Translate this page into:
Functionalized multi walled carbon nanotubes supported copper-titania nanoparticles for oxidation of cinnamyl alcohol under mild reaction conditions
⁎Corresponding author at: Department of Biochemistry, University of Malakand, Pakistan. GSM: +923416062388. zahoor@uom.edu.pk (Muhammad Zahoor) mohammadzahoorus@yahoo.com (Muhammad Zahoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Alcohols oxidation is one of the important organic transformation in fine chemical industries. Prevailing processes are hazardous due to involvement of stoichiometric oxidants and homogeneous catalysts. In the present work, oxidation of cinnamyl alcohol was carried out using unconventional, affordable, and feasible heterogeneous catalysts.
Method
Copper-titania (Cu-Ti) nanoparticles were prepared and supported on functionalized multi walled carbon nanotubes (F-CNTs). Various instrumental techniques such as X-ray Diffractometery (XRD), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray (EDX) Analysis and Brunauer Emmett Teller (BET) surface area analyzer were used to characterize the synthesized catalysts. Both catalysts; Cu-Ti and Cu-Ti/F-CNTs were evaluated for their potencies in conversion of cinnamyl alcohol (CnOH) to cinnamaldehyde (CnHO). Different derivatives of CnOH (with attached electron withdrawing and donating groups) were also oxidized in presence of prepared catalysts to determine the substituents effect and get maximum yield. The prepared catalyst was used five times to determine its reuseablity.
Results
The presence of copper and titania in the synthesized catalyst structure was confirmed through XRD and EDX analysis. The agglomeration level was confirmed from SEM analysis. Little reduction in surface area on parental carbon nanotubes was observed due to deposited metals. Appreciable yield of CnHO were obtained at the optimal reaction conditions: temperature = 70 °C, catalyst amount = 0.1 g, pO2 = 760 Torr, substrate solution concentration and volume = 1 mmol CnOH/10 mL ethanol, stirring speed = 900 rpm, and time interval = 60 min. The conversion rate was improved to 100% through attachment of electron donating groups at ortho and para position of parental compound benzene ring. No appreciable decrease in activity of catalyst were observed after 4th cycle.
Conclusion
Cu-Ti/F-CNTs showed excellent catalytic activity, selectivity, true heterogeneous nature, low cost, and recyclability, hence it could be used as a potent catalyst for CnOH to CnHO conversion.
Keywords
Cinnamaldehyde
Cinnamyl alcohol
Liquid phase
Oxidation
1 Introduction
The oxidation of alcohols into aldehydes have vast applications in the synthesis of several useful intermediates used in food, agriculture, and pharmaceutical industries (Dunn et al., 2010). In traditional methods mostly dichromate and manganate ion based oxidants are frequently used that are hazardous and harmful to the environment (Nyamunda et al., 2012). Therefore, scientists are trying to use environment friendly catalysts (Shi et al., 2012). Different researchers have documented the conversion of CnOH to CnHO using expensive platinum and palladium catalysts (Mallat et al., 1995; Grunwaldt et al., 2003). Mallat and Baiker (1995) investigated the selective aerobic oxidation of CnOH to CnHO using platinum supported on alumina as a catalyst with 88.5% selectivity. About transition metal catalysts such as platinum and palladium, activity loss as a result of deactivation triggered by poisonous by-products of reaction, low selectivity of the platinum group metals and poor catalytic activities have been reported (Sneeden et al., 1955).
Keeping in view the above mentioned shortcomings, efforts have been made to add another metal to form bimetallic catalysts and are considered as alternative being capable of effective conversion of alcohol into aldehydes. According to recent reports, binary metallic alloys are beneficial as catalyst with greater degrees of selectivity having greater lifetime (Hu et al., 2010; Worz et al., 2010; Mallat et al., 1994; Wenkin et al., 2002; Abbadi and Van Bekkum, 1995). The Cu-Ti transition metal nanoparticles may display better catalytic performance, good durability and needs simple separation techniques to separate from reaction mixture after use.
According to literature survey, no data is available on Cu-Ti oxide or functionalized multiwalled carbon nanotubes supported copper-titania nanoparticles (Cu-Ti/F-CNTs) manipulated for the catalytic oxidation of CnOH. Therefore, in the current work, Cu-Ti oxide nanoparticles were supported on functionalized multiwall carbon nanotubes (Cu-Ti/F-CNTs) and were applied for catalytic oxidation of CnOH to CnHO in various solvents (acetonitrile, n-hexane, water and ethanol) in presence of molecular oxygen. The nanoparticles exhibited better effectiveness in terms of selectivity, stability, productivity, heterogeneity, cost and recyclability, therefore, could be efficiently used for oxidation of alcohols at industrial level.
2 Materials and methods
All the chemicals/reagents were purchased from Sigma Aldrich, Merck, and Alfa Aesar. Multiwall carbon nanotubes (MWCNTs; area = 220 m2/g; O.D. × L 6–13 nm × 2.5–20 μm) were also purchased from Sigma Aldrich. Specific filters (C.R.S.Inc.202268) and (C.R.S.Inc.202223) were used for purification of gases.
2.1 Synthesis of catalysts
Co-precipitation method was used for the Cu-Ti nanoparticles preparation. CuCl2·2H2O and TiCl4 (0.1 M) equimolar solutions were titrated against ammonium hydroxide (NH4OH) until the formation of condensed metal hydroxide precipitates. The precipitates were filtered, washed with 0.1 N HCl, and triply distilled water (TDW) in modified Soxhlet apparatus using glass thimble until neutral pH and dried overnight in oven. Multi walled carbon nanotubes and para-aminobenzoic acid were sonicated at 60 °C for 2 h and then refluxed for 1 h in order to obtain functionalized multi walled carbon nanotubes (F-CNTs). Copper-titania were dispersed in ethanol/water (50% V/V) by sonication and required amount of this F-CNTs were added which were further sonicated for 30 min at 30 °C. The Cu-Ti/F-CNTs was filtered, washed with 0.1 N HCl and TDW in modified Soxhlet apparatus using glass thimble until neutral pH and then dried.
2.2 Characterization of the nanoparticles
Morphological and elemental analysis of the prepared Cu-Ti/F-CNTs were carried out by Scanning Electron Microscopy (SEM, JSM 5910, JEOL, Japan) and Energy Dispersive X-ray Spectroscopy (EDX, JSM 5910, JEOL, Japan), respectively. For the phase determination of the catalyst, X-Ray Diffractometer (XRD, JDX-3532, JEOL, Japan) was used with Cu-Kα as a radiation source with λ = 0.15418 nm, while operation voltage was 20–40 kV, in the 2θ range of 0-60° at a step size of 0.05°. Quanta chrome, USA (NOVA2200e) surface area analyzer was used for the determination of BET surface area of the supported nanoparticles.
2.3 Catalytic test
Substrate solution (1mmole CnOH/10 mL ethanol) with 0.1 g catalyst were introduced into 100 mL three necked double walled round bottom batch reactor combined with a quick fit thermometer and a condenser. The mixture was vigorously stirred (300–1500 rpm) with continuous flow of oxygen (40 mL/min) at 70 °C for 60 min. The CnOH to CnHO conversion was investigated using gas chromatography (GC, Clarus 580, Perkin Elmer, USA) provided with a flame ionized detector and capillary column (cross-linked methyl siloxane capillary column; length: 30 m, ID: 0.32 mm, and film thickness: 0.25 µm). Hydrogen and nitrogen generators (PGXH2 100, Perkin Elmer, USA) and (G6010E, Parker domnick hunter, UK) respectively were used for hydrogen and nitrogen supply. The percent conversion of the collected product was determined by applying the following formula.
The chemical reaction occurred is shown in scheme 1.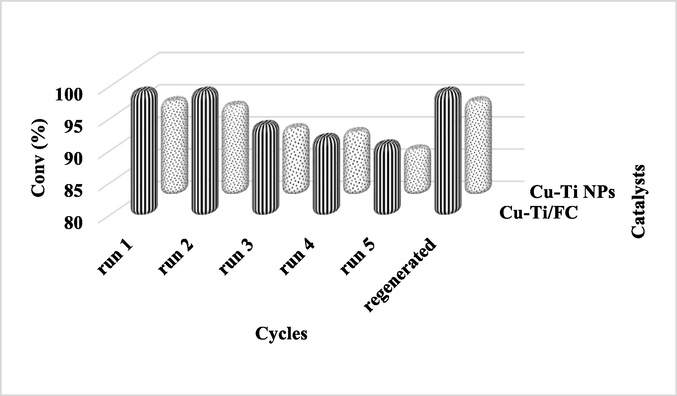
Oxidation of CnOH to CnHO under optimum conditions (0.1 g catalyst, 10 mL substrate solution (1 mmol of CnOH/10 mL ethanol) at 70 °C and stirring at 900 rpm for 60 min in the presence of O2).
3 Results and discussion
3.1 Characterization
Fig. 1 a & b represent the SEM images of copper-titania nanoparticles, clearly indicating the irregular shapes of the nanoparticles (copper-titania) in unsupported nanoparticles, while smooth dispersion and agglomeration of nanoparticles on the surface of the F-CNTs in case of supported nanoparticles. The agglomeration level in the functionalized multi walled carbon nanotubes supported copper-titania nanoparticles (Cu-Ti/F-CNTs) was lower than unsupported nanoparticles. Average grain intercept method was used to calculate the average particle size (23 nm) of the nanoparticles from SEM images (Iqbal et al., 2019). Similar study was also carried out by Etape et al. (2017) and investigated the structural morphologies of the nanoparticles through SEM.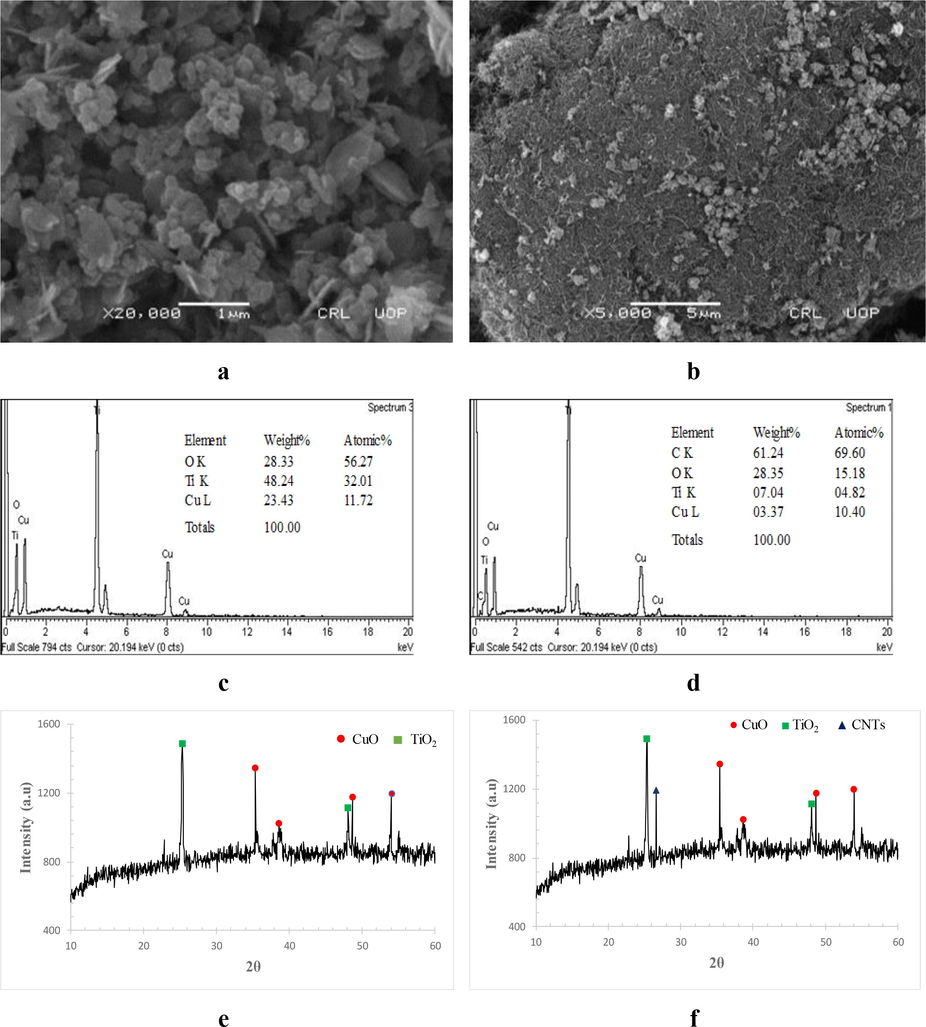
Instrumental characterization of catalysts: a) SEM image Cu-TiO2 (b) SEM image of Cu-TiO2/F-CNTs (c) EDX of Cu-TiO2, (d) EDX of Cu-TiO2/F-CNTs (e) XRD pattern of Cu-TiO2, and (f) XRD pattern of Cu-TiO2/F-CNTs.
The elemental composition of nanoparticles is presented in Fig. 1 c & d, confirming the presence of metals used in its synthesis. The Cu and Ti were present in high quantities while the C peak was present only in Cu-Ti/F-CNTs due to the use of carbon based supporting material (F-CNTs). The oxygen peaks suggests the metal oxides formation and the introduction of carboxylic functional group during the functionalization of multi walled carbon nanotubes with p-amino benzoic acid.
Fig. 1 e & f shows the XRD patterns of both supported and unsupported nanoparticles. A sharp peak is present in case of supported nanoparticles at 2θ = 26.6°, confirms the presence of supporting material while such peak was absent in unsupported copper-titania nanoparticles. The presence of characteristic peaks at 2θ = 25.3° and 48.1° while absence of peaks at 2θ = 27°, 36° and 55° confirmed the presence of TiO2 nanoparticles in anatase phase in both supported and unsupported catalysts. Similarly, peaks at 2θ = 35.4°, 38.3°, 48.7° and 54° revealed the formation of copper oxide nanoparticles. Khan et al. (2017) also reported the similar study for brookite phase of titania. The BET surface area of Cu-Ti/F-CNTs and Cu-Ti nanoparticles was 213.1 m2/g and 31.8 m2/g, respectively. Similar results have also been reported by Dasireddy and Likozar (2018) and Hossain et al. (2014).
3.2 Oxidation of cinnamyl alcohol as benchmarking experiment
About 1 mmol of CnOH in 10 mL solvent were loaded to reactor without catalyst at 70 °C (shaking speed; 900 rpm, pO2; 760 torr and time duration; 60 min) to confirm the autocatalysis of CnOH to CnHO. Furthermore, for optimization of reaction conditions, a series of experiments were performed to investigate the effect of parameters such as temperature, reaction time, solvent, oxygen pressure, stirring speed, catalyst amount, % conversion, and selectivity. Similarly, the same reaction was also catalysed with F-CNTs under the standard set of reaction parameters. Very low catalytic activity was observed for F-CNTs, which were then doped with Cu-Ti to enhance its catalytic activities. The F-CNTs enhanced the mobility of electrons which was considered to be the most probable reason of enhancing the catalytic activities of the composite. In the present work it was observed that Cu-Ti/F-CNTs was an excellent catalyst in term of selectivity and conversion as compared to other reported catalysts as tabulated in table 1. *Conversion/Selectivity.
Catalysts
*Conv/Sel(%)
Reaction Conditions
References
Au–Pd/TiO2
82/64
Temp; 100 °C, Solvent; Toluene,
Time; 7 h(Wu et al., 2016)
Fe2O3/AC
44/89
Temp; 80 °C, Solvent; Water,
Time; 2 h(Sadiq et al., 2014)
Modified Palladium (II) oxide
100/77
Temp; 100 °C, Solvent; Toluene,
Time; 1 h(Stuchinskaya and Kozhevnikov, 2003)
Ru-Al-Mg
Hydrotalcites98/100
Temp; 60 °C, Solvent; Toluene,
Time; 8 h(Kaneda et al., 1998)
Cu-Ti/F-CNTs
99/100
Temp; 70 °C, Solvent; Ethanol,
Time; 1 hPresent Study
3.3 Effect of various reaction parameters on cinnamyl alcohol oxidation
3.3.1 Time profile study
The time profile analysis for CnOH oxidation was monitored periodically as shown in Fig. 2. The experiments were performed at 70 °C by suspending 0.1 g of catalyst in 10 mL of substrate solution (1 mmol CnOH/10 mL ethanol), oxygen partial pressure 760 Torr and agitation speed of 900 rpm. The conversion rate linearly increased up to 60 min, after that the conversion of CnOH to CnHO gradually decreased due to by-products formation (3-phenylproanol and trans-β methyl styrene) as detected by GC, which altered the selectivity towards the major product. Therefore, 60 min time interval was used as optimum time in the subsequent reactions. Previously Sadiq et al. (2010) carried out aerobic oxidation of toluene using Pt/ZrO2 in aqueous media where they studied time effect on % conversion as well.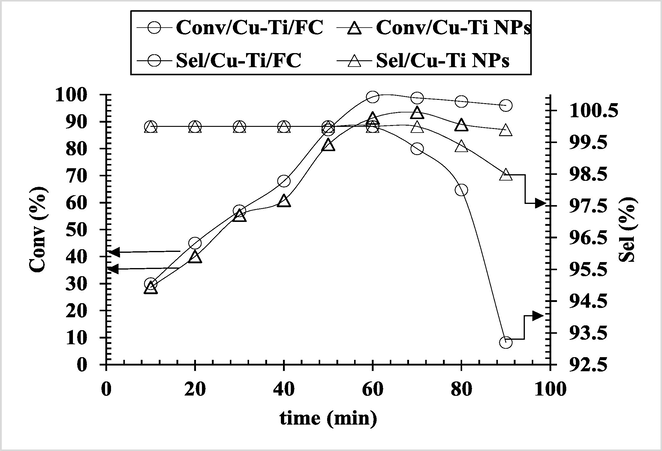
Time effect on the % selectivity and conversion of CnOH to CnHO (Reaction Conditions: Cat; 0.1 g, CnOH; 1 mmol, Temp; 70 °C, Oxidant; O2 (760 Torr), Stirring; 900 rpm).
3.3.2 Temperature study
Conversion of CnOH to CnHO was studied in the range 30–90 °C in ethanol by keeping all other reaction conditions constants. Product analysis showed a gradual increase in % conversion with same higher selectivity towards the major product up to 70 °C (red circle) as shown in Fig. 3. Above 80 °C, the conversion decreased due to two reasons firstly, solvent evaporation which affected the catalyst activity by influencing the diffusion rate as well as mass transfer of reactants, secondly the appearance of by-products like 3-phenylproanol, trans-β methyl styrene and other impurities as observed in GC chromatogram perhaps due to oxidation of solvent. Therefore, all further reactions were carried out at 70 °C. Elmaci et al. (2017), also studied the temperature effect on alcohol oxidation and concluded that conversion rate increased linearly with temperature.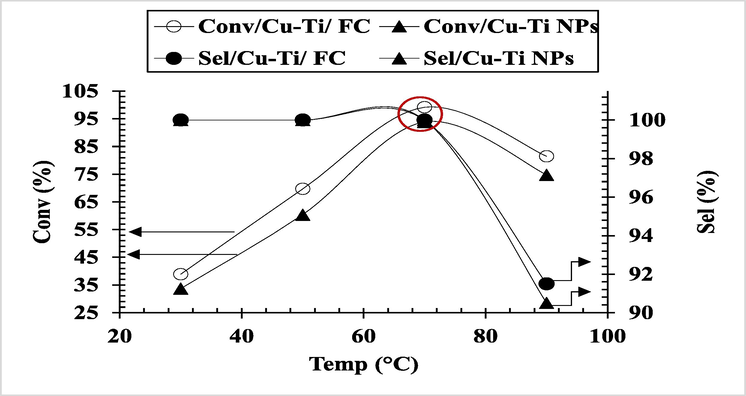
Temperature effect on % selectivity and conversion of CnOH to CnHO (Reaction conditions: Reaction Conditions: Cat; 0.1 g, CnOH; 1 mmol in 10 mL solvent, Time; 60 min, Stirring; 900 rpm; Oxidant; O2 under 1 atm pressure).
3.3.3 Influence of the oxygen pressure
Fig. 1aS (supporting material) describes the effect of partial pressure of O2 on the % conversion of CnOH to CnHO in ethanol. With the increase in pressure of oxygen the conversion rate increases up to 760 Torr. The linear relation of pO2 with % conversion shows that the reaction follow a first order kinetics with Langmuir-Hinshelwood (L-H) mechanism (Eq. (2)).
The experimental data well fitted into Eq. (3) with good regression values of R2 = 0.9954 and 0.9935 for both the catalysts Cu-Ti/F-CNTs and Cu-Ti respectively (Fig. 4). Which clarifies that both reactants [CnOH]l and [O2]g are adsorbed on the surface of catalyst and follow the L-H mechanism.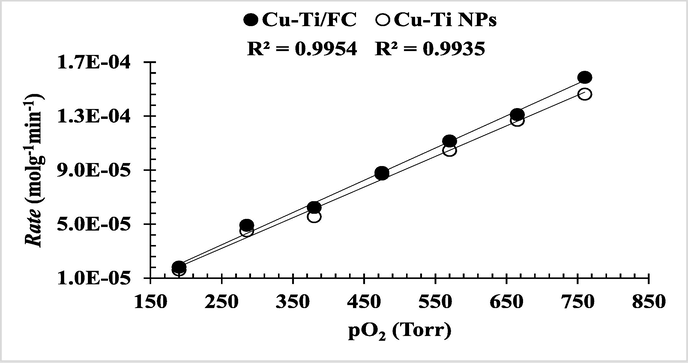
Langmuir-Hinshelwood model for predicting the effect of partial pressure on reaction rate.
3.3.4 Stirring speed effect
Stirring speed has a significant effect on the % conversion of CnOH to CnHO in presence of a solid catalyst. For the determination of optimum stirring speed, the reactions were carried out at different stirring speed (300–1500 rpm) keeping all the other reaction parameters constant. As depicted from Fig. 1bS, the conversion rate increases with the increase in the shaker speed that became steady at the stirring speed of 900 rpm and above. All subsequent experiments were therefore carried out at 900 rpm to exclude the probability of mass transfer. Similarly, stirring effect has also been evaluated by Dimitratos et al. (2009) and according to them the rate of stirring linearly increases the rate of reaction and maximum rate was achieved when the stirring speed was 500 rpm.
3.3.5 Catalyst loading study
Effect of catalyst amount on the percent conversion of CnOH to CnHO was investigated in the range 10–200 mg keeping all the parameters constant. An increased conversion was observed with the amount of catalyst loading without loss in selectivity. Maximum conversion rate with higher selectivity was obtained at catalyst amount of 0.1 g as shown in the Fig. 1cS. Further increase in the catalyst amount caused a decrease in the conversions along with negative effects on selectivity that may probably be due to deactivation of the nano catalyst surface by adsorption of oxidized products. Hammoumraoui et al. (2011) also investigated the effect of catalyst concentration in reaction mixture and found that the catalyst amount increased affect both the selectivity and conversion rate till an optimal value beyond which negative effect were observed.
3.3.6 Effect of solvents
Fig. 1dS shows the comparison of various solvent (water, acetonitrile, ethanol, n-hexane) media used in the conversion of CnOH to CnHO under optimized reaction conditions. It was observed that nature of solvent affect the CnOH to CnHO conversion rate in the following order; ethanol > acetonitrile > n-hexane > water. Butt (1965) obtained similar results and interpreted the thermal conductivity data for pore size, temperature and pressure effect. The solubility of oxygen in various solvents also effects the CnOH conversion. Sato et al. (2014) described the oxygen solubility in some common organic solvents (benzene < cyclohexane < heptane). High conversion in ethanol has been noted in the present study, which is due to their less solubility difference that has no significant effect on the CnOH to CnHO conversion. Ilyas and Sadiq (2007) also determined the effect of solvents and reported maximum conversion in case of n-heptane used as a solvent.
3.3.7 Effect of substitution on the % conversion of cinnamyl alcohol
Electron withdrawing (EWG) groups and Electron donating (EDG) groups favours the oxidation of CnOH to CnHO in all cases, while the % conversion depends upon the position of the EWD and EDG. The % conversion was maximum when the EDG were present at ortho and para position rather than at meta positions. The % conversion of the EDG substituted derivatives of cinnamyl alcohol follows the order; meta < ortho < para. Higashimoto et al. (2010) conducted a study where partially deprotonated benzylic carbon was stabilized by the resonance effect when the EDG were present at ortho and para position although such effect was not observed in case of meta substituted derivative of cinnamyl alcohol. EWG at different positions also affect the %conversion of reactant. EWG are deactivating and affecting the reactivity of both para and ortho positions by regaining the electron density from them. Therefore, when EWG are present at ortho and para positions, then the substituted cinnamyl alcohols becomes less reactive and % conversion decreases as compared to the meta substituted derivatives as shown in Table 2.
S.No
Substrate
Product
% Conversion
1
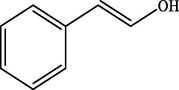
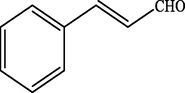
99.2
2
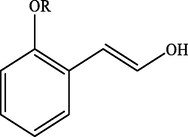
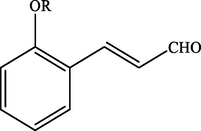
100
3
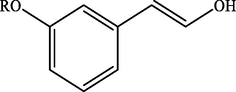
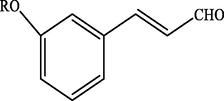
84
4
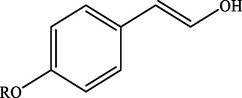
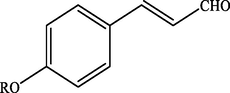
100
5
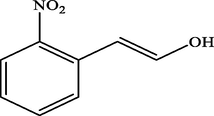
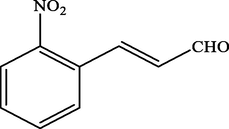
47
6
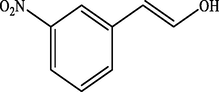
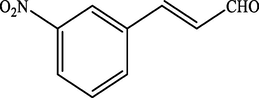
74
7
60
3.4 Leaching and regeneration of the catalyst
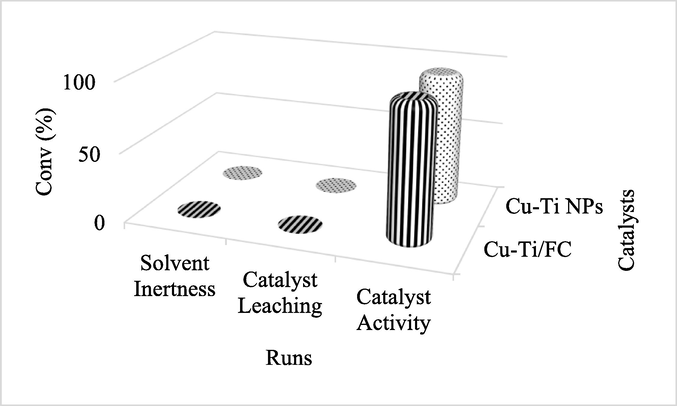
The catalyst was added into solvents media under optimal reaction parameters for an hour and analysed for any possible reaction. The single peak in the chromatograph confirmed the inertness of solvent. Leaching experiments were carried out to check the heterogeneity of the catalyst. CnOH was introduced to the filtrate then the reaction mixture was stirred under same experimental reaction conditions. No conversion suggested the absence of catalyst in filtrate pointing towards the true heterogeneous nature of Cu-Ti/F-CNTs in the applied solvents. When 0.1 g of Cu-Ti/F-CNTs nanoparticles were added to the reaction mixture under conditions, 99.2% conversion of CnOH occurred as shown in Fig. 5. Finally, the used nanoparticles were filtered and used as such for further 5 runs and observed very small change in the activity of the catalyst (washed with 1NHCl/TDW, dried for 2 h at 100 °C) under similar reaction conditions as shown in Fig. 6 were observed confirming the reusability and recyclability of the prepared catalyst.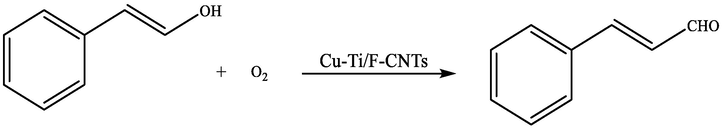
Catalyst leaching study in oxidation of cinnamyl alcohol to cinnamaldehyde.
Reusability of the Cu-Ti and Cu-Ti/F-CNTs catalysts for CnOH to CnHO oxidation.
4 Conclusion
In the current study, Cu-Ti/F-CNTs were synthesized, characterized and utilized as nano catalysts for the selective CnOH to CnHO oxidation applying mild reaction conditions using various solvents such as acetonitrile, ethanol, water and n-hexane. Optimal reaction conditions were achieved for efficient catalysis as; Catalyst amount of 0.1 g, CnOH conc; 1 mmol/10 mL ethanol, Time; 60 min, Temp; 70 °C, and Stirring speed; 900 rpm when Oxidant used was O2 (760 Torr). Maximum conversion of 99.2% with selectivity of 100% was achieved at optimal conditions using selox apparatus. The simple synthesis procedure, high catalytic performance with high conversion rate and selectivity, low cost, heterogeneous behaviour, multiple usage (5 times) makes these nanoparticles valuable for CnOH to CnHO oxidation. However, further studies are needed to effectively utilize the prepared catalyst.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2020/283) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbadi, Van Bekkum, H., 1995. Highly selective oxidation of aldonic acids to 2-keto-aldonic acids over Pt-Bi and Pt-Pb catalysts. Appl. Catal. A: Gen., 124, 409–417. https://doi.org/10.1016/0926-860X(94)00285-1.
- Future trends for green chemistry in the pharmaceutical industry. Green Chem. Pharm. Ind.. 2010;333–355
- [CrossRef] [Google Scholar]
- Selective photocatalytic oxidation of benzene to phenol using carbon nanotube (CNT)-supported Cu and TiO2 heterogeneous catalysts. J. Taiwan Inst. Chem. Eng.. 2018;82:331-341.
- [CrossRef] [Google Scholar]
- Solvent-free oxidation of benzyl Alcohol using Au–Pd catalysts prepared by sol immobilisation. PCCP. 2009;11:5142-5153.
- [CrossRef] [Google Scholar]
- Liquid phase aerobic oxidation of benzyl Alcohol by using manganese ferrite supported-manganese oxide nanocomposite catalyst. Catal. Commun.. 2017;89:56-59.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of CuO, TiO2, and CuO-TiO2 mixed oxide by a modified oxalate route. J. Appl. Chem.. 2017;2017
- [CrossRef] [Google Scholar]
- In situ EXAFS study of Pd/Al2O3 during aerobic oxidation of cinnamyl alCnOHol in an organic solvent. J. Catal.. 2003;213:291-295.
- [CrossRef] [Google Scholar]
- Selective oxidation of glycerol to dihydroxyacetone over Pt-Bi/C catalyst: optimization of catalyst and reaction conditions. Ind. Eng. Chem. Res.. 2010;49:10876-10882.
- [CrossRef] [Google Scholar]
- Catalytic oxidation of cyclohexane to cyclohexanone and cyclohexanol by tert-butyl hydroperoxide over Pt/oxide catalysts. Bull. Mater. Sci.. 2011;34:1127-1135.
- [CrossRef] [Google Scholar]
- Electrochemical reduction of carbon dioxide over CNTsupported nanoscale copper electrocatalysts. J. Nanomater.. 2014;2014:1-9.
- [CrossRef] [Google Scholar]
- Efficient and selective oxidation of benzylic alcohol by O2 into corresponding aldehydes on a TiO2 photocatalyst under visible light irradiation: Effect of phenyl-ring substitution on the photocatalytic activity. J. Catal.. 2010;274(1):76-83.
- [CrossRef] [Google Scholar]
- Liquid-phase aerobic oxidation of benzyl alcohol catalyzed by Pt/ZrO2. Chem. Eng. Technol.: Ind. Chem.-Plant Equipment-Process Eng.-Biotechnol.. 2007;30:1391-1397.
- [CrossRef] [Google Scholar]
- Abbadi, Van Bekkum, H., 1995. Highly selective oxidation of aldonic acids to 2-keto-aldonic acids over Pt-Bi and Pt-Pb catalysts. Appl. Catal. A: Gen. 124, 409–417. https://doi.org/10.1016/0926-860X(94)00285-1.
- Sol–gel deposition and characterization of multilayer 2% Cu doped TiO2 nano structured thin films. J. Mater. Sci.: Mater. Electron.. 2017;28:9471-9477.
- [CrossRef] [Google Scholar]
- Heterogeneous oxidation of allylic and benzylic alcohols catalyzed by Ru− Al− Mg hydrotalcites in the presence of molecular oxygen. J. Org. Chem.. 1998;63(6):1750-1751.
- [CrossRef] [Google Scholar]
- Selective oxidation of cinnamyl alCnOHol to cinnamaldehyde with air over Bi-Pt/alumina catalysts. J. Catal.. 1995;153:131-143.
- [CrossRef] [Google Scholar]
- Catalyst potential: a key for controlling alCnOHol oxidation in multiphase reactors. Catal. Today. 1995;24:143-150.
- [CrossRef] [Google Scholar]
- Partial oxidation of cinnamyl alCnOHol on bimetallic catalysts of improved resistance to self-poisoning. Stud. Surf. Sci. Catal.. 1994;561–570
- [CrossRef] [Google Scholar]
- Catalytic oxidation of cinnamyl alCnOHol to cinnamaldehyde using hydrogen peroxide. IRACST – Eng. Sci. Technol.: Int. J.. 2012;2:414-420.
- [Google Scholar]
- Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev.. 2012;41:3381-3430.
- [CrossRef] [Google Scholar]
- Efficiency of iron supported on porous material (prepared from peanut shell) for liquid phase aerobic oxidation of Alcohol. Mod. Res. Catal.. 2014;2014
- [CrossRef] [Google Scholar]
- Liquid-phase oxidation of Alcohol with oxygen catalysed by modified palladium (II) oxide. Catal. Commun.. 2003;4(8):417-422.
- [CrossRef] [Google Scholar]
- Liquid phase oxidation of cinnamyl Alcohol to cinnamaldehyde using multiwall carbon nanotubes decorated with zinc-manganese oxide NPs. Appl. Catal. A. 2010;539:97-103.
- [CrossRef] [Google Scholar]
- Solubility of oxygen in organic solvents and calculation of the Hansen solubility parameters of oxygen. Ind. Eng. Chem. Res.. 2014;53:19331-19337.
- [CrossRef] [Google Scholar]
- Platinum− bismuth-catalyzed oxidation of glycerol: Kinetics and the origin of selective deactivation. J. Phys. Chem. C. 2010;114:1164-1172.
- [CrossRef] [Google Scholar]
- The role of bismuth as promoter in Pd–Bi catalysts for the selective oxidation of glucose to gluconate. J. Mol. Catal. A: Chem.. 2002;180:141-159.
- [CrossRef] [Google Scholar]
- Oxidation of cinnamyl alcohol using bimetallic Au–Pd/TiO2 catalysts: a deactivation study in a continuous flow packed bed microreactor. Catal. Sci. Technol.. 2016;6:4749-4758.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.101273.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







