Translate this page into:
“From villain to hero”: Harnessing the gaseous grace of nitric oxide for prolonged elegance in Antirrhinum majus L. cut spikes
⁎Corresponding author. tahir.inayatullah@gmail.com (Inayatullah Tahir)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The current investigation meticulously examines the efficacy of Nitric Oxide (NO) on postharvest senescence in cut spikes of Antirrhinum majus. The spikes were harvested having two or more buds at one day before anthesis stage. The spikes underwent treatment with distinct concentrations of sodium nitroprusside (SNP) viz,10 μM, 20 μM and 30 μM, while a control group was immersed in distilled water. The findings illuminate a substantial delay in senescence in cut spikes of Antirrhinum majus when subjected to SNP treatment, resulting in a notably prolonged longevity. The study postulates that the augmented postharvest longevity attributed to SNP is intricately connected to the suppression of bacterial proliferation, improved uptake of solution and enhanced membrane integrity. Moreover, the SNP treatments induced a discernible elevation in the activities of antioxidant enzymes while concurrently diminishing lipoxygenase activity, thereby alleviating oxidative stress in the floral tissues. Furthermore, the treated spikes exhibited heightened levels of soluble proteins, total phenols and sugars fractions in the floral tissues compared to the control. The concentration of 20 µM SNP emerged as the most effective, ensuring a remarkable postharvest longevity of 18 days for the spikes. In contrast, the untreated control spikes exhibited early signs of senescence and endured for a mere 8 days. This research not only sheds light on the intricate interplay of NO in postharvest senescence but also underscores the nuanced effects of SNP concentrations on the vitality and longevity of Antirrhinum majus cut spikes.
Keywords
Nitric oxide
Oxidative stress
Bacterial density
Phenols
Membrane stability
1 Introduction
Senescence poses a significant challenge to the marketability of cut flowers. It is a genetically predetermined phenomenon, which signifies the concluding stage in the development of flowers. During this stage, a series of irreversible events are set in motion, ultimately resulting in cellular breakdown and the demise of the organ. The progression of senescence is choreographed by fluctuations in the concentrations of growth regulators and their interactions serve as regulatory cues for the initiation or termination of particular reactions. (Lone et al., 2021). Various biochemical and physiological indicators influencing senescence process encompass memebrane integrity, respiratory activity, uptake of water, sugar fractions and protein content. Additionally, the antioxidant enzyme activity of floral tissues play a crucial role in this process (Hemati et al., 2019). The visual appeal and duration in vases are pivotal postharvest attributes of flowers essential for their marketability and satisfaction of customers (Haq et al., 2023). Postharvest longevity in flowers can be extended through diverse methods such as genetic mutations of specific genes, transgenic technology and the application of postharvest treatments. Nonetheless, employing postharvest treatments offers a convenient and quick approach to delaying senescence in cut flowers (Haq et al., 2023). Hence, there is a critical need for meticulous attention to optimize postharvest treatments that enhance flower longevity. Transitioning from a perceived antagonist to a protagonist and now regarded as new superstar of biochemistry, Nitric oxide (NO) has emerged as a groundbreaking molecule that governs a wide range of biological processes in both the animal and plant realms (Verma et al., 2020). NO performs a pivotal function in a diverse array of physiological and developmental processes, participating in various mechanisms intricately associated with the senescence of flowers (Hasanuzzaman et al., 2016). Previous studies substantiate anti-senescencent role of Nitric oxide (NO) in different flower systems, positioning it as an innovative alternative to potentially hazardous substances such as silver thiosulphate (STS) in postharvest studies (Deng et al., 2019). The application of exogenous NO has demonstrated a remarkable increase in the vase performance of different cut flowers like rose (Deng et al., 2019). Sodium nitroprusside (SNP) plays a role in inhibiting ethylene output, a key trigger for senescence that restricts the display life of ornamental plants (Naing et al., 2017a). Exogenous inclusion of Nitric Oxide (NO) facilitates the accumulation of compatible solutes, contributing to the stabilization of macromolecules, providing thermal stability to enzymes, and preventing the misfolding of proteins and the dissociation of enzyme complexes (Haq et al., 2021). Additionally, SNP mitigates the peroxidation of membrane lipids and maintains optimal hydration of petal tissues, thereby preventing membrane outpouring and petal wilting (Hassan et al., 2020). Apart from its anti-senescent properties, sodium nitroprusside (SNP) functions as an antibacterial antidote, effectively curbing microbial proliferation. Notably, in Consolida cut flowers, solutions containing SNP demonstrated the least bacterial growth, contributing to a prolonged vase life (Haq et al., 2021). Antirrhinum majus commonly known as snapdragons, are favored ornamental plants appreciated for their varied petal hues and pleasing fragrance, rendering them a splendid choice for creating cut flower arrangements. However, the longevity of A.majus cut spikes is comparatively brief due to incomplete opening of flowers, abscission of petals, color bleaching and bending of spike (Farooq et al., 2022). This limited postharvest longevity poses a substantial postharvest concern for cut snapdragon flowers, presenting challenges and risks for both flower producers and consumers (Farooq et al., 2022).Therefore, the extension of the postharvest longevity of A. majus becomes paramount for preserving its intrinsic qualities and ensuring its prolonged marketability. Thus, recognizing the multifaceted implications of SNP in modulation of postharvest senescence, the present study was undertaken to elucidate the effectiveness of SNP in alleviating postharvest senescence symptoms in A. majus cut spikes, ultimately enhancing their elegance.
2 Materials and methods
2.1 Experimental setup
Uniform and healthy spikes of Antirrhinum majus from the experimental plots situated within the Kashmir University Botanical Garden (KUBG) were collected during the morning hours. These selected spikes were characterized by having the most mature bud at one day before anthesis stage (stage III). To preserve their freshness, the spikes were promptly immersed in distilled water and transported to the laboratory. Each spike was then trimmed to a standardized length of 30 cm to ensure uniform spike length. Subsequently, the spikes were divided into four sets, with each set comprising 10 flasks, and two spikes were placed within each flask. Test solutions of Sodium nitroprusside (SNP) were prepared carefully, with concentrations of 10 μM, 20 μM and 30 μM following rigorous standardization protocols. A control group consisting of 10 flasks with spikes immersed in distilled water was established to serve as a reference for comparative analysis. The first three sets of spikes were pulsed with 10, 20 and 30 μM of SNP for 24 h and then these pulsed spikes were transferred to distilled water containing flasks. The experiment was conducted under controlled conditions, encompassing a relative humidity of 60 ± 10 %, a consistent 12- hour light cycle and an average temperature of 23 ± 2 °C. The day on which the spikes were transferred to the test solutions was designated as day zero (D0). On day 2 and 6, comprehensive evaluations were conducted, encompassing a wide array of parameters, including vaselife, floral diameter, membrane stability index, soluble proteins, total phenols, sugar fractions, membrane stability index, soluble proteins, total phenols, sugar fractions (total, reducing and non-reducing), solution uptake, bacterial density, ethylene content, catalase activity, ascorbate peroxidase activity, superoxide dismutase activity and lipoxygenase activity.
2.2 Vaselife and floral diameter
The evaluation of the vase life of cut A. majus spikes involved visual monitoring of senescence indicators such as wilting and tepal abscission. The average vase life was determined by recording the number of days from the transfer of the spike until 70 % of the florets exhibited senescence on each spike. Additionally, floral diameter measurements were taken on day 2 and day 6 post-spike transfer, with the measurement involving the calculation of the average of two perpendicular distances across a flower.
2.3 Bacterial density and solution uptake
The assessment of bacterial density was carried out by measuring the optical density of 1 ml of solution from each treatment including control at 600 nm using spectrophotometer. The E. coli was taken as reference organism (where 1 OD was equivalent to 1x108 CFU/ml). Solution uptake was determined by calculating the difference between the volume of the vase solution at the end of the experiment and the initial volume of the vase solution (Naing et al., 2017b).
2.4 Ethylene measurement
Ethylene was quantified by employing Whitehead et al. (1992) protocol. The flower petals at different developmental stages of A.majus were collected and kept in 3 glass vials sealed tightly with parafilm. After a 2 h incubation, 1 ml of the gaseous mixture was extracted from each vial and injected into gas chromatograph (Nucon model 5700) fitted with a flame ionization detector. The concentration of ethylene was assessed using a calibration curve derived from known ethylene standard.
2.5 Membrane stability index (MSI)
The assessment of Membrane Stability Index (MSI) involved measuring solute leakage from petal tissues. This was determined by incubating 100 mg of petal tissue in 5 ml of deionized water at 25 °C for 30 min and subsequently at 100 °C for 15 min, following the methodology described by Sairam, 1994. The MSI was calculated using the formula: Here, C1 denotes the conductivity of the samples after incubation at 25 °C, and C2 denotes the conductivity at 100 °C. The conductivity values were recorded using the Elico CM180 Conductivity meter.
2.6 Quantification of soluble proteins
To estimate protein content, 1 g of petal tissue was crushed in a 100 mM phosphate buffer with a pH of 7.2, comprising 10 % polyvinyl pyrrolidone (PVP), 1 mM EDTA, 150 mM NaCl, 10 % glycerol, 1 % Triton X-100, and 1 mM Dithiothreitol (DTT). The resulting mixture underwent centrifugation in a refrigerated centrifuge at 12,000xg and 5 °C for 15 min. Protein estimation was carried out using a portion of the collected supernatant, following the method outlined by Lowry et al., 1951.
2.7 Quantification of phenols and sugars
To determine sugar fractions and phenols, 1 g of chopped petal tissue from each treatment was immersed in hot 70 % ethanol, macerated, and subjected to three rounds of centrifugation. From an appropriate portion of the resulting supernatant, total phenols, reducing sugars, non-reducing sugars, and total sugars were assessed. The quantification of total phenolics followed the method outlined by Swain and Hillis in 1959, utilizing gallic acid as a standard. Nelson's method from 1944 was applied to estimate reducing sugars, with glucose serving as the standard. To determine total sugars, non-reducing sugars were converted to reducing sugars using invertase. The quantity of non-reducing sugars was calculated as the difference between total and reducing sugars.
2.8 Extraction and assessment of enzyme Activity
2.8.1 Superoxide dismutase (SOD) activity
The activity of superoxide dismutase was assessed following the procedure outlined by Dhindsa et al. (1981), which involves monitoring the suppression of the photochemical reduction of nitroblue tetrazolium (NBT). The SOD activity was quantified as units min−1 mg−1 protein, by taking absorbance at 560 nm.
2.8.2 Catalase activity (CAT)
The assessment of catalase activity followed the procedure outlined by Aebi (1984) and was quantified as μM H2O2 red. min-1 mg−1 protein.
2.8.3 Ascorbate peroxidase activity (APX)
The ascorbate peroxidase activity was assessed by a method of Chen and Asada (1989) and the enzyme activity was expressed as μmol min−1 mg−1 protein.
2.8.4 Lipoxygenase activity (LOX)
The activity of Lipoxygenase enzyme was assessed following by following a protocol outlined by Axerold et al. (1981), and the units were quantified as μmol min-1mg−1 protein.
2.9 Experimental design and statistical analysis
The completely randomized experimental design was followed during the study and statistical significance between individual treatments was determined through Duncan’s test at P < 0.05 and each value represents a mean of three replicates.
3 Results
3.1 Vaselife
Flower senescence in A. majus was characterized by incomplete flower opening, pigmentation suppression, spike bending and ultimately petal abscission. The introduction of sodium nitroprusside (SNP) into the preservative solutions led to a remarkable extension in the vaselife of the excised spikes in comparison to the control as shown in Fig. 1. The application of SNP demonstrated a significant enhancement in vaselife, with the most substantial improvement observed at 20 µM. Nevertheless, a gradual reduction in longevity was observed beyond this concentration. The treated spikes exhibited a lifespan of 11, 18 and 13 days for 10 µM, 20 µM and 30 µM SNP respectively. It is noteworthy that all SNP treatments significantly prolonged vaselife compared to the control, having vaselife of only 8 days (Fig. 2A).
Photograph showing the effect of various standardized concentrations of SNP on postharvest longevity in cut pikes of A. majus.
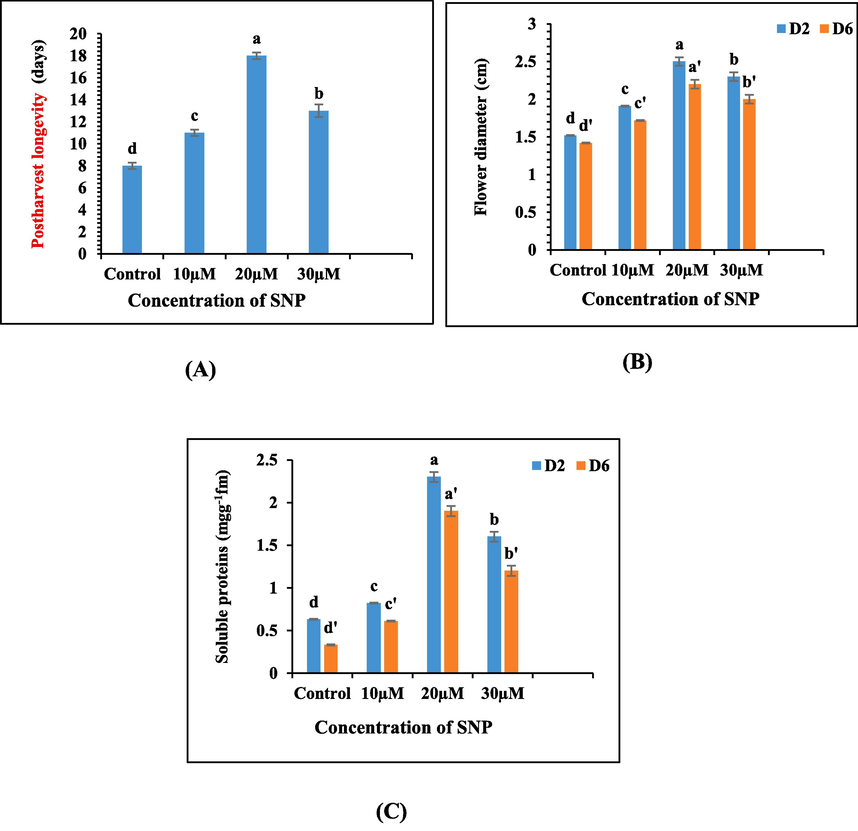
(A-C): Graphs illustrating the impact of varying concentrations of SNP on the duration of postharvest longevity, floral diameter and content of soluble proteins in the floral tissues of A. majus cut spikes.
3.2 Floral diameter
Regular assessments conducted on day 2 revealed a notable expansion in floral diameter among the groups treated with sodium nitroprusside (SNP), particularly at a concentration of 20 µM, in contrast to the control group. However, as the experiment progressed towards day 6, a significant reduction in floral diameter became apparent, with the control displaying the most pronounced decrease compared to the flowers treated with SNP (Fig. 2B).
3.3 Soluble proteins
In our study, soluble protein content in petal tissues was assessed following exposure to various concentrations of sodium nitroprusside (SNP) at regular intervals. Initially, an elevation in SNP concentration corresponded to an upsurge in protein content, reaching its peak at 20 µM SNP. However, as the SNP concentration surpassed this threshold, there was a subsequent decline in soluble protein content. On day 6, protein content decreased across all treatment groups, regardless of the specific treatment applied. The control group exhibited the most significant decrease, whereas the spikes treated with SNP test solutions showed a relatively smaller reduction in soluble protein content (Fig. 2C).
3.4 Total phenols
The cut spikes immersed in sodium nitroprusside (SNP) test solutions exhibited a conspicuous enrichment of phenolic compounds in their petal tissues when compared to the control. The most substantial surge in phenolic concentration was discerned in the floral buds treated with 20 µM SNP, followed by those treated with 30 µM SNP surpassing the phenolic content of other tested concentrations as well as the control group. Significantly, phenolic content displayed a temporal decline as time advanced, with the most pronounced decrease observed in the untreated spikes (Fig. 3A).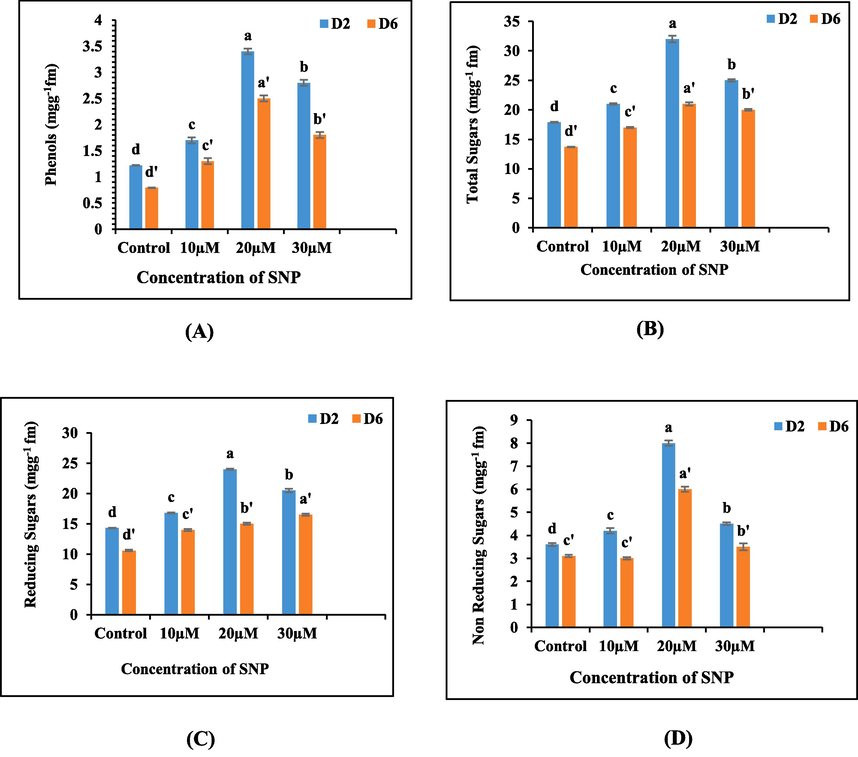
(A-D): Graphs illustrating the impact of varying concentrations of SNP on (A) phenols, (B) total sugars (C) reducing sugars and (D) non-reducing sugars in the floral tissues of cut spikes of A. majus.
3.5 Sugars fractions (Total, reducing and non-reducing)
The application of sodium nitroprusside (SNP) treatments caused considerable augmentation in the content of total, reducing and non-reducing sugars compared to the control. Intriguingly, on day 2 the cut spikes subjected to 20 µM SNP treatments displayed the highest concentrations of total, reducing and non-reducing sugar components. These findings underscore the overall elevation in sugar content within the petal tissues of floral buds treated with SNP. Nevertheless, as the experiment progressed to day 6, a declining trend in the sugar fractions was witnessed across all treatment groups, including the control Fig. 3 (B-D).
3.6 Membrane stability index (MSI)
The membrane stability index (MSI) of the excised spikes experienced a remarkable enhancement when subjected to sodium nitroprusside (SNP) treatments, surpassing the MSI values of the control group. The most pronounced augmentation in MSI was evident at concentrations of 20 µM and 30 µM SNP, signifying the notable influence of SNP treatments on preserving membrane integrity. However, at day 6, a steep descent in MSI values was observed across all sets of treatments under observation, with the most substantial decrease observed in the untreated spikes (Fig. 4A).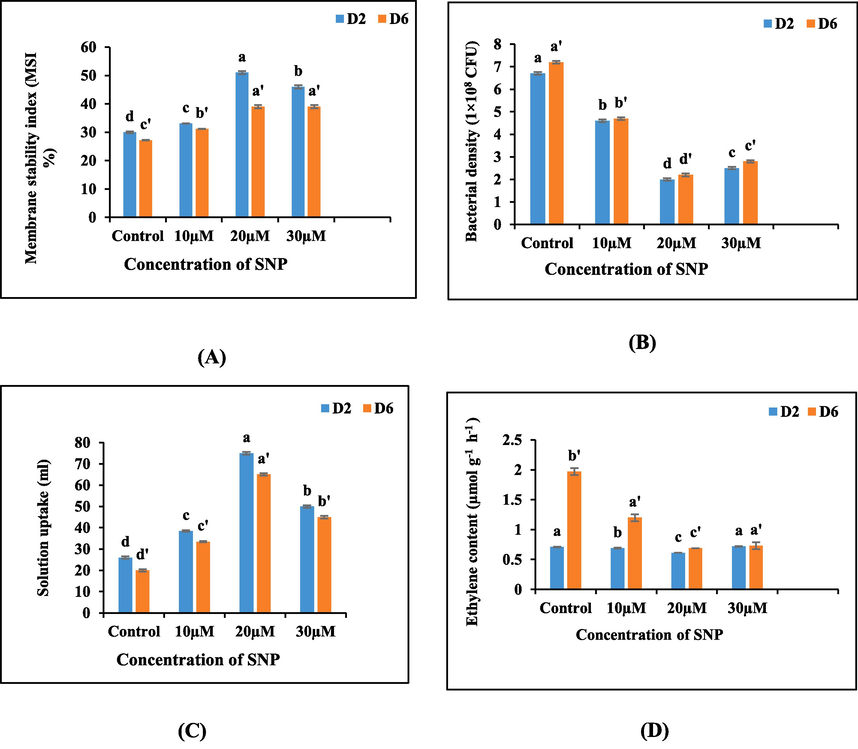
(A-D): Graphs illustrating the impact of varying concentrations of SNP on (A) membrane stability index, (B) bacterial density, (C) solution uptake, and (D) ethylene content in the petal tissues of cut spikes of A. majus.
3.7 Bacterial density and solution uptake
The utilization of various concentrations of sodium nitroprusside (SNP) exerted a dual impact on both solution uptake and bacterial density, establishing a unique interplay between these two factors. The pulsing of SNP led to a reduction in bacterial density when compared to the control group, ultimately resulting in an augmented uptake of the solution. Specifically, the concentration of 20 µM SNP coincided with the lowest bacterial density, concomitant with the highest degree of solution uptake. However, as the experiment proceeded to day 6, all treatments including the control group experienced a substantial elevation in bacterial density, coinciding with the lowest level of solution uptake (Fig. 4B,4C).
3.8 Ethylene content
The incorporation of sodium nitroprusside (SNP) treatments led to a conspicuous attenuation of ethylene production within the excised spikes in comparison to the control. The most substantial decline in ethylene synthesis was witnessed in the spikes exposed to 20 µM SNP. Nonetheless, as the experiment advanced, an escalation in ethylene production was observed across all treatment groups including the control group, signifying a diminishing efficacy of SNP over time (Fig. 4D).
3.9 Catalase (CAT) activity
The ongoing investigation unveiled a noteworthy augmentation in catalase activity within the floral buds of the excised spikes treated with sodium nitroprusside (SNP) in comparison to those treated with distilled water. Specifically, the highest catalase activity was detected in the floral buds exposed to 20 µM SNP, surpassing the activity levels of other concentrations as well as the control group. However, as the study progressed, a gradual descent in catalase activity was observed in all experimental groups, including the control, indicating a diminishing trend in catalase function over time (Fig. 5A).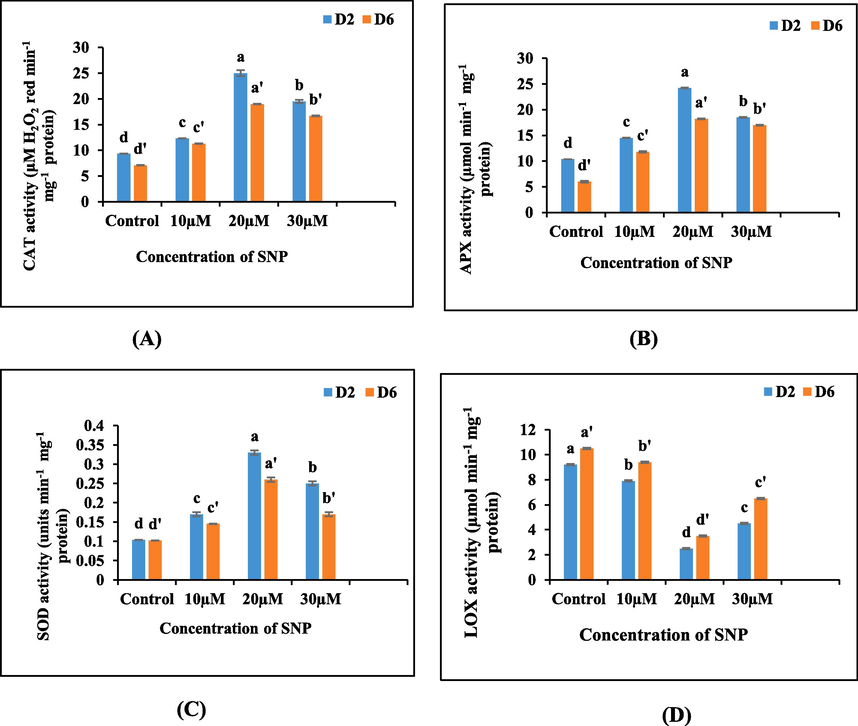
(A-D): Graphs illustrating the impact of varying concentrations of SNP on the (A) catalase, (B) ascorbate peroxidase, (C) superoxide dismutase and (D) lipoxygenase activity in the petal tissues of cut spikes of Antirrhinum majus.
3.10 Ascorbate peroxidase (APX) activity
The application of sodium nitroprusside (SNP) treatments resulted in a significant augmentation APX activity in the petal tissues. The APX activity increased with the application of SNP, reaching its zenith at 20 µM and beyond this concentration, a precipitous decline in APX activity became apparent. As the study progressed, there was an overall decline in APX activity in all the treatments and the control, signifying a reversal of the initial increase and indicating a decline in APX activity over time (Fig. 5B).
3.11 Superoxide dismutase (SOD) activity
The application of sodium nitroprusside (SNP) treatments led to a gradual augmentation in superoxide dismutase (SOD) activity within the cut spikes compared to the untreated ones. Moreover, the spikes treated with a lower concentration of SNP (20 µM) exhibited the highest SOD activity among the various SNP treatments employed. However, as the study progressed, a decline in activity of SOD was noted across all treatment groups, with the untreated spikes experiencing the most significant decrease (Fig. 5C).
3.12 Lipoxygenase (LOX) activity
The utilization of sodium nitroprusside (SNP) exerted a pronounced influence on the lipoxygenase (LOX) activity of the excised spikes. These findings showcased a substantial reduction in LOX activity following the administration of SNP treatments, particularly at a concentration of 20 µM SNP, where the lowest levels of activity were noted. Furthermore, irrespective of the specific treatment, there was a consistent upward trajectory in LOX activity with the passage of time across all experimental groups (Fig. 5D).
4 Discussion
The extended postharvest viability of cut flowers poses a significant challenge to the advancement and growth of the floriculture industry (Hussen and Yassin, 2013). Cut flowers serve as a convenient and practical experimental model for studying the process of flower senescence. Detaching cut flowers from their parent plants results in the cessation of a continuous supply of nutrients and water, as reported by Patel et al. in 2018. Consequently, inclusion of postharvest treatments becomes crucial to enhance their longevity (Nair et al., 2006). In this investigation, exogenous inclusion of 20 μM sodium nitroprusside (SNP) was determined to have a positive impact on the postharvest life and floral dimensions of A.majus cut spikes. These findings align with outcomes observed in gerbera cut flowers (Liu et al., 2009). The extension of vase life through SNP is attributed to an improved water balance achieved by reducing transpiration and enhancing solution uptake, as noted by Hemati et al. (2019). The increased solution uptake is rationalized by the germicidal characteristics of SNP, preventing vascular blockage by suppressing bacterial proliferation (Naing et al. in 2017b). Earlier investigations have demonstrated that SNP contributes to the prolonged longevity of various flowers by reducing ethylene output and downregulating senescence-inducing genes (Naing et al., 2017a). Furthermore, the increase in floral diameter in SNP-treated flowers could be associated with the enhancement of sugar content in tepal tissues. This sugar enrichment ensures the turgidity of flowers through endosmosis, resulting in a subsequent rise in floral diameter. (Reid, 2003).
Our findings indicate that spikes treated with SNP retained higher levels of proteins as compared to untreated group. The protein enrichment within petal tissues can be linked with decreased in protease activity in flowers treated with sodium nitroprusside (SNP), as recognized in Gladiolus cut flowers (Dwivedi et al., 2016). Moreover, SNP enhances the synthesis of proteins, mRNA and the activity of antioxidant enzymes, and thus alleviate flower senescence (Zeng et al., 2011). Decreased protease activity functions as a preventive measure against protein breakdown, leading to an increase in protein accumulation within petal tissues. This occurrence is simultaneously linked to a reduction in α-amino acids, as reported by Pak and Van Doorn in 2005. The phenomenon of color bleaching is a critical factor affecting the market viability of cut flowers and invariably resulting in termination of postharvest longevity across various floral varieties (Salehi Salmi et al., 2018). Pertinently, petals attain their coloration through the buildup of phenolic compounds, facilitating the synthesis of a variety of pigments within epidermal cells (Tanaka et al.,2008). During the current investigation, tepal tissues treated with SNP exhibited a higher concentration of phenols, as reported by earlier studies in Dianthus (Naing et al., 2017b). The increase in phenolic content observed after SNP treatments can be associated with the heightened expression of phenylalanine ammonia lyase (PAL), responsible for the synthesis of the phenolic compounds (Naing et al., 2017a.) This upregulation of PAL serves as a protective response against oxidative stress, ultimately resulting in the postponement of the initiation of senescence (Ahmadi Soleimanie et al., 2020).
Sugars act as crucial metabolites in maintaining the flower quality, serving as the primary energy source in the cut flower metabolism (Zuliana et al., 2008). Typically, sugar content tends to decrease with senescence due to oxidative processes (Cavasini et al., 2018). However, treatments with sodium nitroprusside (SNP) led to a sugar enrichment in floral tissues. Nitric oxide (NO) has been reported to increase sugar concentration in floral tissues of different cut flowers including gerbera contributing significantly to the extended longevity of these flowers (Hemati et al., 2019). Sugars serve twofold purpose in augmenting flower quality. They bolster the relative moisture content as well as foster the production of anthocyanins, pivotal for petal pigmentation, thereby, enhancing the longevity and aesthetic allure of the flowers (Hemati et al., 2019). Additionally, sugars function as osmoprotectants, regulating osmotic fluctuations, protecting membranes, and mitigating ROS induced damage during stress (Singh et al., 2015).
The loss of membrane integrity adversely affects the flower longevity, serving as a critical indicator that signals the initiation of senescence (Khandan-Mirkohi et al., 2021). Our research indicated elevated membrane stability index (MSI) values in petal tissues treated with SNP compared to the control. These values were associated with a reduction in Lipoxygenase (LOX) activity. Lipoxygenase (LOX) oxidizes fatty acids and triggers the onset of senescence (Shabanian et al., 2019). Conversely, treatments involving SNP effectively curbed fatty acid oxidation by attenuating LOX activity in floral tissues. SNP induces a conformational change in LOX enzymes, transitioning them from the active Fe 2+ (ferric) form to the inactive Fe3+ (ferrous) form rendering it inactive (Zhu et al., 2006).
The postharvest quality of flowers is significantly influenced by oxidative stress, which affects key physiological processes and triggers the onset of petal senescence (Arora et al., 2007). Floral tissues exhibiting reduced activity of antioxidant enzymes, display early senescence symptoms due to accumulation of ROS (Saeed et al., 2014). Antioxidant enzymes act as key ROS scavengers and consequently delaying senescence instigation (Cavaiuolo et al., 2013). Conversely, a decrease in antioxidant enzymes weakens the antioxidant system, initiating senescence, as reported in Freesia by Shu et al. in 2010. In the current study, floral tissues treated with sodium nitroprusside (SNP) exhibited a noticeable upsurge in SOD, CAT and APX activity. These findings suggest that SNP contributes to strengthening the antioxidant response to counteract the detrimental effects of ROS as observed in Gladiolus and Brassica (Kazemzadeh-Beneh et al., 2018).
5 Conclusions
The preceding findings and discussions underscore the considerable potential of sodium nitroprusside (SNP) in mitigating postharvest senescence in A. majus cut spikes by orchestrating antioxidant and biochemical mechanisms. Our study proposes 20 μM SNP as a suitable postharvest treatment for preserving the quality of cut spikes of A.majus. Our research confirms the multifunctional role of SNP in alleviating postharvest senescence in A. majus cut spikes and offers valuable insights into the fundamental physiological responses linked to SNP concerning flower senescence, which can potentially revolutionize the postharvest practices within the floriculture industry.
6 Consent to participate
All authors consent to participate in the manuscript publication
7 Consent for publication
All authors approved the manuscript to be published
8 Data availability statement
The raw data is available when requested from the author.
Ethics approval
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
SF, AUQ and MLL drafted the experimental design and SF performed the experiments. WW, SP, and FA analyzed the data and helped in manuscript writing. IT finalized the draft and supervised the experiment. AAA, helped in the statical analysis of the data. All authors read this manuscript before submission.
Credit authorship contribution statement
Sumira Farooq: Writing – original draft. Aehsan Ul Haq: Methodology. Mohammad Lateef Lone: Methodology. Wajahat waseem: Data curation, Writing – review & editing. Shazia Parveen: Data curation, Writing – review & editing. Foziya Altaf: Data curation, Writing – review & editing. Inayatullah Tahir: Supervision, Writing – review & editing. Abdulaziz Abdullah Alsahli: Formal analysis.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R236) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preharvest application of sodium nitroprusside improves tomato fruit quality and alleviates chilling injury during cold storage. Int. J. Veg. Sci.. 2020;26:364-378.
- [Google Scholar]
- Oxidative stress mechanisms during flower senescence. Plant Stress. 2007;1:157-172.
- [Google Scholar]
- Lipoxygenase from soybean. In: Lowenstein J.M., ed. Methods Enzymol. New York: Academic Press; 1981. p. :441-451.
- [Google Scholar]
- The antioxidants changes in ornamental flowers during development and senescence. Antioxidants. 2013;2:132-155.
- [Google Scholar]
- Carbohydrate reserves on postharvest of lisianthus cut flowers. Orn. Hort.. 2018;24:12-18.
- [Google Scholar]
- Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol.. 1989;30:987-998.
- [Google Scholar]
- The involvement of NO in ABA-delayed the senescence of cut roses by maintaining water content and antioxidant enzymes activity. Sci. Hortic.. 2019;247:35-41.
- [Google Scholar]
- Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot.. 1981;32:93-101.
- [Google Scholar]
- Effect of sodium nitroprusside on differential activity of antioxidants and expression of SAGs in relation to vase life of gladiolus cut flowers. Sci. Hortic.. 2016;210:158-165.
- [Google Scholar]
- Farooq, S., ul Haq, A., Lone, M.L., Parveen, S., Altaf, F., & Tahir, I. (2022). Salicylic Acid Enhances the Vase Life and Improves the Postharvest Attributes—A Case Study of Antirrhinum majus L.
- Haq, A.U., Lone, M.L., Farooq, S., Parveen, S., Altaf, F., Tahir, I., et al. (2021). Nitric oxide effectively orchestrates postharvest flower senescence: a case study of Consolida ajacis. Funct. Plant Biol., 1.
- Flower Senescence Coordinated by Ethylene: An Update and Future Scope on Postharvest Biology in the “Buttercup” Family. J. Plant Growth Regul. 2023:1-21.
- [Google Scholar]
- Nitric oxide: a Jack of all trades for drought stress tolerance in plants. In: Ahmad P., ed. Water Stress Crop Plants, 1. West Sussex: Wiley; 2016. p. :628-648.
- [Google Scholar]
- Involvement of ethylene synthetic inhibitors in regulating the senescence of cut carnations through membrane integrity maintenance. J. Hortic. Res.. 2020;28:39-48.
- [Google Scholar]
- The roles of sodium nitroprusside, salicylic acid and methyl jasmonate as hold solutions on vase life of Gerbera jamesonii ‘Sun Spot’. Adv. Hortic. Sci.. 2019;33:187-195.
- [Google Scholar]
- Review on the impact of different vase solutions on the postharvest life of rose flower. Int. J. Agric. Res. Rev.. 2013;1:13-17.
- [Google Scholar]
- Biochemical, physiological changes and antioxidant responses of cut gladiolus flower 'White Prosperity' induced by nitric oxide. Adv. Hortic. Sci.. 2018;32:421-431.
- [Google Scholar]
- Effects of salicylic acid and humic material preharvest treatments on postharvest physiological properties of statice cut flowers. Sci. Hortic.. 2021;283:110009
- [CrossRef] [Google Scholar]
- Nanosilver pulse treatments inhibit stem-end bacteria on cut gerbera cv. Ruikou flowers. Postharvest Biol. Technol.. 2009;54:59-62.
- [Google Scholar]
- Nitric oxide effectively curtails neck bending and mitigates senescence in isolated flowers of Calendula officinalis L. Physiol. Mol. Biol. Plants. 2021;27:835-845.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Characterization of the role of sodium nitroprusside (SNP) involved in long vase life of different carnation cultivars. BMC Plant Biol.. 2017;17:149.
- [Google Scholar]
- Involvement of sodium nitroprusside (SNP) in the mechanism that delays stem bending of different gerbera cultivars. Front. Plant Sci.. 2017;8:2045.
- [Google Scholar]
- Effect of chemical preservatives on enhancing vase-life of gerbera flowers. J. Trop. Agric.. 2006;41:56-58.
- [Google Scholar]
- A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem.. 1944;153:375-380.
- [Google Scholar]
- Delay of Iris flower senescence by protease inhibitors. New Phytol.. 2005;165:473-480.
- [Google Scholar]
- Patel, D.K., S.L., C., V., G.N. (2018). Effect of botanicals on vase life of cut flowers: a review. Bull. Environ. Pharmacol. Life Sci., 8, 01–08.
- Reid, M.S. (2003). Flower development: from bud to bloom. In ‘VIII Int. Symp. Postharvest Physiol. Ornamental Plants 669’. pp. 105–110. (ISHS: Belgium).
- Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul.. 2014;72(1):89-95.
- [Google Scholar]
- Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol.. 1994;32:584-593.
- [Google Scholar]
- Extending vase life of cut rose (Rosa hybrida L.) cv. Bacara by essential oils. Adv. Hortic. Sci.. 2018;32:61-69.
- [Google Scholar]
- Salicylic acid modulates cutting-induced physiological and biochemical responses to delay senescence in two gerbera cultivars. Plant Growth Regul.. 2019;87(2):245-256.
- [Google Scholar]
- Distinct respiration and physiological changes during flower development and senescence in two Freesia cultivars. HortScience. 2010;45:1088-1092.
- [Google Scholar]
- Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev. Environ. Sci. Biotechnol.. 2015;14:407-426.
- [Google Scholar]
- The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J. Sci. Food Agric.. 1959;10(1):63-68.
- [Google Scholar]
- Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J.. 2008;54:733-749.
- [Google Scholar]
- Nitric oxide in plants: an ancient molecule with new tasks. Plant Growth Regul.. 2020;90(1):1-13.
- [Google Scholar]
- Ethylene sensitivity in germinating peanut seeds: the effect of short-chain saturated fatty acids. J. Plant Physiol.. 1992;139(4):479-483.
- [Google Scholar]
- The physiological responses of carnation cut flowers to exogenous nitric oxide. Sci. Hortic.. 2011;127:424-430.
- [Google Scholar]
- Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol. Technol.. 2006;42:41-48.
- [Google Scholar]
- Effects of aminooxyacetic acid and sugar on the longevity of pollinated Dendrobium pompadour. Asian J. Plant Sci.. 2008;7:654-659.
- [Google Scholar]







