Translate this page into:
Gamma ray irradiation assisted decomposition for isoproturon pesticide in aqueous solutions: A detailed study
⁎Corresponding author. mzaben@ksu.edu.sa (Maha I. Al-Zaben)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Isoproturon (IP), a systemic herbicide, is also considered as a potential groundwater contaminant. In the present study, the radiation-induced degradation of IP employing gamma-rays is investigated. Several factors such as radiation dose, pH and the presence of co-contaminants such as H2O2, isopropanol, tert-butanol, coffee waste (CW), carbonized coffee waste (CWC) and TiO2, which might affect the degradation process are examined. The IP concentration decreased with the increase of radiation dose and the degradation obtained at pH = 7.0 is found to be 90%. The degradation efficiency decreases with the increase of initial concentration at the same radiation dose. Co-contaminants such as H2O2, TiO2 and CW had increased the degradation efficiency. The process followed by first order reaction kinetics. Furthermore, the increase of the concentration of H2O2, isopropanol, tert-butanol and the weight of TiO2, CW and CWC resulted in the decrease of the degradation values. The pH value was found to effect the removal efficiency and the degradation process was enhanced under neutral condition. IP concentration of 30µ M was selected as standard, while 0.63 kGy was selected as the standard radiation dose.
Keywords
Isoproturon
Environmental pollution
Gamma-ray
Pesticide degradation
Kinetic
Decomposition
1 Introduction
Scientific research in various fields for the improvement of life has led to an increase in the industrialization in multiple areas of material science, pharmaceutical sciences which leads to various forms of pollutions, and also towards the development of various spectroscopic protocols for their quantification and evaluation of the extent of damage caused (Alabbad et al., 2014; Adil et al., 2013; Varala et al., 2016; Khan et al., 2017; Rahman and Nasir, 2020; Rahman and Varshney, 2020; Azmi et al., 2016, 2013). Moreover, the growing demand for food has directed the focus of the scientific community to study pathways and measures of increasing the productivity of food crops, which led to the development of pesticides and herbicides, as one of the approaches to protect the food corps from pests and weeds, which led to the pollution of soil. Moreover, their usage during the last few decades has increased dramatically due to the increase in the demand of production and supply of food stocks that face losses due to the weeds and pests (Meftaul et al. 2019). This in turn, led to the presence of these chemicals and their residues in various environmental matrices that pollute the soil and seep deep into the earth’s crust even polluting the groundwater which is consumed as drinking water in most parts of the world. Also the rainwater usually act vehicle that transports these harmful pesticides and herbicides into rivers, channels, lakes, sea and prove as risk not only to human health but to the entire environment (Vieira et al., 2020; Herrero-Hernández et al., 2017; Anifandis et al., 2018). Hence, removal of pesticide/herbicide from the environment is a matter of increasing concern (Zolgharnein, Shahmoradi, and Ghasemi 2011).
Among the various pesticides and herbicides used, Isoproturon, (3-(4-isopropylphenyl)-1,1-dimethylurea), (IP) (Fig. 1) a herbicide belonging to the phenyl urea derivatives family, is widely used in agriculture for wheat, barley and rye plants (Tomlin 2000). It is chemically very stable to light, acids and alkalies, however upon treatment with strong alkalies along with heating it undergoes hydrolytic cleavage, often used in the control of annual grasses and many broad-leaved weeds in cereals and wheat crops.zz (Tomlin, 1994; von Wirén-Lehr et al., 2001; Worthing et al., 1990; MacBean, 2012) It is a widely used urea herbicide for pre- and post-emergence control of black-grass, silky bentgrass, wild oats, annual meadow grass, ray grass, various broad-leaf weeds in wheat and barley (spring and winter), and winter rye (Tomlin, 1994, 2000). It is slightly water-soluble and can easily migrate through the soil to crops. This can result in the herbicide entering the human food chain and ending up in our plates. Depending on rainfall conditions and soil properties, it can also reach the groundwater, which pollutes the groundwater. Lately, it has been observed that there were accumulation and intolerable concentrations in drinking water, which can be due to the absence of microbial activity, which tends to slow down the natural degradation processes of the herbicides and pesticides (Krämer, Goodrow, and Kremmer 2004). Due to the environmental hazards it inflicts and health risks it poses, it is classified as WHO Class III hazardous material (Tomlin, 1994; von Wirén-Lehr et al., 2001).
The chemical structure of Isoproturon (IP).
Several methods such as Advanced Oxidation Processes (AOPs) (Neto and De Andrade, 2011) and microbial degradation methods (Lu et al., 2014; Miriti et al., 2014) and fungal degradation techniques (Badawi et al., 2009; Ellegaard-Jensen et al., 2014; Mendoza-Huizar, 2015) including photocatalytic degradation over TiO2/Al-MCM-41 composite systems using solar light (Sharma et al., 2008) and photo-Fenton degradation (Paterlini and Nogueira, 2005; Ruggieri et al., 2011) have been reportedly employed to eliminate IP from the environment. Induced absorption of solar radiation for the photo-degradation IP was obtained by incorporating absorbing species such as Fe(III) aqua-complexes (Andrianirinaharivelo, Pilichowski, and Bolte 1993). Further, this study of induced photo-degradation of IP by Fe(III) was investigated under both artificial and solar light, which yielded that the Fe(III) aqua-complexes absorbs light in the near UV–visible spectral region (Galichet et al., 2002; Tomlin, 2000; Andrianirinaharivelo, Pilichowski, and Bolte, 1993; Kari, Hilger, and Canonica, 1995; Matthus et al., 1989). Mascolo et al. studied the degradation of the IP employing simple ozonation (Lopez, James, and Fielding 2001). The degradation of isoproturon photo induced by Fe(III) was investigated under both artificial and solar light (Galichet et al. 2002). Utilized sunlight and porous nanosilica supported TiO2 photocatalyst to study degradation of IP in water. The optimized parameters were then applied for two other pesticides including imidacloprid and phosphamidon for aqueous solutions (Sharma et al., 2009).
To continue this study further, we decided to investigate the conditions suitable for the use of ionizing radiation (gamma rays) for the radiolytic decomposition of IP, an herbicide commonly used in the Kingdom of Saudi Arabia. The other factors such as radiation dose, pH and the presence of co-contaminants such as H2O2, isopropanol, tert-butanol, coffee waste (CW), carbonized coffee waste (CWC) and TiO2, are studied.
2 Experimental
2.1 Materials and methods
Analytical grade of isoproturon used in this study from Herbicide Selectomobeed (Saudi Arabia) had a purity of over 97%. A stock solution containing 65 mg/L isoproturon was prepared using distilled water. The concentration of isoproturon remaining in the solution after shaking at 240 rpm for 30 min (to ensure a balance). Isoproturon solutions were prepared at different initial concentrations and different additives of different concentrations were added into 30 µM isoproturon solution to examine their effects on degradation. Sulfuric acid 98% from BDH, NaOH from winlab (GPR) were added in the put into isoproturon solution to test the effects of pH value on degradation efficiency.
2.2 Instruments
Gamma-ray was obtained from 60Co source, Gamma cell 220 NO. from Nordion International Inc. located at central laboratory of the College of Sciences, King Saud University.
The UV absorbance at 190–400 nm were measured by UV spectrophotometer (UV/Visible spectrophotometer Ultraspec 2000), Pharmacia Biotech in King Saud University, at central lab of the College of Sciences at chemistry department.
2.3 Specifications of Gamma-ray irradiator – its current dose rate, specify the dosimetry used to calculate the dose
Gamma-ray was obtained from 60Co source, Gamma cell 220 NO.246 from from Nordion International Inc. located at central laboratory of the College of Sciences, King Saud University. Aqueous solutions were placed in 25 mL airtight glass vessels, which were placed in radiation field at a specific distance from the source, and 0.1, 0.21,0.31, 0.63, 0.94 and 1.25 kGy were selected as the radiation doses. The dose rates of the dots, depending on the distance between 60Co column and the dots, were determined by means of a modified Fricke dosimeter using G (Fe3+) = 15.6. All of irradiation studies were conducted at room temperature.
The other information of materials and about this study are given in the supplementary file in sections 2.4–2.5.
3 Results and discussion
3.1 G value calculations and dose constant
The mathematical equations employed are given in the supplementary files.
According to Eq. (3), G values of IP were obtained, as shown in Table 1. Conc. of IP = 30 µM; pH = 7.0.
Radiation (kGy)
0.21
0.31
0.63
0.94
1.25
G (μmol J−1)
0.08
0.08
0.06
0.04
0.03
The results showed that G values decreased with the enhancement of radiation dose. At the higher dose, the relative concentration of active radicals is lower.
By plotting the natural logarithm of residual concentration as a function of radiation dose, (Fig. 2), a linear relationship could be derived (R2 = 0.99), which indicates that the degradation of IP takes place as a pseudo first-order reaction with respect to radiation dose and could be described by Eq. (4) (Nickelsen et al. 1994).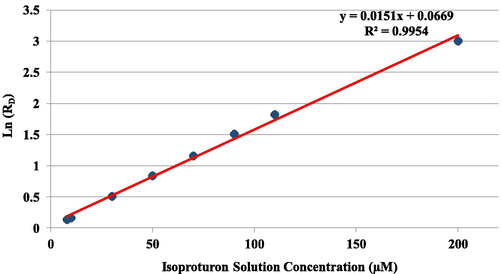
Plot of natural logarithm of residual concentration as a function of radiation dose.
RD – the residual concentration of Isoproturon at any radiation dose (µM);
D – radiation dose (kGy);
R0 – the initial concentration of Isoproturon (µM);
k – decay constant (kGy−1).
D0.5, D0.9 and D0.99 values were calculated and used for analysis of IP degradation by using Eqn’s (5), (6) and (7):
This was obvious from the decay constant (k) for each aimed concentration and calculated values of D0.5, D0.9 and D0.99 (the irradiation dose required to remove 50% and 90% and 99% of initial IP concentration in water), which substantiated this trend.
In general, the irradiation dose necessary to eradicate 50%, 90% or 99% of initial IP concentration in water decreased as the IP concentration in the solution is reduced showing 0.369 kGyD0.5−1, 1.22 kGyD0.9−1 and 2.45 kGyD0.99−1 at the IP concentrations of 30 µM at pH 7 (Changotra et al. 2018).
Hence, a 30 µM IP solution and the 0.63 kGy irradiation dose is considered optimum for maximum degradation efficiency of IP, the solution pH = 7 was maintained throughout the study.
3.2 Optimization of parameters for Radiation-induced decomposition of IP by gamma-ray irradiation
UV absorbance of IP in the wavelength range of 190 – 400 nm before and after gamma-ray irradiation was recorded and the absorbance at wavelengths 202 nm and 239 nm was found to confirm the presence of IP. However, it was observed that upon irradiation with gamma-rays, the absorbance at both 202 nm and 239 nm decreased with the elapsing of each radiation dose and as the number of doses increased the concentration of IP apparently was found to decrease. Fig. 3 represents an overlay of the UV spectra’s obtained from each radiation dose.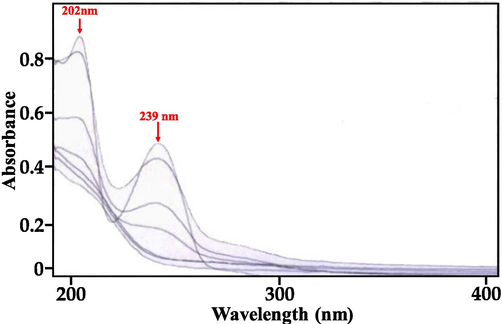
Degradation of IP studied using UV–Vis spectrophotometer.
The optimum irradiation dose for efficient degradation was studied by irradiating the solutions with varying concentrations in the range of 2–200 µM, which were irradiated with gamma rays with two different irradiation doses of 0.31 kGy and 0.63 kGy.
During this study, it was found that degradation process is effected by the initial concentration of the IP solutions and also on the intensity of irradiation. The maximum degradation obtained was 88%, when the concentration of IP solution is only 8 µM for the radiation with intensity of 0.31 kGy, however, a ∼90% degradation was obtained for a solution with a IP concentration of 30 µM, when the irradiation dose was 0.63 kGy. The results of this study are given in Table 2. and are graphically illustrated in Fig. 4.
IP Solution concentration (µM)
Degradation efficiency (%)
Irradiation Dose (kGy)
0.31
0.63
2
64
64.29
6
70
80
8
88
83
10
85
88
30
54
89.71
50
40
71
70
33
61
90
27
49
110
22
46
200
9
22
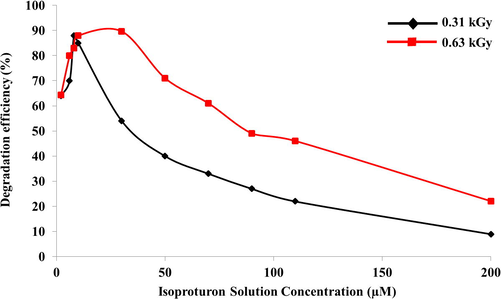
Degradation efficiency of IP solutions of varying concentrations.
These outcomes signify that the optimum irradiation dose suitable for best results is 0.63 kGy, it also points out that most of the IP residues were lowered in solutions when exposed to different doses of high energy irradiation; specifically, at the higher target. Furthermore, the degradation pattern was studied with regard to varying the dose in the range 0.1 – 1.25 kGy along with varying pH, while maintaining the initial concentration of IP solutions of 30 µM. The results obtained are tabulated in Table 3 and are graphically illustrated in Fig. 5.
Radiation dose (kGy)
Degradation efficiency (%)
pH
3.0
7.0
10.0
0.1
11.5
11.52
11.52
0.21
45.3
45.27
49.59
0.31
64.2
63.17
68.11
0.63
85.1
89.71
84.57
0.94
83.47
84.57
84.77
1.25
84.2
85.19
86.21
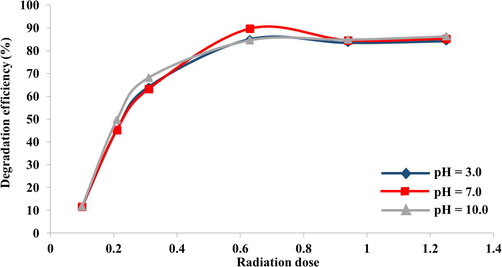
Degradation efficiency of IP solutions of varying pH and radiation dose Conditions: Initial concentration of IP solutions of 30 µM.
The dilapidation efficiency under neutral conditions was found to be almost similar to the results obtained under acidic conditions and alkaline conditions, however, under neutral conditions the dilapidation efficiency was found to be the best.
At pH 7.00, a decrease of 90% was accomplished at 0.63 kGy, while at the same radiation dose, at pH 3.0 and 10.00, the degradation competences were 85% and 84.5%, respectively. The pH value became lower with intensifying radiation dose after gamma-ray irradiation. The modification of pH value resulted in concentration alteration of [H+] and [OH−]. As is well known, eaq− +H+→H• (with rate constant of 2.3 × 1010 L(L S)−1). It was observed that at 0.10 kGy, the degradation efficiency was only 11.5%. The maximum degradation efficiency of IP 90% was obtained when 0.63 kGy was adopted as the radiation dose at pH = 7.0 as shown in Fig. 5.
These results imply that most of the IP residues were reduced in solutions when exposed to different doses of high energy irradiation however, it has been observed that the pH doesn’t influence in enhancing the degradation, however the pH = 7 was found to be the best and is most optimum for excellent degradation of IP.
3.3 The factors affecting degradation of Isoproturon
3.3.1 Effect of H2O2, isopropanol and tert. butanol on IP degradation
Furthermore, the effect of various additives, for which various concentrations of H2O2, isopropanol and tert. butanol in the range of 0.001–0.1 μM has been studied while the pH of the solution to be maintained at 7.0, at 30 µM concentration of IP and the radiation dose was 0.63 kGy. The results obtained have been tabulated in Table 4, and graphically illustrated in Fig. 6. The degradation efficiency of IP was found to be enhanced in the presence of H2O2 in the range of (0.001–0.004 µM), however further increase led to depreciation in the degradation efficiency. pH = 7.0; Conc. of Isoproturon = 30 µM; Radiation dose; 0.63 kGy.
Concentration (µM)
Degradation efficiency (%)
H2O2
tert. butanol
Isopropanol
0.001
99
52.11
31.22
0.002
98.37
48.91
30.039
0.004
93.41
38
25
0.008
80.32
22.46
12.84
0.01
68.63
20
10
0.02
42.75
20
8.1
0.03
34.98
17
7
0.04
30.13
15
5
0.08
12.88
12
2.15
0.1
5.9
9.46
1
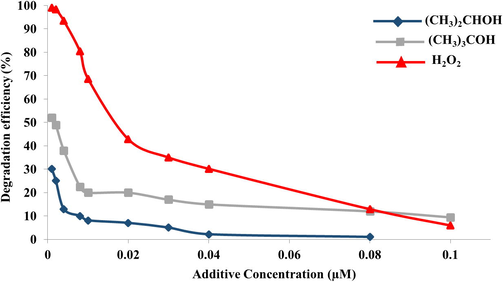
Degradation pattern of IP solutions effected by various solvents.
The degradation efficiency was found to be 99% when the IP solution was spiked with 0.001 µM, which is much better than ∼90% degradation obtained with the gamma irradiation of un-spiked IP solution. Table 4 that shows the G values decreased with increased of concentration of H2O2. This demonstrates the fact that during irradiation, the additional hydrogen peroxide will act as extra source of oxygen and hydroxyl radicals that result in more efficient degradation of IP. However, as the concentration of H2O2 was further increased, there is a resultant increase oxygen and hydroxyl radicals which led to a greater scavenging effect among the oxygen and hydroxyl radicals formed by the H2O2 and due to the radiolysis of H2O, which lead to decrease in the degradation of IP.
However, in the case of isopropanol and tert-butanol, the results revealed that the degradation of IP is drastically effected with minute quantities as less as 0.001 µM of tert. butanol which yielded a degradation efficiency of 52.1%, moreover with the same concentration isopropanol, the degradation efficiency was even lower i.e. 31% as shown in Table 4. Hence, it can be said that the presence of isopropanol and tert. butanol adversely effects the degradation of IP at the 0.63 kGy radiation dose. Fig. 6 illustrates the pattern of effect of degradation of IP, in terms of decrease in G values, which in turn means the reduction in the degradation of IP with enhancement of concentration of isopropanol and tert. butanol.
The probable reason can be as reported by Buxton et al., wherein it was noticed that isopropanol can scavenge •OH and H• as shown in Eqs. (8) and (9) reactions, whereas tert. butanol is capable to scavenge •OH, according to the reaction shown in Eq. (10) (Buxton et al. 1988):
Since both •C(CH3)2OH and •CH2C(CH3)2OH are inert species, H• and e−aq play the predominant roles along with tert. butanol, while e−aq plays the imperative role in the presence of isopropanol.
3.3.2 Effect of CW, CWC and TiO2 on IP degradation by gamma-ray irradiation
Furthermore, effect of various additives on the degradation of IP was studied by incorporating CW, CWC and TiO2 in the range of 1–200 mg to the IP solution with concentration of 30 µM while the pH was maintained at 7.0. The degradation studies were carried out by gamma-ray irradiation at a radiation dose of 0.63 kGy.
The results obtained revealed that the degradation of IP in the presence of 1 mg of CW and TiO2 was found to be 93% and 100% respectively, which was higher than the degradation obtained in their absence i.e. 89%.
However, with respect 1 mg of CWC, the degradation of IP was found to be 82%, which is less than the value obtained in its absence i.e. 89%, indicating that CWC addition adversely effects the degradation efficiency of gamma radiation. Further studies carried out by increasing the amount of additives which was found to adversely affect the degradation efficiency of the gamma radiation. The G-values obtained are tabulated in the Table 5 and are graphically illustrated in the Fig. 7. pH = 7.0; Conc. of Isoproturon = 30 µM; Radiation dose; 0.63 kGy.
Amount of additive (mg)
Degradation efficiency (%)
TiO2
CW
CWC
1
100
93
82
5
100
93
82
50
100
93
82
100
98
85
67.45
200
97.91
78.02
67.26
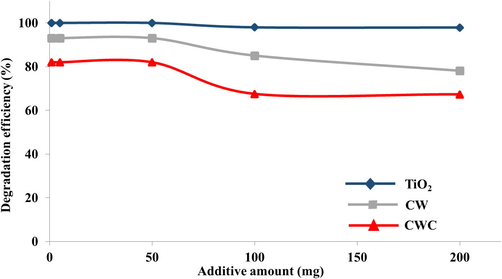
Degradation pattern of IP solutions effected by various additives.
4 Conclusions
From the results of this study, we can conclude that the gamma-ray irradiation could effectively degrade Isoproturon, a potent environmental hazard. Efficient decomposition, i.e. up to 90% decomposition, of this pesticide by employing gamma radiation can be obtained when the concentration is 30 µM, with a radiation dose of 0.63 kGy and a neutral pH (pH = 7) was found to be essential for effective degradation. The reaction could be described by pseudo first-order reaction kinetics, the dose-constant was 1.87 kGy−. Furthermore, it was found that low concentrations of H2O2 increased the degradation efficiency. Moreover, it is found that the addition of coffee waste (CW), and TiO2 to the IP solution also influence degradation efficiency, wherein a 100% degradation efficiency was obtained when 1 mg of additive was added, however upon further increase of weight there was a decrease in the efficiency. Based on the above experimental results, gamma-ray irradiation can be employed as an efficient protocol to decompose IP, along with conventional methods such as biological, adsorption, floatation etc. for the purification of water and treatment of wastewater that is polluted due to the presence of herbicide. Further, utilization of this technology for degradation of various other pesticides and herbicides can be studied and we hope to employ it for facilitating process for the removal of the pollutants.
Acknowledgements
The authors would like to acknowledge the Researchers Supporting Project (RSP-2020/155), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nano silver-doped manganese oxide as catalyst for oxidation of benzyl alcohol and its derivatives: synthesis, characterisation, thermal study and evaluation of catalytic properties. Oxid. Commun.. 2013;36:778-791.
- [Google Scholar]
- Gold & silver nanoparticles supported on manganese oxide: Synthesis, characterization and catalytic studies for selective oxidation of benzyl alcohol. Arabian J. Chem.. 2014;7:1192-1198.
- [Google Scholar]
- Nitrilotriacetic acid transformation photo-induced by complexation with iron (III) in aqueous solution. Transition Met. Chem.. 1993;18:37-41.
- [Google Scholar]
- The in vitro impact of the herbicide roundup on human sperm motility and sperm mitochondria. Toxics. 2018;6:2.
- [Google Scholar]
- Utility of cefixime as a complexing reagent for the determination of Ni (II) in synthetic mixture and water samples. Environ. Monit. Assess.. 2013;185:4647-4657.
- [Google Scholar]
- Optimized and validated spectrophotometric method for the determination of palladium (II) in synthetic mixture and automobile workshop area samples. J. Assoc. Arab Univ. Basic Appl. Sci.. 2016;19:29-36.
- [Google Scholar]
- Metabolites of the phenylurea herbicides chlorotoluron, diuron, isoproturon and linuron produced by the soil fungus Mortierella sp. Environ. Pollut.. 2009;157:2806-2812.
- [Google Scholar]
- Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:513-886.
- [Google Scholar]
- Assessment of reaction intermediates of gamma radiation-induced degradation of ofloxacin in aqueous solution. Chemosphere. 2018;208:606-613.
- [Google Scholar]
- Fungal–bacterial consortia increase diuron degradation in water-unsaturated systems. Sci. Total Environ.. 2014;466:699-705.
- [Google Scholar]
- Iron (III) photo-induced degradation of isoproturon: correlation between degradation and toxicity. Pest Manag. Sci.. 2002;58:707-712.
- [Google Scholar]
- Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain) Sci. Total Environ.. 2017;609:161-171.
- [Google Scholar]
- Determination of the reaction quantum yield for the photochemical degradation of Fe (III)-EDTA: Implications for the environmental fate of EDTA in surface waters. Environ. Sci. Technol.. 1995;29:1008-1017.
- [Google Scholar]
- Miswak mediated green synthesized palladium nanoparticles as effective catalysts for the Suzuki coupling reactions in aqueous media. Journal of Saudi Chemical Society. 2017;21:450-457.
- [Google Scholar]
- Enzyme-linked immunosorbent assays based on rabbit polyclonal and rat monoclonal antibodies against isoproturon. J. Agric. Food. Chem.. 2004;52:2462-2471.
- [Google Scholar]
- By-products formation during degradation of isoproturon in aqueous solution. I: ozonation. Water Res.. 2001;35:1695-1704.
- [Google Scholar]
- Enhanced degradation of herbicide isoproturon in wheat rhizosphere by salicylic acid. J. Agric. Food. Chem.. 2014;63:92-103.
- [Google Scholar]
- The pesticide manual: a world compendium. In.: BCPC; 2012.
- Photodegradation of ferric ethylenediaminetetra (methylenephosphonic acid)(EDTMP) in aqueous solution. Water Res.. 1989;23:845-851.
- [Google Scholar]
- Pesticides in the urban environment: a potential threat that knocks at the door. Sci. Total Environ.. 2019;134612
- [Google Scholar]
- Chemical reactivity of isoproturon, diuron, linuron, and chlorotoluron herbicides in aqueous phase: A theoretical quantum study employing global and local reactivity descriptors. J. Chem.. 2015;15
- [Google Scholar]
- Isolation and characterization of linuron degrading bacteria from soils under horticultural production in Kenya. Int. J. Environ. Bioremed. Biodegrad.. 2014;2:220-227.
- [Google Scholar]
- Electrochemical oxidation of herbicides. In: Larramendy Marcelo, ed. Herbicides. Theory and Applications; 2011.
- [Google Scholar]
- High energy electron beam generation of oxidants for the treatment of benzene and toluene in the presence of radical scavengers. Water Res.. 1994;28:1227-1237.
- [Google Scholar]
- Multivariate analysis of photo-Fenton degradation of the herbicides tebuthiuron, diuron and 2, 4-D. Chemosphere. 2005;58:1107-1116.
- [Google Scholar]
- Effective removal of acetaminophen from aqueous solution using Ca (II)-doped chitosan/β-cyclodextrin composite. J. Mol. Liq.. 2020;301:112454
- [Google Scholar]
- Assessment of ampicillin removal efficiency from aqueous solution by polydopamine/zirconium (iv) iodate: optimization by response surface methodology. RSC Adv.. 2020;10:20322-20337.
- [Google Scholar]
- Photocatalytic degradation of linuron in aqueous suspensions of TiO2. RSC Adv.. 2011;1:611-618.
- [Google Scholar]
- An efficient and novel porous nanosilica supported TiO2 photocatalyst for pesticide degradation using solar light. J. Hazard. Mater.. 2009;171:626-633.
- [Google Scholar]
- Photocatalytic degradation of isoproturon herbicide over TiO2/Al-MCM-41 composite systems using solar light. Chemosphere. 2008;72:644-651.
- [Google Scholar]
- The Pesticide manual : a world compendium : incorporating the agrochemicals handbook. Information Sciences: Cambridge, UK): British Crop Protection Council ; Royal Society of Chemistry, Information Sciences; 1994.
- The pesticide manual: A world compendium. Cambridge, UK: British Crop Production Council; 2000.
- Sulfated tin oxide (STO)–Structural properties and application in catalysis: A review. Arabian J. Chem.. 2016;9:550-573.
- [Google Scholar]
- Herbicide drift exposure leads to reduced herbicide sensitivity in Amaranthus spp. Sci. Rep.. 2020;10:1-11.
- [Google Scholar]
- The pesticide manual: a world compendium. Cambridge, UK: British Crop Protection Council; 1990.
- Pesticides removal using conventional and low-cost adsorbents: a review. Clean-Soil, Air, Water. 2011;39:1105-1119.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.08.020.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







