Translate this page into:
GC-HRTOF-MS metabolite profiling and antioxidant activity of methanolic extracts of Tulbaghia violacea Harv
⁎Corresponding authors. makhuvele.rhulani70@gmail.com (Rhulani Makhuvele), pnjobeh@uj.ac.za (Patrick Berka Njobeh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tulbaghia violacea is a bulbous herb that is used extensively in traditional medicine to alleviate various illnesses. The current study aimed to evaluate the total phenolic and flavonoid contents of methanolic extracts of the bulb, leaves, rhizome, and stem of the plant, T. violacea, and to compare the phytochemical profile of these extracts utilizing gas chromatography/high-resolution time-of-flight mass spectrometry (GC-HRTOF-MS). The antioxidant potential of the plant extracts was also determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS). All methanolic T. violacea extracts were rich in terpenoids, flavonoids, and saponins; however, only leaf extract contained tannins. Cardiac glycosides and anthraquinones were not observed in any of the tested extracts. All plant extracts showed weak antioxidant capabilities in both DPPH and ABTS assays with IC50 values from 146.4 ± 1.11 to 303.15 ± 1.98 µg/mL and 136.25 ± 0.03 to 246.09 ± 0.01 µg/mL, respectively. Methanolic bulb extract of T. violacea contained significant phenolic content (22.85 ± 3.15 mg GAE/g), followed by rhizomes (17.46 ± 1.75 mg GAE/g), whereas the leaf (9.47 ± 0.64 mg GAE/g) and stem (5.83 ± 0.77 mg GAE/g) extracts had the least phenolic contents. Similarly, the bulb extracts possessed significant flavonoid content (37.59 ± 1.27 QE/g), followed by rhizome (26.40 ± 0.21 QE/g) and leaves (22.67 ± 1.26 QE/g), and then stems with flavonoid content of 8.65 ± 2.11QE/g. These results were significantly different at P < 0.05. The GC-HRTOF-MS revealed that stem extract is rich in sulphur-containing compounds (51.2 %), followed by fatty acid amides (23.64 %), esters (10.50 %) and flavonoids (10.51 %). The rhizome extract showed the presence of sulphur-containing compounds (40.11 %), fatty acid amides (37.24 %), fatty acid esters (14.33 %), phenol (3.70 %), butyl alcohol (3.04 %) and sterols (0.77 %), while the bulb extract possessed a high quantity of sulphur compounds (93.34 %) with a lesser amount of fatty acid amide (0.29 %), fatty acid esters (2.91 %), flavonoids (0.81 %) and miscellaneous compounds (1.35 %). Additionally, the leaf extract also possessed sulphur compounds (48.37 %), fatty acid esters (15.77 %) and fatty acid amides (21.97 %), vitamins (9 %), terpenoids (1.37 %), sterols (1.63 %) and phenols (0.19 %). The findings from this study indicate that bulb extract of T. violacea holds potential pharmacological properties, which can induce detoxifying enzymes and can protect against reactive oxygen species due to its high number of sulphur-containing compounds.
Keywords
Tulbaghia violacea
Phytochemicals
Antioxidant activity
Phenolic content
GC-HRTOF-MS
- GC-HRTOF-MS
-
gas chromatography/high-resolution time-of-flight mass spectrometry
Abbreviations
1 Introduction
Plants present excellent alternatives for the discovery of drugs, nutraceuticals, and feed additives due to the broad spectrum of protective and preventative properties they possess. They contain several other secondary metabolites, which are known for their disease-fighting capabilities when consumed regularly. These phytochemicals include phenolic compounds, alkaloids, terpenes, and essential oils (Awuchi and Twinomuhwezi, 2021). Herbs provide not only essential nutrients to the body, but also phytochemicals that help in the prevention and reduction of chronic diseases, thus promoting general wellbeing (Thakur et al., 2020). Such uses are currently regaining interest around the world, in that many individuals are turning to these products for the treatment of several health ailments (Restani, 2018). Furthermore, medicinal plants have low toxicity and they are good sources of pharmaceutical compounds that act against various diseases (Nyakudya et al., 2020). Generally, the food and allied industries are limiting the use of chemicals, synthetic food and feed additives due to their negative impact on human and animal health, and are opting for natural plant-based additives and preservative agents that can withstand other climatic conditions such as drought (Dikhoba et al., 2019; Lasram et al., 2019).
Tulbaghia violacea Harv. is a bulbous drought-resistant plant in the family Alliaceae and is widely known as wild garlic, society garlic, or sweet garlic (Madike et al., 2019). The leaves and bulbs of T. violacea have been employed in herbal medicine to alleviate various diseases, which include tuberculosis, fever, cough, asthma, hypertension, sinus, headache, oesophageal cancer, rheumatism and gastrointestinal disorders (Moodley et al., 2013; Saibu et al., 2015; Madike et al., 2017). Research has demonstrated that T. violacea possess antifungal, antibacterial, antioxidant and anti-inflammatory properties (Aremu and Van Staden, 2013; Takaidza et al., 2015; Krstin et al., 2018; Ncise et al., 2021). Mcgaw et al. (2000) revealed that T. violacea water and ethanol extracts possess anthelminthic properties. Furthermore, the T. violacea leaf extract also showed anticancer effect against several cancer cell lines tested by inducing apoptosis (Motadi et al., 2020). Several sulphur-containing compounds were isolated and identified from T. violacea and they include thiosulfinate marasmicin (2,4,5,7-tetrathiaoctan-4-oxide), (R(S)R(C))– S-(methylthiomethyl) cysteine-4-oxide and Methyl alpha-d-glucopyranoside. Thiosulfinate marasmicin has been reported to possess antimicrobial activities, while methyl alpha-d-glucopyranoside has shown anticarcinogenic activity (Ncise et al., 2021). The current research aimed to compare the secondary metabolites of the methanolic bulb, rhizome, stem, and leaf extracts of T. violacea, using GC-HRTOF-MS profiling, and explore their antioxidant properties.
2 Materials and methods
2.1 Chemicals and reagents
Ascorbic acid, acetic acid, chloroform, methanol, 1,1-diphenyl-2-picrylhydrazyl salt (DPPH), 2,2′-azinobis 3-ethylbenzthiazoline-6-sulphonic acid (ABTS), sodium bicarbonate (Na2CO3), potassium persulphate (K2S2O8), sodium nitrite (NaNO2), quercetin, and Trolox were procured from Sigma Aldrich, Germany. Chloroform, sulfuric acid (H2SO4), ferric chloride (FeCl3), ammonium hydroxide (NH₄OH), and sodium hydroxide (NaOH) were bought from Merck, Germany. Folin-Ciocalteu, aluminium chloride (AlCl3) and gallic acid were purchased from Protea Lab, Johannesburg, South Africa.
2.2 Plant collection and processing
Different plant part materials of Tulbaghia violacea were gathered from the Walter Sisulu Botanical Garden in Roodepoort (26°05′33.5″S 27°50′33.6″E), South Africa, in October 2021. The identity of the plant was authenticated by the Nursery Manager, Mr Solomon Nenungwi, of the South African National Biodiversity Institute, Roodepoort in Johannesburg. The voucher specimen (RM01) was filed in the University of Johannesburg herbarium (JRAU). The plant materials were gently cleansed with tap water to eliminate excess dust and soil and then rinsed with distilled water. The leaves, stems, rhizomes, and root bulbs were separated and then dried at room temperature for about 21 days. Thereafter, each plant material was milled to a powder and stored in glass containers in the dark at room temperature until use.
2.3 Sample extraction and preparation
An amount of 10 g of each powder plant material (leaves, stems, rhizomes, and root bulbs) of T. violacea was extracted to exhaustion using 80 % methanol by maceration at room temperature for 48 h. The crude extracts were then filtered through Whatman No. 1 filter paper. Methanol was concentrated using a rotary evaporator (Buchi, Germany) and the extract was frozen at −80 °C before freeze-drying in a Telstar Lyoquest freeze drier (Labotec, RSA) for 48 to 96 h, depending on the amount of the extract obtained. Freeze-dried extracts were weighed and stored in a tight glass container away from light at 4 °C until required. The percentage extract yield of the extracts was expressed by dividing the total mass extracted after freeze-drying by the mass of the dried plant sample used for extraction. Stock solutions of 10 mg/mL extract were prepared and dissolved in 80 % methanol for each extract.
2.4 Qualitative phytochemical analysis
All plant extracts were screened for detection of the bioactive constituents, including terpenoids, flavonoids, tannins, saponins, and quinones, executed as described by María et al. (2018), while glycosides, phenols and cardiac glycoside were carried out as per Roghini and Vijayalakshmi (2018), following standard methods.
2.5 Antioxidant activity
2.5.1 DPPH free radical-scavenging assay
DPPH assay was conducted to determine the antioxidant activities of T. violacea extracts by the method described by (Phuyal et al., 2020). Ascorbic acid and Trolox were used as the reference standard for antioxidant. The DPPH scavenging activity of the extracts was calculated by using the equation below, and the inhibitory concentration at a 50 % decrease of free radicals (IC50) value was determined. The results were expressed as mean IC50 values.
Percentage DPPH scavenged (%) = ((Absorbance control–Absorbance sample)/ Absorbance control) × 100.
2.5.2 The ABTS radical scavenging assay
The assay was conducted as outlined by Dikhoba et al. (2019). Ascorbic acid and Trolox were used as reference antioxidant standards. The ABTS scavenging activity of the extracts were calculated using the equation below and the IC50 value was determined. The results were expressed as mean IC50 values.
Percentage ABTS scavenged (%) = ((Absorbance control–Absorbance sample)/ Absorbance control) × 100.
2.6 Determination of the total phenolic content
Total phenolic contents of T. violacea extracts were determined using the Folin–Ciocalteu (FC) test as described by Sankhalkar and Vernekar (2016). The total phenolic content (mg/mL) was calculated using gallic acid as standard and was expressed as mg equivalents of GA per g of dry matter (mg GAE/g).
2.7 Determination of total flavonoid content
The total flavonoid content of stems, rhizomes, bulbs, and leaves extract of T. violacea was determined using the aluminium chloride method according to Sankhalkar and Vernekar (2016). Total flavonoid content was determined from the calibration curve of quercetin using a linear equation and expressed as mg quercetin equivalent per gram of dry weight (mg QE/g).
2.8 Phytochemical profiling of methanolic extracts by GC-HRTOF-MS
The analysis of secondary metabolites of extracts of T. violacea was performed as outlined by Adebiyi et al. (2019) using the Pegasus GC-HRTOF-MS. The compounds were identified using LECO ChromaTOF® software.
2.9 Statistical analysis
Data obtained from this study were examined using the IBM SPSS statistics 27. The experiment was repeated thrice, and the results were presented as mean ± standard deviation (SD). Analyses of variance were performed using one-way ANOVA to determine the significant differences between the mean values (P < 0.05).
3 Results
3.1 Phytochemical analysis
The phytochemical screening of methanolic plant extract of stem, rhizome, bulb and leaves of T. violacea showed the existence of different secondary metabolites (Table 1). Flavonoids, terpenoids and saponins were the most plentiful secondary metabolites in all the extracts. Phenolics and tannin were only observed in the leaf extract, while absent in all other extracts as evident after adding ferric chloride, which did not change the colour to black or green. Cardiac glycosides and anthraquinones were not found in any of the various extracts. Furthermore, these different plant parts of T. violacea varied in their percentage yield extracts, which ranged from 14.88 % to 60.09 % (w/w). + = positive test; - = negative test.
Phytochemical tests
Stem
Rhizome
Bulbs
Leaves
Flavonoids
Basic
+
+
+
+
Acid
+
+
+
+
Terpenoids
Salkowski test
+
+
+
+
Lieberman Bouchard test
+
+
+
+
Tannins
–
–
–
+
Phenols
–
–
–
–
Saponins
+
+
+
+
Cardiac glycoside
–
–
–
–
Quinones
+
+
+
–
Anthraquinones
–
–
–
–
Extraction yields (% w/w)
60.09
14.88
21.95
53.35
3.2 Antioxidant activity
The results for DPPH and ABTS free radical scavenging effects of the methanolic extracts of T. violacea are presented in Table 2. The plant extracts and reference standards (ascorbic acid and Trolox) revealed different antioxidant activities. Ascorbic acid and Trolox showed significant higher antioxidant potency based on both the DPPH and ABTS scavenging assay with the IC50 value of 15.83 ± 0.08 and 41.732 ± 0.49 µg/mL at P < 0.05, respectively. All tested plant extracts of T. violacea showed significant poor DPPH and ABTS radical scavenging activities in comparison with ascorbic acid and Trolox (P < 0.05). Their IC50 value ranged from 146.4 ± 1.11 to 303.15 ± 1.98 µg/mL in the DPPH assay and 136.25 ± 0.03 to 246.09 ± 0.01 µg/mL in the ABTS assay. Values are expressed in means ± standard deviation (n = 3). The results show statistical significance (*) at P < 0.05.
Sample
DPPH IC50 (µg/mL)
ABTS IC50 (µg/mL)
Total phenolic content (mg gallic acid equivalent/g)
Total flavonoid content (mg quercetin acid equivalent/g)
Stem
290.57 ± 2.91*
221.96 ± 0.90*
5.83 ± 0.77*
8.65 ± 2.11*
Rhizome
157.16 ± 1.76*
136.25 ± 0.03*
17.46 ± 1.75*
26.40 ± 0.21*
Bulbs
146.4 ± 1.11*
246.09 ± 0.01*
22.85 ± 3.15*
37.59 ± 1.27*
Leaves
303.15 ± 1.98*
153.86 ± 0.2*
9.47 ± 0.64*
22.67 ± 1.26*
Trolox
41.732 ± 0.49*
38.67 ± 0.06*
–
–
Ascorbic acid
15.83 ± 0.08*
52.83 ± 0.08*
–
–
3.3 Total phenolic content
The total phenolic contents of stem, rhizome, bulb and leaves of T. violacea plant extract were determined from the calibration curve of gallic acid with the linear regression equation (y = 10.742x + 0.0337; R2 = 0.9565). The results in Table 2 indicated that methanolic bulbous extract of T. violacea possessed significant higher phenolic content (P < 0.05) followed by the rhizomes (17.46 ± 1.75 mg GAE/g), while the leaf (9.47 ± 0.64 mg GAE/g) and stem (5.83 ± 0.77 mg GAE/g) extracts had less phenolic contents.
3.4 Total flavonoid content
The results for the total flavonoid content of methanolic T. violacea extracts are listed in Table 2. The flavonoid content of T. violacea extracts was established using linear equation (y = 0.4469 + 0.0048) R2 = 0.9989, obtained from calibration curve of quercetin. The bulb extracts possessed significant high flavonoid content (37.59 ± 1.27 QE/g), followed by rhizome (26.40 ± 0.21 QE/g) and leaves (22.67 ± 1.26 QE/g), and lastly, stems with flavonoid content of 8.65 ± 2.11 QE/g. These extracts showed significant differences in their flavonoid content (P < 0.05).
3.5 Phytochemical profiling of T. violacea extracts using GC-HRTOF-MS
A total of 23 different bioactive compounds were reported from the stem, 16 from the rhizome, 17 from the bulb and 20 from the leaves of T. violacea methanolic extracts using GC-HRTOF-MS. Table 3 provides the respective identified chemical composition, m/z, molecular formula, retention time, and percentage composition. All extracts of T. violacea mostly contained a complex mixture of sulphur-containing compounds, fatty acid esters, fatty acid amide, terpenoids, sterol, flavonoids, and phenols. The major compounds observed in stem extract include disulfide, methyl (methylthio), methyl (7 %), 4 h-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl (10,51 %) methane, (methylsulfinyl)(methylthio)- (13,92 %), isomer 2: 2,3,5,7-tetrathiaoctane 3,3-dioxide (14,54 %), 2,3,5,7-tetrathiaoctane 3,3-dioxide (8,93 %), and isomer2: 9-octadecenamide, (z)- (19,34 %). For rhizome extract, the major compounds were methane (methylsulfinyl)(methylthio)-(10,67 %), 9,12-octadecadienoic acid, methyl ester, (e,e)- (8,86 %), 9-octadecenamide, (z)- (33,41 %) and 2,3,5,7-tetrathiaoctane 3,3-dioxide (25,47 %). The bulb extract revealed the presence of methylthio (methylthio-methyl) sulfone (14,02 %), 2,4,5,7-tetrathiaoctane (34,19 %), disulfide, methyl (methylthio) methyl (8,69 %) and hexadecanoic acid, methyl ester (6,55 %). Furthermore, leave extract contained methane, (methylsulfinyl)(methylthio)- (41,04 %), hexadecanoic acid, methyl ester (9,35 %), dl-a-tocopherol (9 %), 2,3,5,7-tetrathiaoctane 3,3-dioxide (35,47 %), 9-octadecenamide, (z)- (17,04 %) and disulfide, methyl (methylthio) methyl (5,63 %). The chromatographic profiles and relative abundances of the Plant extracts are presented in figure below. Methanolic stem extract of T. violacea contained 23 compounds (Fig. 1 and Fig. 5), of which 51.2 % of the compounds present belonged to the sulphur-containing compound group followed by fatty acid amides (23.64 %) and esters (10.50 %), flavonoids (10.51 %), terpenoids (1.79 %) and sterols (1.06 %). The rhizome extract showed the presence of 16 compounds on GC–MS metabolic profiling (Fig. 2 and Fig. 6). The sulphur-containing compounds (40.11 %) were the major compounds present followed by fatty acid amides (37.24 %), fatty acid esters (14.33 %), phenol (3.70 %), butyl alcohol (3.04 %) and sterols (0.77 %). The sulphur compounds (93.34 %) were also observed as the major compounds present in the bulb methanolic extracts of T. violacea (Fig. 3 and Fig. 7) with 17 compounds identified. Other compounds, including fatty acid amide (0.29 %), fatty acid esters (2.91 %), flavonoids (0.81 %) and miscellaneous compounds 91.35 %), were observed but in relatively low quantities. Furthermore, the methanolic leaf extract of T. violacea contained a total of 20 compounds (Fig. 4 and Fig. 8) with sulphur-containing compounds (48.37 %) being the most abundant constituents. Additionally, the leaf extract also possessed fatty acid esters (15.77 %) and fatty acid amides (21.97 %), vitamins (9 %), terpenoids (1.37 %), sterols (1.63 %) and phenols (0.19 %). Other miscellaneous volatile compounds, such as 3,5,5-trimethylhexyl s-2-(dimethylamino) ethyl propylphosphonothiolate, phosphine, tris (trifluoromethyl)-, 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy) tetrasiloxane and tetradonium bromide were also reported in the present study. The bold colour indicates the most abundant compound in the sample. Nd means the compound is not determined.
Retention time (min)
Compound Name
Nature of compound
Observed Ion m/z
Molecular formula
Peak Area (%)
Stem
Rhizome
Bulb
Leaves
1
3,81
Dimethyl trisulfide
Sulphur compound
126
C2H6S3
3.70
nd
nd
1.26
2
4,44
1-Butanamine, 2-methyl-N-(2-methylbutylidene)-
Butyl alcohol
155
C10H21N
nd
1.25
1.00
nd
3
4,56
1-Butanamine, 3-methyl-N-(3-methylbutylidene)-
Butyl alcohol
154
C10H21N
nd
1.79
nd
nd
4
5,64
3,5,5-Trimethylhexyl S-2-(dimethylamino)ethyl propylphosphonothiolate
Miscellaneous compounds
264
C16H36NO2PS
nd
nd
1.18
nd
5
5,73
Disulfide, methyl (methylthio)methyl
Sulphur compound
140
C3H8S3
7.00
3.97
8.69
5.63
6
6,02
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-
Flavonoid
144
C6H8O4
10.51
nd
0.81
nd
7
7,32
Benzene, 1,3-bis(1,1-dimethylethyl)-
Benzene, phenylpropanes
190
C14H22
0.14
nd
nd
0.17
8
8,39
2-Methoxy-4-vinylphenol
Phenol
150
C9H10O2
nd
3.57
nd
nd
9
8,88
Methylthio(methylthio-methyl) sulfone,
Sulphur compound
172
C3H8O2S3
nd
nd
14.02
nd
10
9,65
Phosphine, tris(trifluoromethyl)-
Miscellaneous compounds
219
C3F9P
nd
0.03
0.01
0.04
11
10,88
3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane
Miscellaneous compounds
503
C18H52O7Si7
nd
nd
0.16
nd
12
11,29
Phenol, 2,5-bis(1,1-dimethylethyl)-
Phenol
206
C14H22O
0.17
nd
nd
0.19
13
11,31
Phenol, 2,6-bis(1,1-dimethylethyl)-
Phenol
206
C14H22O
nd
0.13
0.04
nd
14
11,46
2,4,5,7-Tetrathiaoctane
Sulphur compound
186
C4H10S4
nd
nd
34.19
nd
15
11,47
Methane, (methylsulfinyl)(methylthio)-
Sulphur compound
125
C3H8OS2
13.92
10.67
0.97
41.04
16
14,22
Tetradonium Bromide
Miscellaneous compounds
269
C17H38BrN
nd
nd
nd
0.93
17
14,23
Glycine, N, N-dimethyl-, ethyl ester
Amine
131
C6H13NO2
nd
0.51
nd
nd
18
15,20
Isomer2: 2,3,5,7-Tetrathiaoctane 3,3-dioxide
Sulphur compound
218
C4H10O2S4
14.54
nd
nd
nd
19
16,87
Hexadecanoic acid, methyl ester
Fatty acid methyl esters
270
C17H34O2
6.55
nd
1.86
9.35
20
16,89
2,4,5,6,8-Pentathianonane
Sulphur compound
217
C4H10S5
2.11
nd
nd
nd
21
17,35
Dibutyl phthalate
Plasticizer
225
C16H22O4
nd
nd
0.10
nd
22
17,52
Nonanamide
Amide
156
C9H19NO
0.98
nd
nd
nd
23
17,56
Pentadecanoic acid, 14-methyl-, methyl ester
Fatty acid methyl esters
269
C17H34O2
nd
4.85
nd
nd
24
17,59
Dodecanoic acid, ethyl ester
Fatty acid ethyl esters
227
C14H28O2
0.27
nd
nd
0.15
25
17,93
Undecanoic acid, methyl ester
Fatty acid methyl esters
199
C12H24O2
nd
nd
0.06
nd
26
17,99
Neophytadiene
Diterpene
278
C20H38
nd
nd
nd
1.37
27
18,59
Tridecanoic acid, methyl ester
Fatty acid methyl esters
227
C14H28O2
nd
0.62
nd
nd
28
18,62
9,12-Octadecadienoic acid, methyl ester
Fatty acid methyl esters
294
C19H34O2
nd
nd
0.99
nd
29
18,62
9,12-Octadecadienoic acid, methyl ester, (E, E)-
Fatty acid methyl esters
294
C19H34O2
nd
8.86
nd
1.51
30
18,62
Isomer1: 9,12-Octadecadienoic acid, methyl ester, (E, E)-
Fatty acid methyl esters
294
C19H34O2
2.65
nd
nd
nd
31
18,68
9-Octadecenoic acid (Z)-, methyl ester
Fatty acid methyl esters
275
C19H36O2
0.58
nd
nd
nd
32
18,69
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-
Fatty acid methyl esters
292
C19H32O2
nd
nd
nd
3.88
33
18,87
1-Decanamine, N-decyl-N-methyl-
Alicyclic compound
269
C21H45N
nd
0.26
nd
nd
34
18,87
Ethylamine, N-decyl-N-methyl-2-(2-thiophenyl)-
Amine
257
C17H31NS
nd
nd
nd
0.56
35
18,91
Tridecanoic acid, 12-methyl-, methyl ester
Fatty acid methyl esters
241
C15H30O2
nd
nd
nd
0.88
36
19,25
Isomer2: 9,12-Octadecadienoic acid, methyl ester, (E, E)-
Fatty acid methyl esters
271
C19H34O2
0.45
nd
nd
nd
37
19,35
Isomer1: 9-Octadecenamide, (Z)-
Fatty acid amide
269
C18H35NO
1.30
nd
nd
nd
38
19,52
Hexadecanamide
Fatty acid amide
255
C16H33NO
3.00
nd
nd
nd
39
19,53
Dodecanamide
Fatty acid amide
198
C12H25NO
nd
3.83
nd
4.93
40
19,60
Isomer1: 2,3,5,7-Tetrathiaoctane 3,3-dioxide
Sulphur compound
219
C4H10O2S4
1.00
nd
nd
nd
41
19,61
2,3,5,7-Tetrathiaoctane 3,3-dioxide
Sulphur compound
219
C4H10O2S4
8.93
25.47
35.47
0.44
42
21,10
Isomer2: 9-Octadecenamide, (Z)-
Fatty acid amide
281
C18H35NO
19.34
nd
nd
nd
43
21,12
9-Octadecenamide, (Z)-
Fatty acid amide
225
C18H35NO
nd
33.41
0.29
17.04
44
21,85
Bis(2-(Dimethylamino)ethyl) ether
Amine
156
C8H20N2O
nd
nd
nd
nd
45
24,60
2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-
Sesquiterpenoids
223
C15H26O
1.16
nd
nd
nd
46
26,78
dl-a-Tocopherol
Vitamins
430
C29H50O2
nd
nd
nd
9.00
47
27,52
Ergost-5-en-3-ol, acetate, (3β,24R) -
Sterols
402
C30H50O2
nd
nd
nd
0.46
48
28,10
β-Sitosterol
Sterols
414
C29H50O
0.52
0.77
0.16
1.17
49
28,23
cis-3,14-Clerodadien-13-ol
Sterols
290
C20H34O
0.54
nd
nd
nd
50
28,70
a-Amyrin
Terpenoids
427
C30H50O
0.63
nd
nd
nd
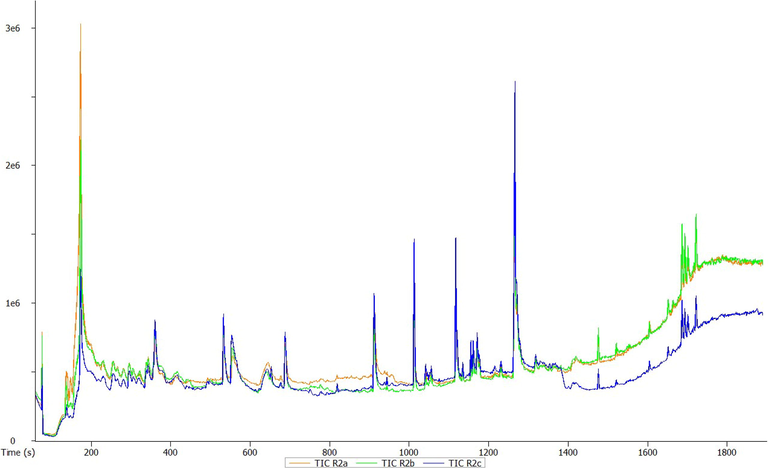
GC-HRTOF-MS chromatographic profile of methanolic stem extract of T. violacea.
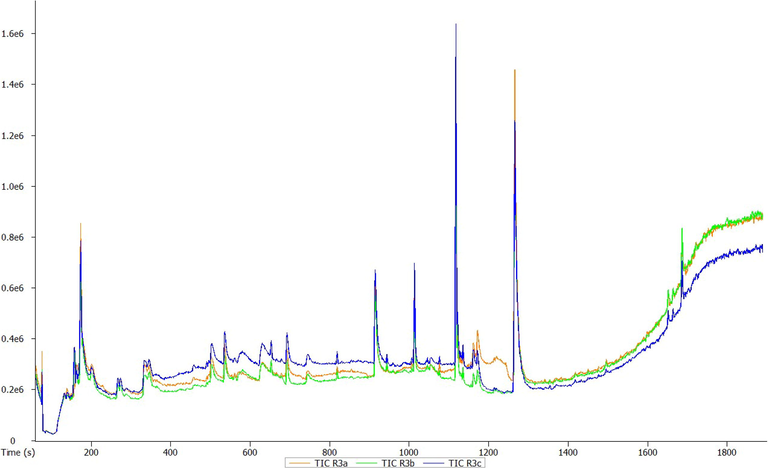
GC-HRTOF-MS chromatographic profile of methanolic rhizome extract of T. violacea.
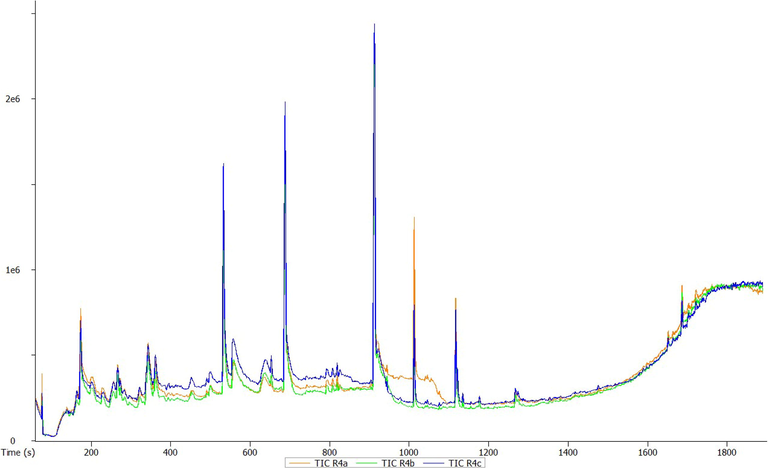
GC-HRTOF-MS chromatographic profile of methanolic bulb extract of T. violacea.
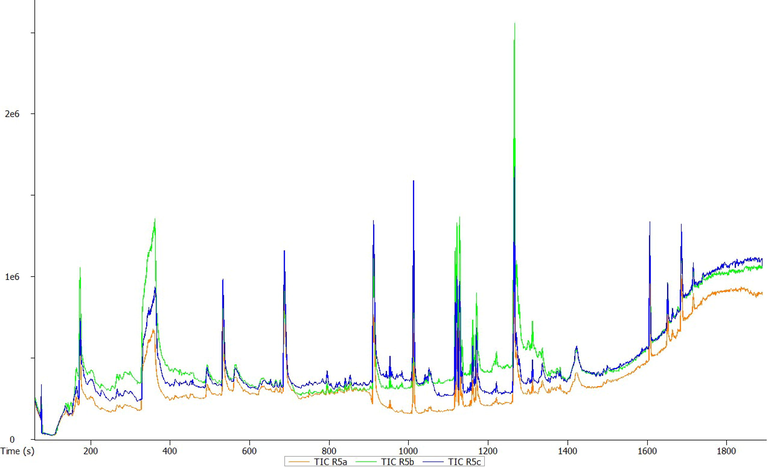
GC-HRTOF-MS chromatographic profile of methanolic leaves extract of T. violacea.
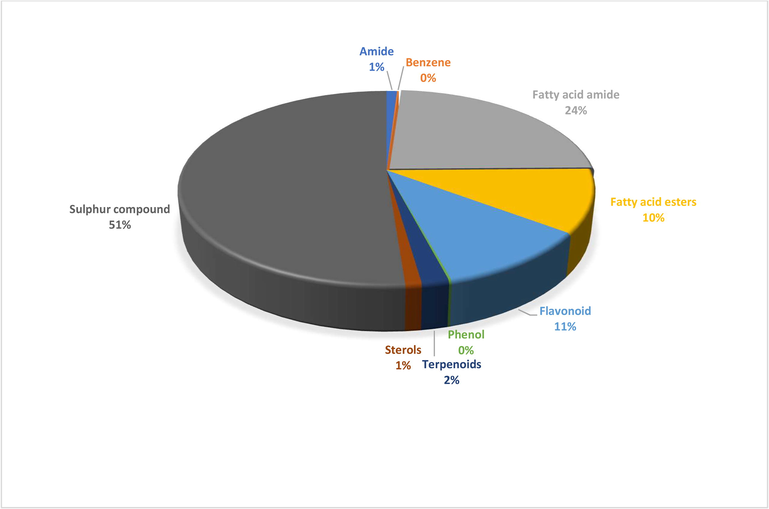
Distribution of secondary metabolites in stem extract of T. violacea.
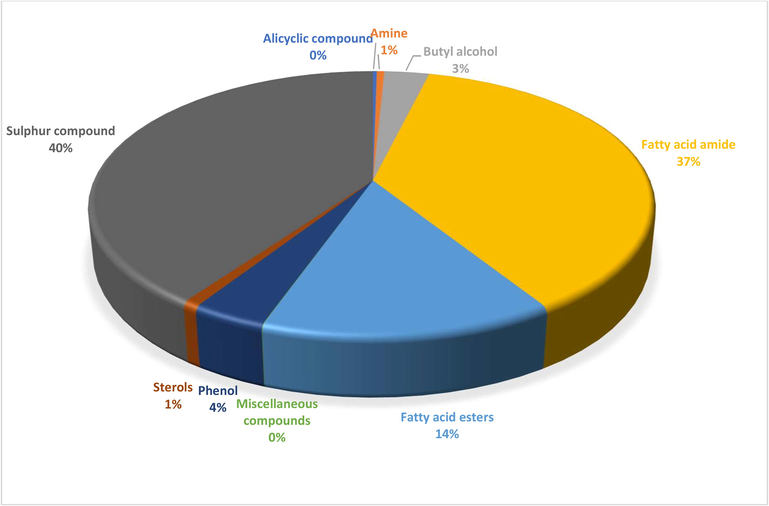
Distribution of secondary metabolites in rhizome extract of T. violacea.
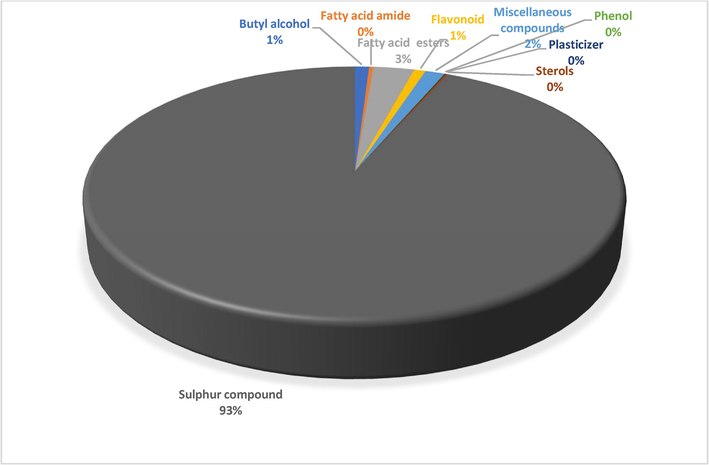
Distribution of secondary metabolites in bulb extract of T. violacea.
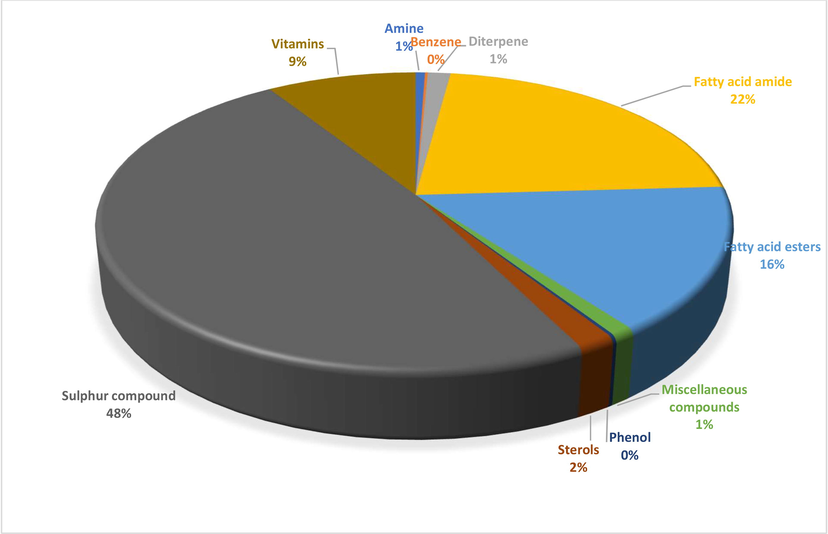
Distribution of secondary metabolites in leaves extract of T. violacea.
4 Discussion
Various bioactive compounds of plant origin are well known for their biological actions that contribute to human and animal health. Phenolics, flavonoids, terpenoids, alkaloids, steroids, saponins, and essential oils play a significant role in combating many diseases. The current study compared the phytochemical profile, antioxidant, flavonoid and phenolic content of five different T. violacea extracts. The study found that methanolic stem, rhizome, bulb and leaf extracts of T. violacea are rich in flavonoids, terpenoids and saponins, whereas tannins were only observed in the leaf extract. These discoveries are in line with research by Madike et al. (2017). Tannins are water-soluble phenol with an astringent taste and have displayed important pharmacological and nutraceutical roles. They have antioxidant, antimicrobial, cardioprotective, antidiabetic, antimutagenic as well as antinutrive properties (Sieniawska and Baj, 2017). Cardiac glycosides and anthraquinones were not found in all the extracts and these outcomes are in line with what was stated earlier by (O et al., 2013), but contrary to the findings reported by Ncube et al. (2011) and Madike et al. (2017), who reported the presence of cardiac glycosides in different parts of T. violacea. Geographical position and environmental conditions, such as temperature, soil composition, drought, rainfall as well as radiation, have significant effects on the biosynthesis or bioactivity of these compounds in the plant. They affect plant morphology and physiology as well as gene expression. The expression of genes responsible for the biosynthesis of secondary metabolites is regulated by different stress levels (Li et al., 2020). Similar plant species may contain different compound contents and quantities depending on their geographic location (Ghasemzadeh et al., 2018).
Plants that exhibit high antioxidant activities play a significant role in food preservation and human health due to their capacity to scavenge free radicals in a cell. High IC50 values mean less antioxidant activities, whereas low IC50 values indicate higher antioxidant activities (Dikhoba et al., 2019). Our results show that all the extracts of T. violacea had weak antioxidant effects in DPPH and ABTS assays since they had IC50 values of more than 100 µg/mL. These findings correspond with the work done by (O et al., 2013), in which the authors observed poor ABTS and DPPH scavenging activity. The antioxidant effect of the plant extracts also corresponds to their phenolic content. In this study, we observed that phenolics were not detected using qualitative phytochemical screening and total phenolic contents were considered low. The absence or low total phenolic contents noted in this study could explain the weak antioxidant effects of T. violacea extracts established when conducting both ABTS and DPPH radical scavenging assays. However, the bulb extract showed to have better phenolic and flavonoid contents when compared with those of the leaf and stem extracts. The phenolic contents of methanolic stem and leaf extract of T. violacea were low in this study and it is similar to the results reported earlier by Madike et al. (2017). This might likewise be a result of the environmental conditions in which the plant grew.
The GC-HORTF-MS results revealed the occurrence of many sulphur compounds in all extracts of T. violacea and only a few of these compounds have been earlier reported in the literature (Pino et al., 2008; Olorunnisola, 2012; Eid, 2015; Staffa et al., 2020). Eid (2015) reported the presence of 2,4,5,6,8-pentathiononane, hexadecenoic acid methyl ester and 9-octadecenamide in T. violacea extracts. These sulphur compounds identified in the methanolic extracts of T. violacea can be considered as degradation by-products of marasmicin produced via enzymatic cleavage (Eid, 2015). Furthermore, dimethyl trisulfide was also reported in T. violacea species by Pino et al. (2008), Olorunnisola (2012), Eid (2015) and Staffa et al. (2020). The medicinal properties of T. violacea demonstrated herein are attributed to the abundance of sulphur-containing compounds and fatty acids identified using GC-HORFT-MS. During oxidative stress, which is a condition whereby the amount of free radical generated and destroyed is not balanced, the antioxidant compounds can regulate the process by scavenging the free radical. The sulphur-containing compounds are known for their ability to induce phase II detoxification enzymes and protect against free radicals that are linked to carcinogenicity, mutagenicity, cardiovascular, and metabolic diseases (Cerella et al., 2014; Miękus et al., 2020). Fatty acid esters, hexadecanoic acid, and methyl esters were reported to have antioxidant, hypocholesterolemic, nematicide and anticancer activity (Kim et al., 2020; Siswadi & Saragih, 2021). 9,12-octadecadienoic acid, methyl ester is reported to have anti-inflammatory activity, while 9–octadecenamide showed antioxidant activity. Tocopherol, commonly known as vitamin E, has been reported for its antioxidant activity (Siswadi and Saragih, 2021).
5 Conclusion
Different secondary metabolites, including terpenoids, flavonoids, saponin, and tannin, were observed in solvent extracts of the medicinal plant, T. violacea, in this study. The results of phytochemical screening showed that methanolic bulb extract of T. violacea contains significant total phenolic and flavonoid contents when compared to those of rhizome, leaf and stem extracts. The plant extracts of T. violacea were revealed to have weak antioxidant activities in this study. Different compounds were profiled from the bulb (17), rhizome (16), leaf (20) and stem (23) extracts of T. violacea plant materials by GC-HRTOF-MS with the bulb extract being a good source of phenolic, flavonoids and sulphur-containing compounds, which may be useful in protection against oxidative damage resulting from free radicals. Nonetheless, future studies should focus on the antioxidant activity of individual bioactive compounds, such as sulphur-containing compounds and the fatty acids that could be isolated, purified and characterised. Other biological studies, such as anticancer, antigenotoxicity and detoxification abilities of extracts from the plant, could also be explored.
Funding
The work was supported by the National Research Foundation South Africa (DSI/NRF innovation Postdoctoral scholarship, PDG190211415413) and the University of Johannesburg Global Excellence and Stature Fellowship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Assessment of nutritional and phytochemical quality of Dawadawa (an African fermented condiment) produced from Bambara groundnut (Vigna subterranea) Microchem. J.. 2019;149:104034
- [CrossRef] [Google Scholar]
- The genus Tulbaghia (Alliaceae) - A review of its ethnobotany, pharmacology, phytochemistry and conservation needs. J. Ethnopharmacol.. 2013;149:387-400.
- [CrossRef] [Google Scholar]
- Awuchi, C.G., Twinomuhwezi, H., 2021. the Medical , Pharmaceutical , and Nutritional Biochemistry and Uses of Some Common Medicinal.
- Cerella, C., Kelkel, M., Viry, E., Dicato, M., Jacob, C., Diederich, M., 2014. Naturally Occurring Organic Sulfur Compounds : An Example of Naturally Occurring Organic Sulfur Compounds : An Example of a Multitasking Class of Phytochemicals in Anti-Cancer Research 2–42. https://doi.org/10.5772/26003.
- Antifungal and anti-mycotoxigenic activity of selected South African medicinal plants species. Heliyon. 2019;5(10)
- [CrossRef] [Google Scholar]
- The influence of extraction methods on the composition and antimicrobial activity of the volatile constituents of Tulbaghia violacea Harv., Cultivated in Egypt. J. Pharmacogn. Phytochem.. 2015;4:118-125.
- [Google Scholar]
- Assessment and comparison of phytochemical constituents and biological activities of bitter bean (Parkia speciosa Hassk.) collected from different locations in Malaysia. Chem. Cent. J.. 2018;12:1-9.
- [CrossRef] [Google Scholar]
- Composition and antioxidant activities of volatile organic compounds in radiation-bred coreopsis cultivars. Plants. 2020;9(6):717.
- [Google Scholar]
- Tulbaghia violacea and Allium ursinum extracts exhibit anti-parasitic and antimicrobial activities. Molecules. 2018;23(2):313.
- [Google Scholar]
- Antifungal and antiaflatoxinogenic activities of Carum carvi L., Coriandrum sativum L. seed essential oils and their major terpene component against Aspergillus flavus. Ind. Crops Prod.. 2019;134:11-18.
- [CrossRef] [Google Scholar]
- The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem.. 2020;148:80-89.
- [CrossRef] [Google Scholar]
- Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. Int. J. Pharmacogn. Phytochem. Res.. 2017;9
- [CrossRef] [Google Scholar]
- Genotoxicity of aqueous extracts of Tulbaghia violacea as determined through an Allium cepa assay. S. Afr. J. Sci.. 2019;115
- [CrossRef] [Google Scholar]
- Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J. King Saud Univ. - Sci.. 2018;30:500-505.
- [CrossRef] [Google Scholar]
- Antibacterial anthelmintic and anti-amoebic activity. Ethno-Pharmacol.. 2000;72:247-263.
- [Google Scholar]
- Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules. 2020;25(17):3804.
- [Google Scholar]
- Cardiovascular effects of Tulbaghia violacea Harv. (Alliaceae) root methanolic extract in Dahl salt-sensitive (DSS) rats. J. Ethnopharmacol.. 2013;146:225-231.
- [CrossRef] [Google Scholar]
- Anticancer properties of Tulbaghia violacea regulate the expression of p53-dependent mechanisms in cancer cell lines. Sci. Rep.. 2020;10:1-12.
- [CrossRef] [Google Scholar]
- Interactive effects of light intensity and pH on growth parameters of a bulbous species (Tulbaghia violacea L.) in hydroponic cultivation and its antifungal activities. Med. Plants - Int. J. Phytomedicines Relat. Ind.. 2021;13:442-451.
- [CrossRef] [Google Scholar]
- A comparative study of the antimicrobial and phytochemical properties between outdoor grown and micropropagated Tulbaghia violacea Harv. plants. J. Ethnopharmacol.. 2011;134:775-780.
- [CrossRef] [Google Scholar]
- Nyakudya, T.T., Tshabalala, T., Dangarembizi, R., Erlwanger, K.H., Ndhlala, A.R., 2020. The potential therapeutic value of medicinal plants in the management of metabolic disorders. https://doi.org/10.3390/molecules25112669.
- Chemical composition, antimicrobial and antioxidant properties of the essential oils of Tulbaghia violacea Harv L.F. African J. Microbiol. Res.. 2013;7(18):1787-1793.
- [Google Scholar]
- Chemical composition, antioxidant activity and toxicity evaluation of essential oil of Tulbaghia violacea Harv. J. Med. Plants Res.. 2012;6:2340-2347.
- [CrossRef] [Google Scholar]
- Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum armatum DC. Hindawi The Scientific World Journal 2020
- [CrossRef] [Google Scholar]
- Volatile compounds of tulbaghia violacea harv. J. Essent. Oil-Bearing Plants. 2008;11:203-207.
- [CrossRef] [Google Scholar]
- Restani, P., 2018. Food supplements containing botanicals: benefits, side effects and regulatory aspects, https://doi.org/10.1007/978-3-319-62229-3.
- Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of citrus Paradisi. Int. J. Pharm. Sci. Res.. 2018;9:4859.
- [CrossRef] [Google Scholar]
- Biological activities of species in the genus Tulbaghia: A review. African J. Biotechnol.. 2015;14(45):3037-3043.
- [Google Scholar]
- In vitro cytotoxic and pro-apoptotic effects of water extracts of Tulbaghia violacea leaves and bulbs. J. Ethnopharmacol.. 2015;164:203-209.
- [CrossRef] [Google Scholar]
- Quantitative and qualitative analysis of phenolic and flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacognosy Res.. 2016;8:16-21.
- [CrossRef] [Google Scholar]
- Tannins. In: Pharmacognosy, Applications and Strategy. Elsevier; 2017. p. :199-232.
- [Google Scholar]
- Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. AIP Conf. Proc.. 2021;2353
- [CrossRef] [Google Scholar]
- The effect of Beauveria bassiana inoculation on plant growth, volatile constituents, and tick (Rhipicephalus appendiculatus) repellency of acetone extracts of Tulbaghia violacea. Vet. World. 2020;13:1159-1166.
- [CrossRef] [Google Scholar]
- Phytochemicals, Functional and Preservative Properties of Phytochemicals. Elsevier; 2020. p. :341-361.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102278.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







