO server (http://cello.life.nctu.edu.tw) predicted protein subcellular localisation. The Pfam database (http://pfam.xfam.org) was used to find protein domain families, and the Phytozome v12.1 database was used to assess functions (El-Gebali, 2019). The FGENESH online tool predicted the structural arrangement of HMA3 genes (Solovyev et al., 2006). Promoter 2.0 Prediction Server (http://www.cbs.dtu.dk/services/Promoter) projected promoter position. In addition, each HMA3 gene's promoter was analyzed in silico for 1 kbp upstream of the translation start site from the relevant databases. For scanning of cis-elements present in promoter regions of these genes, the PLACE (Higo et al., 1999) and PlantCare (Lescot et al., 2002) programs were utilized. TSSPlant (Shahmuradov et al., 2017) and FGENESH 2.6 (Solovyev et al., 2006) were also used to predict the transcriptional start site (TSS) and the PolA site, respectively.
2.3 Phylogenetic relationships and identification of conserved protein motifs
Clustal Omega was used to construct multiple sequence alignments of HMA3 proteins in order to discover conserved residues. MEME Suite 5.1.1 (http://meme-suite.org/tools/meme) was used to characterize the proteins' five conserved protein motifs using default parameters, but there were a maximum of five motifs to find (Timothy et al., 2009). The MyHits (https://myhits.sib.swiss/cgi-bin/motif scan) online program was used to scan the motifs for matches with other domains (Sigrist et al., 2010). Using 11 HMA3 homologs from 11 plant species, MEGA (V. 6.0) created a phylogenetic tree using the maximum likelihood (ML) technique for 1000 bootstraps (Tamura et al., 2013).
2.4 Interactions and co-expression of HMA3 protein
The HMA3 protein interactome network was displayed in Cytoscape using the STRING server (http://string-db.org) (Szklarczyk et al., 2019). Genevestigator software was also used to retrieve Arabidopsis HMA3 expression data. Based on the Affymetrix Array Platforms (AT AFFY-ATH1-0), the expression and co-expression connections of HMA3 were investigated in various anatomical, developmental, and perturbational contexts.
2.5 Structural analysis of HMA3 proteins
Structural analysis, such as transmembrane domains, was constructed with Protter ( http://wlab.ethz.ch/protter/start) tool (Omasits et al., 2014). Besides, a two-dimensional secondary structure of MTP1 proteins constructed GORIV ( https://npsa-prabi.ibcp.fr/NPSA/npsa_gor4.html).
3 Results
3.1 Retrieval of HMA3 transporter genes/proteins
Arabidopsis AtHMA3 was searched in the NCBI database to get the FASTA sequence of the protein (NP_194741.2) and mRNA (NM_119158.4). The blast analysis of AtHMA3 protein showed 11 homologs of the heavy metal atpase 3 family by filtering (E-value: 0.0, query cover: 97–100%, percentage identity: 71.73–100%) in 11 plant species, which include Arabidopsis thaliana, Camelina sativa, Capsella rubella, Eutrema salsugineum, Brassica oleracea var. oleracea, Raphanus sativus, Brassica napus, Brassica rapa, Arabidopsis lyrata subsp. lyrata, Eutrema salsugineum and Tarenaya hassleriana (Table 1).
| Sl. | Protein Accession | Species | Protein length | MW (Da) | pI | Instability index | Grand average of hydropathicity (GRAVY) | α-helix | Extended strand | Random coil |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NP_194741.2 | Arabidopsis thaliana | 542 | 58642.37 | 7.44 | 31.50 | 0.355 | 24.17% | 26.38% | 49.45% |
| 2 | NP_001289919.1 | Camelina sativa | 540 | 58517.12 | 6.72 | 29.10 | 0.380 | 23.70% | 27.04% | 49.26% |
| 3 | XP_006303912.1 | Capsella rubella | 539 | 58183.82 | 6.42 | 31.49 | 0.364 | 24.68% | 25.05% | 50.28% |
| 4 | XP_006412748.2 | Eutrema salsugineum | 539 | 58475.96 | 6.72 | 31.97 | 0.307 | 25.97% | 24.49% | 49.54% |
| 5 | XP_013591300.1 | Brassica oleracea var. oleracea | 540 | 58479.85 | 5.74 | 29.62 | 0.312 | 27.59% | 23.70% | 48.70% |
| 6 | XP_018480904.1 | Raphanus sativus | 540 | 58244.61 | 6.43 | 31.31 | 0.328 | 28.33% | 25.00% | 46.67% |
| 7 | XP_022562629.1 | Brassica napus | 540 | 58365.83 | 6.42 | 30.92 | 0.359 | 25.00% | 26.11% | 48.89% |
| 8 | XP_009137892.1 | Brassica rapa | 541 | 58293.66 | 6.98 | 33.89 | 0.325 | 28.47% | 22.00% | 49.54% |
| 9 | XP_020886284.1 | Arabidopsis lyrata subsp. lyrata | 528 | 57120.44 | 6.55 | 33.68 | 0.330 | 25.19% | 26.70% | 48.11% |
| 10 | XP_006409084.2 | Eutrema salsugineum | 525 | 56983.36 | 6.97 | 37.19 | 0.314 | 22.86% | 28.57% | 48.57% |
| 11 | XP_010548593.1 | Tarenaya hassleriana | 538 | 58204.12 | 8.16 | 32.42 | 0.222 | 24.16% | 27.88% | 47.96% |
3.2 Physiochemical features and localization of HMA3 proteins
The 11 HMA3 protein homologs encoded a protein with residues of 525–542 amino acids having 56983.36 to 58642.37 (Da) molecular weight, and 5.74 to 8.16 pI value, 29.10 to 33.89 instability index, and 0.222 to 0.380 grand average of hydropathicity (Table 1). Topological prediction analyses of transmembrane (TM) domains of HMA3 protein homologs showed 4 transmembrane domains in protein representative from each of the plant species (Supplementary Fig. S1). None of the HMA3 protein homologs contains signal peptide. These HMA3 proteins showed positioning similarity in terms of cytoplasmic and non-cytoplasmic regions (Supplementary Fig. S1). In addition, secondary structure prediction showed that all HMA3 proteins contain above ∼ 22–28% α-helices, ∼22–28% extended strands, and ∼ 50% random coils (Table 1).
3.3 Localization and functional annotation of HMA3 proteins
HMA3 protein of Arabidopsis lyrata subsp. lyrata (XP_020886284.1) and Eutrema salsugineum (XP_006409084.2) is located at chromosome 2; however, the rest of the HMA3 homologs positioned at chromosome 4 (Table 2). All of these HMA3 protein homologs are associated with E1-E2 ATPase (PF00122). The CELLO localization predictor showed that these HMA3 proteins are localized in the plasma membrane of roots in all 11 plant species (Table 2). Ontology analysis demonstrated that HMA3 proteins of Arabidopsis thaliana, Camelina sativa, Capsella rubella, Eutrema salsugineum, Brassica oleracea var. oleracea, Raphanus sativus, Brassica napus and Brassica rapa possess several cellular components, including vacuolar membrane, plasma membrane, and a membrane having involvement in the same biological process (cation transport, metal ion transport) and molecular function (nucleotide-binding, ATP binding, ATPase activity, hydrolase activity, metal ion binding). In addition, HMA3 Arabidopsis lyrata subsp. lyrata and Eutrema salsugineum showed the same cellular component (vacuolar membrane, plasma membrane, membrane), biological process (transition metal ion transport, cation transport, zinc ion transport, cadmium ion transport, response to cadmium ion) and molecular function (nucleotide-binding, ATP binding, ATPase activity, hydrolase activity, metal ion binding, metal ion transmembrane transporter activity, cadmium-transporting ATPase activity). Lastly, HMA3 protein of Tarenaya hassleriana showed unique cellular components (plasma membrane, membrane, integral to membrane), biological process (ATP biosynthetic process, cation transport, metabolic process, metal ion transport, zinc ion homeostasis) but molecular function similar to Arabidopsis lyrata subsp. lyrata and Eutrema salsugineum (Table 2).
| Sl. | Protein Accession | Species | Domain | Localization | Cellular component | Biological Process | Molecular function |
|---|---|---|---|---|---|---|---|
| 1 | NP_194741.2 | Arabidopsis thaliana | E1-E2 ATPase (PF00122) | Plasma Membrane | -vacuolar membrane-plasma membrane-membrane | -cation transport-metal ion transport | -nucleotide binding-ATP binding-ATPase activity-hydrolase activity,-metal ion binding |
| 2 | NP_001289919.1 | Camelina sativa | As above | As above | As above | As above | As above |
| 3 | XP_006303912.1 | Capsella rubella | As above | As above | As above | As above | As above |
| 4 | XP_006412748.2 | Eutrema salsugineum | As above | As above | As above | As above | As above |
| 5 | XP_013591300.1 | Brassica oleracea var. oleracea | As above | As above | As above | As above | As above |
| 6 | XP_018480904.1 | Raphanus sativus | As above | As above | As above | As above | As above |
| 7 | XP_022562629.1 | Brassica napus | As above | As above | As above | As above | As above |
| 8 | XP_009137892.1 | Brassica rapa | As above | As above | As above | As above | As above |
| 9 | XP_020886284.1 | Arabidopsis lyrata subsp. lyrata | As above | As above | -plasma membrane-plasmodesma-membrane-integral to membrane | -transition metal ion transport-cation transport-zinc ion transport- cadmium ion transport-response to cadmium ion | -nucleotide binding-ATP binding-ATPase activity-hydrolase activity,-metal ion binding- metal ion transmembrane transporter activity- cadmium-transporting ATPase activity |
| 10 | XP_006409084.2 | Eutrema salsugineum | As above | As above | As above | As above | As above |
| 11 | XP_010548593.1 | Tarenaya hassleriana | As above | As above | -plasma membrane-membrane-integral to membrane | -ATP biosynthetic process-cation transport-metabolic process-metal ion transport-zinc ion homeostasis | As above |
3.4 Gene organization
ARAMEMNON analysis showed the presence of 8–9 exons among the HMA3 gene homologs located at different positions of gene ranged from 1 to 3535 base pairs (Fig. 1, Table 3). Promoter analysis showed marginal and highly like the prediction of promoter position in the gene sequence in different HMA3 homologs across the 11 plant species. The Arabidopsis thaliana HMA3 showed three different positions of promoter marginal predicted at 200, 700, and 1800 bp (Table 3). Highly predicted position of promoters are located in 2200 bp, 2100 bp and 2500 bp in XM_006303850.2 (Capsella rubella), XM_006412685.2 (Eutrema salsugineum), XM_010550291.1 (Tarenaya hassleriana), respectively (Table 3). The position of TSS varied from 22 to 36 bp if found. Also, the PolA was positioned after the coding region in all HMA3 genes showing the position at 2369–4074 bp, if found (Table 3). The identified cis-acting elements were stress, hormone, and other responsive factors. Stress responsive, anaerobic induction, and light responsive regulators were found to be the height number and most common of cis-acting elements in HMA3 genes. That projected their involvement of these activities (Table 4).
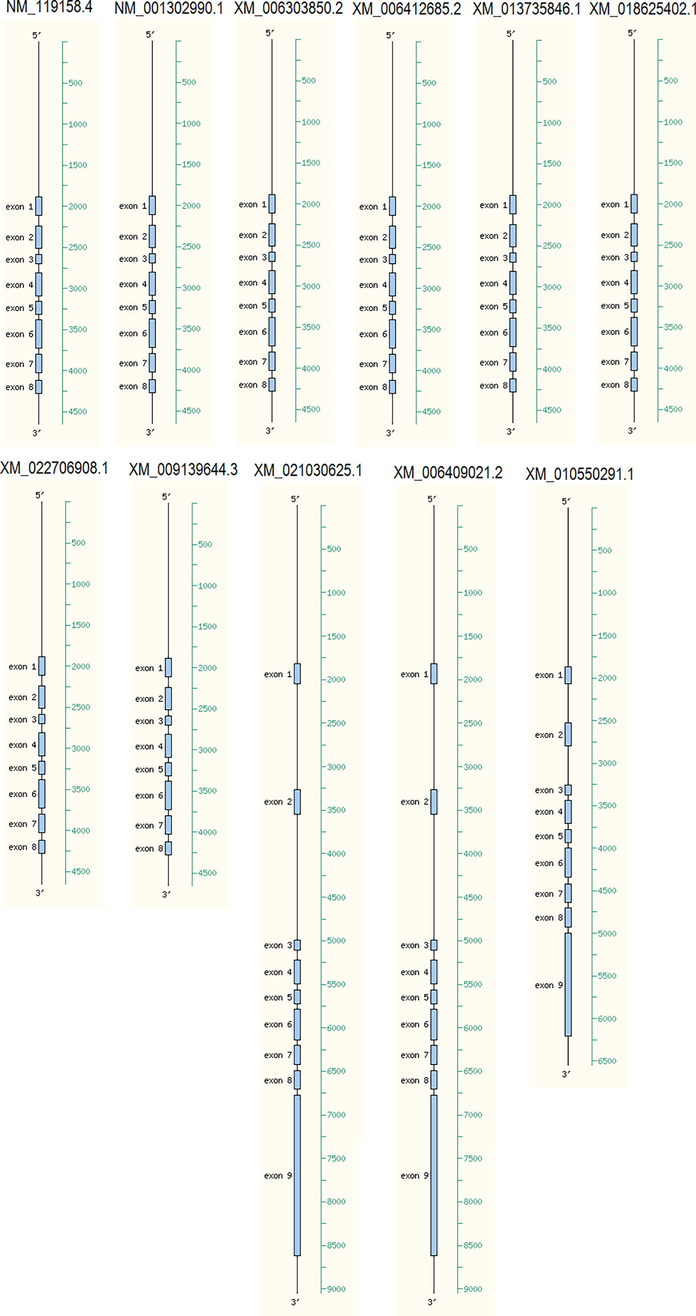
- Gene organization of HMA3 homologs.
| No. | Gene Accession | Chromosome number | Coding region | Promoter position | Position of transcriptional start site (TSS) | PolA |
|---|---|---|---|---|---|---|
| 1 | NM_119158.4 | 4 | 127-1645, 1756-2408 | 200 Marginal prediction700 Marginal prediction1800 Marginal prediction | 24 | 2516 |
| 2 | NM_001302990.1 | 4 | 1-2286 | 1700 Marginal prediction | 24 | 2516 |
| 3 | XM_006303850.2 | 4 | 38-2302 | 2200 Highly likely prediction | – | 2453 |
| 4 | XM_006412685.2 | 4 | 6-2288 | 1100 Marginal prediction2100 Highly likely prediction | – | 2354 |
| 5 | XM_013735846.1 | 4 | 1-2277 | 600 Marginal prediction1100 Marginal prediction1700 Marginal prediction | – | – |
| 6 | XM_018625402.1 | 4 | 71–2335 | 200 Marginal prediction1000 Marginal prediction1700 Marginal prediction | 22 | 2376 |
| 7 | XM_022706908.1 | 4 | 92-2341 | 200 Marginal prediction1100 Marginal prediction | 36 | 2369 |
| 8 | XM_009139644.3 | 4 | 123-2417 | 1800 Marginal prediction | – | 2681 |
| 9 | XM_021030625.1 | 2 | 190-4023 | 1000 Marginal prediction2700 Marginal prediction3200 Marginal prediction3600 Marginal prediction | – | 4074 |
| 10 | XM_006409021.2 | 2 | 135-4142 | 1100 Marginal prediction2200 Marginal prediction2600 Marginal prediction3500 Marginal prediction3900 Marginal prediction | – | 4235 |
| 11 | XM_010550291.1 | 4 | 155-516, 589-3535 | 2500 Highly likely prediction3300 Marginal prediction | – | 3788 |
| Gene Accession | Cis-acting elements | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MYC/Myc | MYB/myb | MRE | TC rich | GT1 | G box/ABRE | W - box | TATC-Box | ERE | AREs | As-1/TGA | AuxRR-core | TGACG | CGTCA | TCA element | ACE | AE-Box | Box −4 | GATA-Motif | GA-Motif | WUN3 | WRE3 | O2 Site | circadian | STRE | AT-rich element | |
| NM_119158.4 | 1 | 4 | 1 | 2 | 1 | 2 | 1 | 1 | 4 | 1 | 1 | |||||||||||||||
| NM_001302990.1 | 3 | 2 | 1 | 1 | 2 | 1 | 1 | 3 | 2 | 2 | 2 | 1 | ||||||||||||||
| XM_006303850.2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||
| XM_006412685.2 | 5 | 2 | 2 | 1 | 2 | 2 | 4 | 1 | 1 | 4 | 2 | 1 | ||||||||||||||
| XM_013735846.1 | 1 | 4 | 1 | 2 | 2 | 1 | 4 | 1 | ||||||||||||||||||
| XM_018625402.1 | 2 | 1 | 2 | 1 | 3 | 1 | 1 | 2 | 1 | 1 | 1 | |||||||||||||||
| XM_022706908.1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | |||||||||||||||
| XM_009139644.3 | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 2 | 1 | 2 | 1 | |||||||||||||
| XM_021030625.1 | 1 | 4 | 1 | 3 | 2 | 1 | 1 | 1 | 1 | |||||||||||||||||
| XM_006409021.2 | 1 | 4 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | ||||||||||||||||
| XM_010550291.1 | 4 | 1 | 2 | 1 | 1 | 4 | 1 | 2 | 1 | 3 | 1 | 1 | ||||||||||||||
Different cis- regulatory elements: MYC/Myc (Dehydration-responsive), MYB/Myb (drought-responsive), MBS (involved in drought induction), TC rich (defense and stress-responsive), GT1 (SALT-responsive), ABRE/G-box (Abscisic acid-responsive), W-box (defense responsive), TATC-Box (gibberellins responsive) ERE (ethylene-responsive), AREs (involved in anaerobic induction), As-1, and AuxRR-core, (auxin-responsive), TGACG and CGTCA (MeJA-responsive), TCA (salicylic acid-responsive), ACE, AE-box, Box-4, GATA-Motif, GA-motif, and G-Box (light-responsive), WUN3, and WRE3 (wound-responsive), O2 site (Zein metabolism regulation), circadian (circadian control), STRE (Expression activator), and At-rich (DNA binding).
3.5 Conserved motif, sequence similarities, and phylogenetic analysis
We have used the MEME tool to search for the five most conserved motifs in identified 11 HMA3 homologs (Fig. 2). All these 5 motifs are 50 residues long located at site 11. These motifs are as follows: motif 1 (INLNGYIKVKTTALARDCVVAKMTKLVEEAQKSQTKTQRFIDKCSRYYTP), motif 2 (HPMAAALIDYARSVSVEPKPDMVENFQNFPGEGVYGRIDGQDIYIGNKRI), motif 3 (NLSHWFHLALVVLVSGCPCGLILSTPVATFCALTKAATSGFLIKTGDCLE), motif 4 (KALNQARLEASVRPYGETSLKSQWPSPFAVVSGVLLALSFLKYFYSPLEW) and motif 5 (CMZDYTEAATIVFLFSVADWLESSAAHKASTVMSSLMSLAPRKAVIAETG). Motif 1, 3 and 5 encodes pfam_fs: E1-E2_ATPase. Motif 4 is linked to freq_pat:PKC_PHOSPHO_Site, while motif 2 shows no information (Fig. 2). The HMA3 protein homologs were aligned to check the similarities of sequence across the plant species. The MTP1 proteins showed 71.7% to 100% similarities among the different plant species, in which the consensus sequence ranged from 70% to 100% (Supplementary Fig. S2). The phylogenetic tree was clustered into four groups (A, B, C, D) based on tree topologies (Fig. 3). In cluster A, HMA3 of Arabidopsis thaliana formed a cluster with the Camelina sativa and Capsella rubella, while group B consist of HMA3 protein homologs of Brassica oleracea var. oleracea, Raphanus sativus, Brassica napus and Brassica rapa. The HMA3 of Eurema salsugineum clustered alone is located in the distance from Arabidopsis thaliana homolog. The cluster D consists of Arabidopsis lyrata subsp. Lyrata HMA4, Eurema salsugineum HMA4 and Tarenaya hassleriana HMA3 (Fig. 3). In this phylogenetic tree, HMA3 of Arabidopsis thaliana, Brassica oleracea var. oleracea, Raphanus sativus, Brassica napus and Brassica rapa showed the highest 100% bootstrap value (Fig. 3).
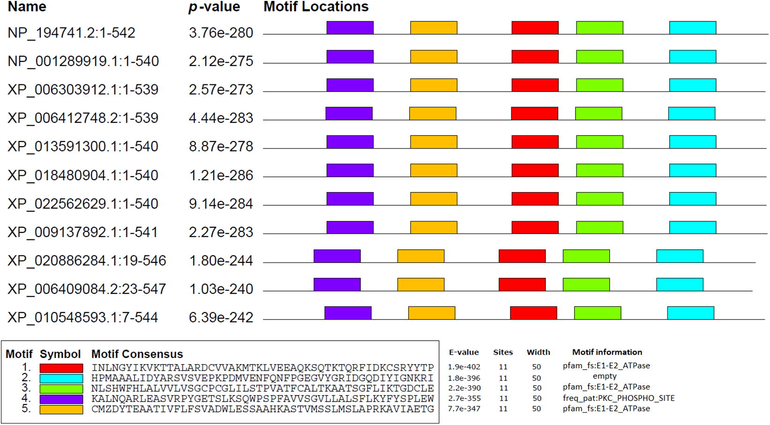
- Schematic representation of the 5 conserved motifs in 11 HMA3 protein homologs across 11 plant species. Scale bar corresponds to 0.1 amino acid substitution per residue. Different motifs, numbered 1–5, are displayed in different colored boxes.
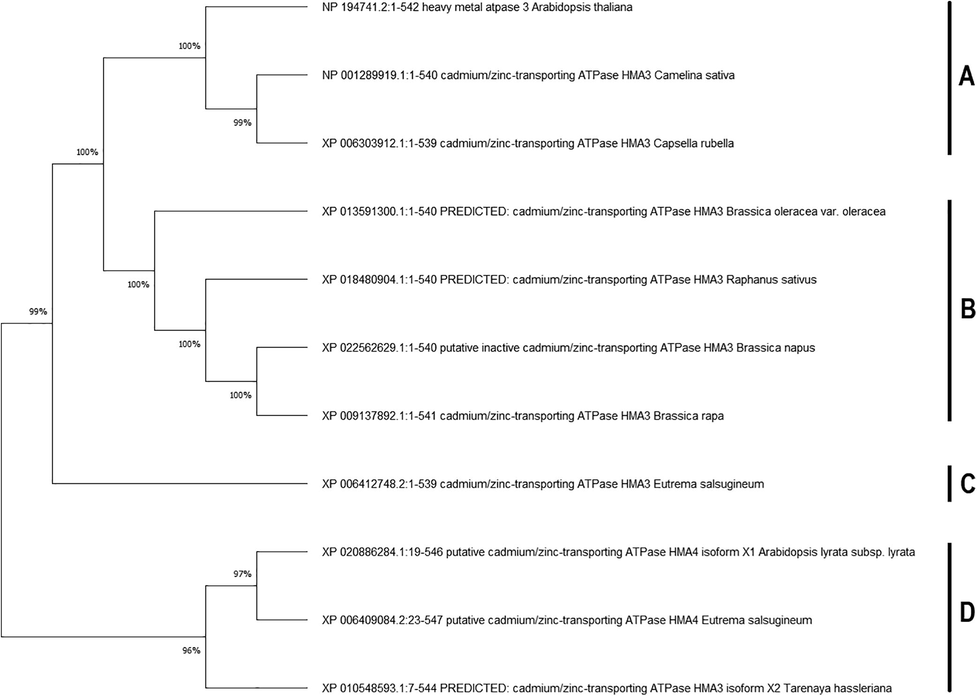
- Phylogenetic trees of HMA3 protein homologs. Trees were constructed by MEGA 6 software with the maximum likelihood (ML) method for 1000 bootstrap values. Trees were used as benchmarks to analyze the clustering of 11 HMA3 sequences. A, B, C and D represents different clusters.
3.6 Predicted interaction partner analysis
Interactome analysis was performed for AtHMA3 (AT4G30120) on STRING server. STRING showed five closely associated putative interaction partners of AtHMA3. These include MTPA2 (metal tolerance protein A2), ZAT (a member of the zinc transporter and cation diffusion facilitator), NRAMP3 (natural-resistance-associated macrophage protein 3), IRT1 (iron-regulated transporter 2) and NRAMP4 (natural-resistance-associated macrophage protein 4) genes (Fig. 4). Further, the analysis showed four local network clusters, including CL:28166 (nickel transport and cation efflux protein), CL:28164 (manganese ion transport and nickel transport), CL:28176 (ion influx/efflux at the host-pathogen interface), CL:28126 (transition metal ion transmembrane transporter). Lastly, reactome pathways of AtHMA3 include ion influx/efflux at host-pathogen interface, zinc efflux, and compartmentalization by the SLC30 family, peptide hormone metabolism, insulin processing, and metal ion SLC transporters (Fig. 4).
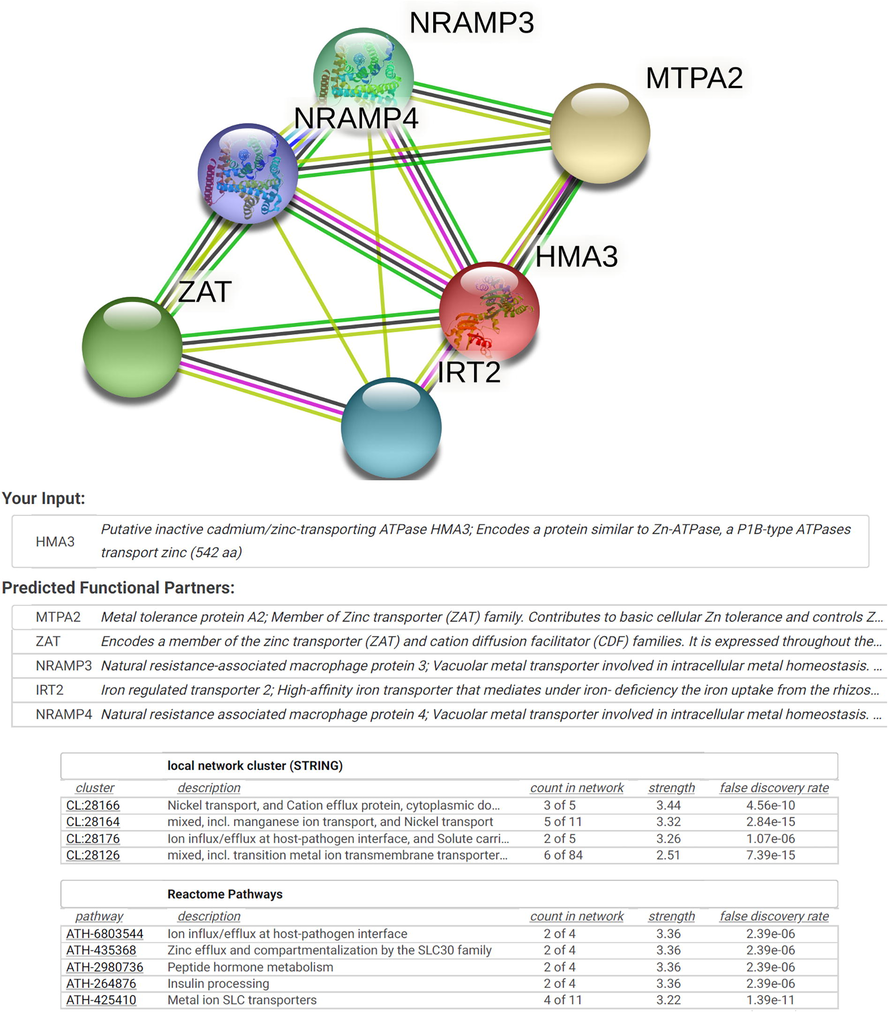
- Gene interaction partners and gene network analysis of AtHMA3 and its homologs. Interactome was generated using Cytoscape for STRING data.
3.7 Expression profiles of HMA3
The genevestigator analysis against the Affymetrix Array Platforms showed expression potential and co-expression data of HMA3 in different anatomical parts, developmental stages, and perturbations. In the anatomical part, lateral roots, cauline leaf, silique inflorescence, and radicle in shoot apex seemed to be highly potential for HMA3 expression (Fig. 5a). Further, giant root cell and sperm cells in cell culture have the potential for HMA3 expression (Fig. 5a). Besides, HMA3 has expression potential during senescence, germinated seed, seedling, young rosette, bolting, and young flower stages of development (Fig. 5b). Among the selected stress, HMA3 only showed significant upregulation under Fe deficiency; while the expression did not notably vary in other stresses, such as anoxia, cold, drought, gamma irradiation, genotoxicity, heat, osmotic stress, salt stress, shift cold stress, submerge stress, wounding stress (Fig. 5c).
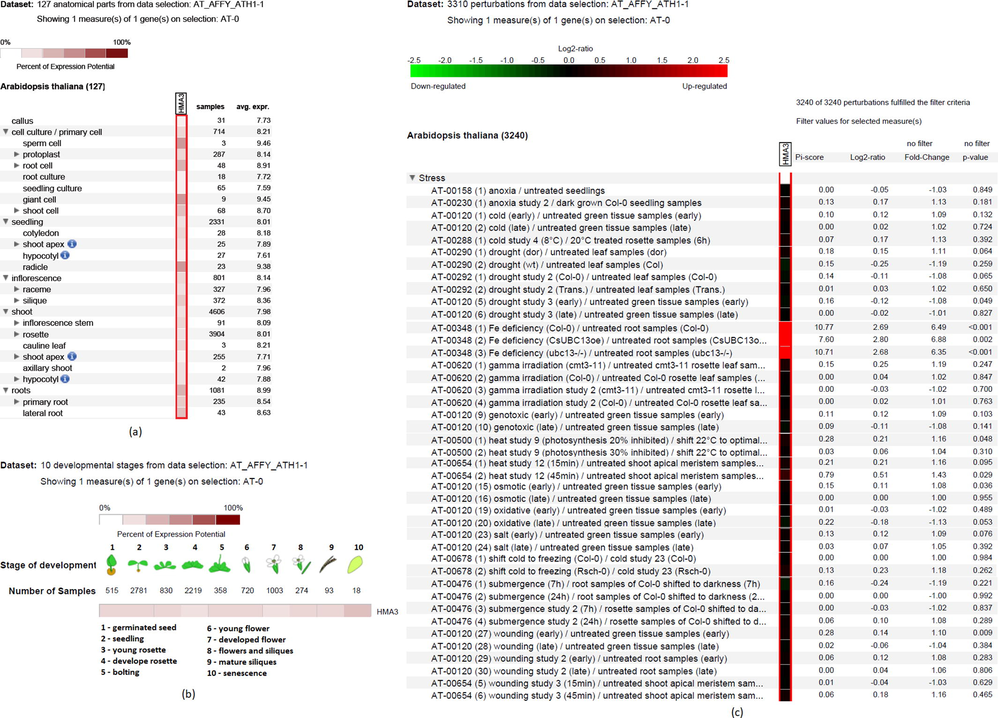
- Expression profiles of AtHMA3 in different anatomical part, developmental stage and perturbations in Genevestigator Affymetrix platform.
Co-expression analysis was filtered to five closely associated genes in different anatomical parts, developmental stages and perturbations (Fig. 6). In the anatomical part, the AtHMA3 gene is closely co-expressed with AT1G30560 (putative glycerol-3-phosphate transporter), AT1G63550 (cystein-rich repeat secretory protein 9), AT3G60270 (cupredoxin superfamily protein), AT5G43370 (probable inorganic phosphate transporter 1–2) and AT3G12900 (2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase superfamily protein). During the development stage, AT3G56891 (heavy metal transport/detoxification superfamily protein), AT5G19040 (adenylate isopentenyltransferase 5, chloroplastic), AT3G45410 (L-type lectin-domain containing receptor kinase I.3), AT3G29250 (short-chain dehydrogenase reductase 4) and AT2G25260 (unknown protein) co-expressed with AtHMA3 (Fig. 6). Under perturbations, the top five genes co-expressed with AtHMA3 are AT1G64480 (calcineurin B-like protein 8), AT5G17100 (cystatin/monellin superfamily protein), AT5G65980 (auxin efflux carrier family protein), AT2G28690 (protein of unknown function, DUF1635), and AT2G12190 (cytochrome P450 superfamily protein) (Fig. 6).
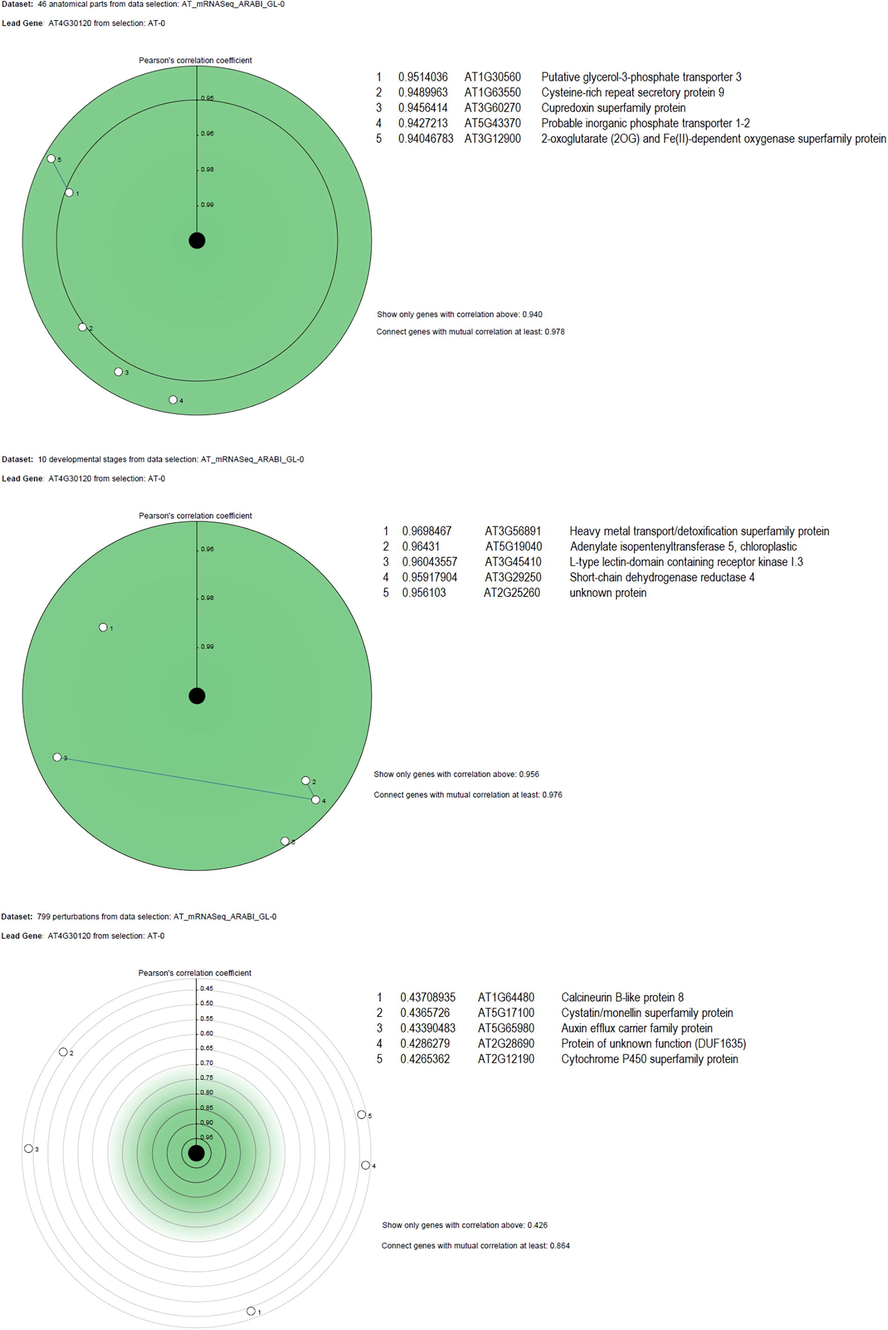
- List of top 5 genes co-expressed with AtHMA3 in different anatomical parts and developmental stages of plants.
4 Discussion
In recent years, the characterization of membrane transporters involved in heavy metal scavenging in plants is emerging. Prior to the wet-lab experiment, the in silico analysis is of utmost interest to narrow down the target of studies. The role of AtHMA3 in vacuolar storage of a few metals is documented (Morel et al., 2009), although the involvement of other metals and dynamic network of other associated genes and gene/protein properties are yet to extensively studies. This in silico characterization and expression profile AtHMA3 and its homologs and closely associated genes unveil significant regulatory findings that can be essential contributors to the downstream genome-editing or biotechnological approach to heavy metal studies.
In this study, we selectively blasted the AtHMA3 sequences resulted in 11 different HMA3 protein homologs having 71.73–100% percentage identity. The similarities in protein size, pI, instability index and hydrophilicity suggest that these HMA3 proteins are biochemically relevant. Domain analysis further revealed the association of these HMA3 protein homologs with E1-E2 ATPase (PF00122) localized in the plasma membrane. The proton-pumping ATPase (H+-ATPase) in the plasma membrane produces the proton motive force through the plasma membrane that is required to enable much of the transport of ions and metabolites (Morsomme et al., 2000). However, HMA3 proteins showed diverse cellular components in HMA3 homologs, the unique feature of these is limited to the membrane, vacuolar membrane and plasma membrane. These cellular components are crucial in mineral absorption and metal homeostasis along with salt tolerance, intracellular pH regulation and cellular expansion in plants (Morsomme et al., 2000; Logan et al., 1997). However, HMA3 proteins are predominantly associated with cation transport, metal ion transport, zinc ion transport and cadmium ion transport, as evident from our ontology analysis. HMA3 gene is involved in cadmium and lead transport along with vacuolar sequestration potentiality in a heterologous system, but not in zinc transport. Vacuolar sequestration may have a detoxification function (Gravot et al., 2004).
In predicting evolutionary relationships and functional genomics possibilities, the knowledge on the position and organization of the coding sequence of a gene is considered a critical factor. In this study, all of the identified sequences of HMA3 proteins demonstrated 4 transmembrane helices confirming similar hydropathy of these HMA3 protein homologs. The metal specificity of each subclade is determined by specific amino acids in the three transmembrane helices closest to C-terminus (Raimunda et al., 2012). In this study, all HMA3 gene homologs belonging to the 11 plant species showed 8–9 exons, suggesting that these HMA3 genes are evolutionarily closer to each other. Although promoter analysis predicted several promoter regions of each HMA3 gene, the highly likely prediction of the promoter was made at 2100–2500 bp in several plant species. Localization of exon and promoter plays an essential part in CRISPR-Cas9 and other genome editing studies in plant science (Yan et al., 2017). In addition, the identification of TSS and PolA in HMA3 homologs will be crucial in understanding the transcriptional and translational genomics. Besides, promoter analysis reveals the involvement of cis-acting elements associated with stress response, hormone, anaerobic induction, and light-responsive regulators in HMA3 genes. The architectures of 1000 bp promoter regions upstream of transcription start sites were determined using in silico analysis (New et al., 2015). In the light of our findings, the frequency with which these cis-elements appeared in each promoter varied, and the number of clusters within each promoter's core-regulatory area could indicate how susceptible each gene was to HMA regulation in plants.
Conserved motifs are identical sequences across species that are maintained by natural selection. A highly conserved sequence is of having functional roles in plants and can be a useful start point to start research on a particular topic of interest (Wong et al., 2015). Out of the five motifs, three motifs are mainly matched with the E1-E2_ATPase associated with H+ pumping. P-type proton ATPase is found in the plasma membranes of plants that, in turn, drives secondary active transport processes across the membrane (Palmgren et al., 2001). One of the motifs is also linked to the protein kinase C phosphorylation site that may play roles in controlling the catalytic activity, stability and intracellular localization of the enzyme (Freeley et al., 2011). Further, the phosphorylation site may be attributed to the release of Zn from intracellular stores leading to phosphorylation kinases and activation of signaling pathways (Thingholm et al., 2020). The presence of common and long-preserved residues suggests that HMA3 homologs between species may have highly conserved structures. Additionally, for sequence-specific binding sites and transcription factor analysis, this information can be targeted. In phylogenetic analysis, HMA3 protein of A. thaliana positioned in the same cluster with C. sativa and C. rubella, suggesting its close relationship during the evolutionary trend. Consistently, HMA3 protein homologs of Brassica sp. and Raphanus sp. clustered within B, suggesting the close evolutionary emergence from a common ancestor within the Brassicaceae family. It appears that the HMA3 of E. salsugineum is relatively distantly related to A. thaliana over the evolutional trends. Thus, our results might infer a functional relationship of HMA3 sequences in metal uptake across different plant species.
The interaction network of a specific gene provides information of all physical associations that can occur among family members. Global gene co-expression analysis is an emerging tool to identify the tissues and the conditions in which significant interactions occur. The interactome map analyzed in String platform showed the most close association with MTPA2, ZAT, NRAMP3, IRT2 and NRAMP2, mainly linked to metal transport in plants. Consistently, the local network of AtHMA3 implies the involvement with metal transporter. As a result, these findings might be useful to characterize HMA3 and to interpret the interactions of multiple genes linked to particular stress of interest in plants. Studies reported that Zn homeostasis is closed associated with P-type ATPase heavy metal transporters (HMA). Again, both HMA2 and HMA4 were reported to be involved with Zn homeostasis in Arabidopsis (Hussain et al., 2004). Besides, AtHMA3 showed some reactome pathways, among which ion influx, zinc influx, and metal ion SLC transporters may attribute to the metal transporter properties of this gene. Overall, this interactome finding might provide essential background for functional genomics studies of metal uptake and transport in plants.
The expression potential of a gene in different conditions is a crucial factor in determining the involvement in a particular trait. The in silico expression analysis in the Genevestigator platform showed interesting outputs concerning the expression of AtHMA3 (AT4G30120) in different anatomical, perturbations, and developmental stages. Being consistent with the AtHMA3 gene ontology, Genvestigator showed that the root is the important location where this gene showed expression potential. In a wet-lab experiment, root-specific expression of HMA3 was reported in rice (Cai et al., 2009). Further, AtHMA3 is most potentially expressed during senescence, but germinated seeds, seedlings, young rosette, bolting and young flower also possess significant potential for AtHMA3 expression. Interestingly, AtHMA3 showed a significant upregulation (>6.0 fold) in response to Fe-deficiency. Till now, HMA3 is known to induce its expression subjected to heavy metals in several plant species (Yao et al., 2018). Nevertheless, our results suggest that AtHMA3 is a potential gene that could contribute to Fe-deficiency tolerance in plants.
5 Conclusion
This in silico work identifies and characterizes 11 HMA3 homologs from each plant species. The analysis showed similar physicochemical properties, gene organization, and conserved motifs related to metal transport. The identified cis-acting elements were linked to stress response, hormone, and other responsive factors. Sequence homology and phylogenetic tree showed the closest evolutionary relationship of Arabidopsis HMA3 with Camelina sativa and Capsella rubella. In addition, the interactome map displayed some partner genes of AtHMA3 involved in metal transport in plants. It was also predicted that AtHMA3 is expressed in root tissue during senescence and was significantly upregulated in response to Fe-deficiency. These findings will provide basic theoretical knowledge for the downstream studies on HMA3 function and characterization related to metal homeostasis in various plants.
Acknowledgment
The current work was funded by Taif University Researchers Supporting Project number (TURSP - 2020/75), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Genomic comparison of P-Type ATPase ion pumps in Arabidopsis and rice. Plant Physiol.. 2003;132:618-628.
- [Google Scholar]
- Cross‐species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J.. 2004;37:251-268.
- [Google Scholar]
- Root-specific expression of rice OsHMA3 reduces shoot cadmium accumulation in transgenic tobacco. Mol. Breeding. 2009;39(3)
- [CrossRef] [Google Scholar]
- Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS Genet.. 2012;8(9):e1002923.
- [CrossRef] [Google Scholar]
- The Pfam protein families database in 2019. Nucleic. Acids. Res.. 2019;47:D427-D432.
- [Google Scholar]
- Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites. Cell. Signal.. 2011;23(5):753-762.
- [Google Scholar]
- Protein identification and analysis tools on the ExPASy server. In: Walker J.M., ed. The proteomics protocols handbook. Louisville: Humana; 2005. p. :571-607.
- [Google Scholar]
- AtHMA3, a plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS Lett.. 2004;561:22-28.
- [Google Scholar]
- Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucl. Acids Res.. 1999;27(1):297-300.
- [Google Scholar]
- P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell.. 2004;16:1327-1339.
- [Google Scholar]
- Plant CARE, a data base of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucl. Acids. Res.. 2002;30:325-327.
- [Google Scholar]
- Plasma membrane transport systems in higher plants: From black boxes to molecular physiology. Physiol. Plant.. 1997;100(1):1-15.
- [Google Scholar]
- AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol.. 2009;149:894-904.
- [Google Scholar]
- The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim Biophys Acta. 2000;1465:1-16.
- [Google Scholar]
- In silico characterization and expression analysis of selected Arabidopsis receptor-like kinase genes responsive to different MAMP inducers. Biol. Plantarum. 2015;59(1):18-28.
- [Google Scholar]
- Omasits, U., Ahrens, C. H., Müller, S., & Wollscheid, B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 30, 884-886 (2014).
- Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817-845.
- [Google Scholar]
- A tetrahedral coordination of zinc during transmembrane transport by P-type Zn2+-ATPases. Biochim. Biophys. Acta. 2012;1818:1374-1377.
- [Google Scholar]
- TSSPlant: a new tool for prediction of plant Pol II promoters. Nucleic. Acids. Res.. 2017;45:e65
- [Google Scholar]
- ROSITE, a protein domain database for functional characterization and annotation. Nucleic. Acids. Res.. 2010;38:D161-6.
- [Google Scholar]
- Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol.. 2006;7:1-10.
- [Google Scholar]
- Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids. Res.. 1997;25:3389-3402.
- [Google Scholar]
- STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucl. Acids. Res.. 2019;47:D607-613.
- [Google Scholar]
- An in silico approach for structural and functional analysis of heavy metal associated (HMA) proteins in Brassica oleracea. Period. Eng. Nat. Sci. (PEN). 2016;4(2):41-59.
- [Google Scholar]
- MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol.. 2013;30(12):2725-2729.
- [Google Scholar]
- Why and how to investigate the role of protein phosphorylation in ZIP and ZnT zinc transporter activity and regulation. Cell Mol. Life Sci.. 2020;77(16):3085-3102.
- [Google Scholar]
- MEME SUITE: tools for motif discovery and searching. Nucleic. Nucleic. Acids. Res.. 2009;37:202-208.
- [Google Scholar]
- Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol. Plant. 2012;34(1):279-289.
- [Google Scholar]
- Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA (38):16500-16505.
- [Google Scholar]
- P1B-ATPases—an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491-502.
- [Google Scholar]
- Conserved functional motifs and homology modeling to predict hidden moonlighting functional sites. Front. Bioeng. Biotech.. 2015;3:82.
- [Google Scholar]
- bHLH104 confers tolerance to cadmium stress in Arabidopsis thaliana. J. Integr. Plant. Biol.. 2018;60(8):691-702.
- [Google Scholar]
- RRP42, a subunit of exosome, plays an important role in female gametophytes development and mesophyll cell morphogenesis in Arabidopsis. Front. Plant Sci.. 2017;2017(8):981.
- [Google Scholar]
- Enhanced expression of SaHMA3 plays critical roles in Cd hyperaccumulation and hypertolerance in Cd hyperaccumulator Sedum alfredii Hance. Planta. 2016;243(3):577-589.
- [Google Scholar]
- Variation in the BrHMA3 coding region controls natural variation in cadmium accumulation in Brassica rapa vegetables. J. Exp. Bot.. 2019;70(20):5865-5878.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101730.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







