Translate this page into:
First record of iridovirus (ISKNV) infections in Fourfinger threadfin from Kuwait
⁎Corresponding author. azadis@hotmail.com (I.S. Azad) aismail@kisr.edu.kw (I.S. Azad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Following an initiative by the Govt. of Kuwait and the Kuwait Institute for Scientific Research (KISR), the institute started simultaneous efforts of domestication of locally caught fish and hatchery production of Fourfinger threadfin (FFT, Eleutheronema tetradactylum), locally called Sheem. A private farm and KISR are working together to optimize the conditions for growth and spawning performances under the environmental conditions of Kuwait. During these efforts, infectious spleen and kidney necrosis virus (ISKNV), an important fish pathogen from Iridoviridae, caused mortalities of the FFT sub-adults (150–200 g). We noticed that the mortality was associated with anorexia, haemorrhages of the snout, fin bases, and scale pockets. We also noticed that this was typical to one of the tanks that had consistently high ammonia (0.2–0.25 ppm) levels in the water, and all 35 sub-adults died within 4–5 days. Diagnostics (molecular and electron microscopy examinations) revealed ISKN iridovirus as the causative. We noticed viral particles of 150–200 nm in the tissue sections of the skin, gills, heart, liver, spleen, kidney, and intestine. The major capsid protein (MCP) gene sequence analysis confirmed the results, revealing 98 % similarity with the laminin-type epidermal growth factor-like protein gene of ISKNV. The present report is the first record of ISKNV infections in FFT and from Kuwait. The study aimed to decipher the cause of illness and mortality of cultured FFT.
Keywords
Sheem
Domestication
Infection
Iridovirus
ISKNV
- KISR
-
Kuwait Institute for Scientific Research
- KFAS
-
Kuwait Foundation for Advancement of Science
- ISKNV
-
Infectious Spleen and Kidney Necrosis Virus
- MCP
-
Major Capsid Protein
- PCR
-
Polymerase Chain Reaction
- RAS
-
Recirculatory Aquaculture System
- WOAH
-
World Organization for Animal Health
- BHI
-
Brain Heart Infusion
- TCBS
-
Thiosulphate Citrate Bile salt Sucrose
- RNRS
-
Ribonuclease Reductase Small sub-unit
- TEM
-
Transmission Electron Microscope
- CEV
-
Carp Edema Virus
- NCBI
-
National Centre for Biotechnology Information
- LTEGFP
-
Laminin Type Epidermal Growth Factor Protein
- DNA
-
Deoxyribose Nucleic Acid
- RNA
-
Ribose Nucleic Acid
- FFT
-
Fourfinger Threadfin
Abbreviations
Data availability
Data will be made available on request.
1 Introduction
Aquaculture, in general, is still in its infancy in the Gulf Cooperation Council member countries. Though Kuwait started aquaculture research through its Mariculture Department of KISR in the early seventies, commercial aquaculture is largely restricted to tilapia farming in the agriculture-aquaculture farming units in the Wafra region of Kuwait. The early 80s saw some efforts of cage culture in the marine environment and land-based mariculture by a private company to culture the European sea bream (Sparus aurata) and the local sobaity bream (Sparidentax hasta). However, after the Iraqi invasion, a few aquaculture entrepreneurs ventured into land-based aquaculture with Asian seabass and as a joint effort with the KISR mariculture unit with the FFT.
Fourfinger threadfin, locally known as Sheem, is a much-valued and preferred fish in the region. This fish species is available in the territorial waters of Kuwait. However, it is fast becoming scanty in Kuwait's Fishery. The Govt. of Kuwait and the KISR started an initiative for the propagation and fishery restoration efforts in 2021. Collection and domestication of the local species from Kuwait Bay began in 2021. To provide a jump-start for the research and development activity of spawning and rearing locally captured FFT, just hatched FFT larvae were procured in November 2021 from Singapore. The batch of larvae that arrived at KISR was negative for iridovirus and viral nerve necrosis through PCR diagnostics (data not shown here). The larvae were grown in land-based re-circulatory aquaculture systems (RAS). Commercial aquaculture of FFT is yet to establish itself, though scanty reports of growing this fish are available (Abu Hena et al 2011). The KISR and a private aquaculture sector have ventured into optimizing the grow-out production and confined spawning of FFT under the environmental conditions of aquaculture in Kuwait. Diseases occur in the aquaculture systems, especially when intensive farming practices are adopted. Viral disease such as iridovirus is very often encountered in Asian sea bass. This group of viruses is reported from fishes inhabiting different environments from freshwater to marine and from table fish production systems to aquaria (Qin et al 2023). The present investigation is aimed to decipher the causes of illness and mortality of cultured FFT.
2 Materials and methods
Fish, Hatchlings of FFT were procured from Singapore in November 2021. The fish were reared in one-ton fiber reinforced plastic (FRP) tanks under two different systems (RAS and semi-flow-through) following optimized protocols. Temperature of the intake water, with the salinity adjusted to range between 30–32 ppt, was regulated at 27 ± 2 °C during the culture period. Briefly, the larvae were grown with green water containing nanochloropsis algal food organisms (3x105 to 5x105 cells /ml) to act as food for the rotifer population (3x103 to 1x104 rotifers/l). Larvae of fish were reared up to 35 days post-hatch (dph) during which time gradual weaning was carried out from 22 dph with decapsulated artemia (3–6 artemia nauplii/ml). Initial daily water exchange was 10–30 %. Feeding was done twice a day at 8.00 and 14.00 pm. Weaning to an artificial diet (BioMar, Greece) containing 42 % crude protein was initiated at 28–30 dph depending on the size groups. Rearing was continued in both production systems.
Clinical Manifestations, Forty-five juvenile FFT (35–43 g) were reared in each of three two-ton tanks. The tanks were fed from a storage tank, via a biofilter tank, where 100 % water exchange took place every 2–3 days. One moribund fish and two fresh-dead specimens from the tank with severe clinical manifestations were used for the investigations. Following positive diagnosis for ISKNV from the severely affected tank, two fish each from the other two tanks were also sacrificed for diagnosis.
Microbiological Observations, Aseptic blood samples from the affected fish with severe clinical manifestations were used for the detection of any possible systemic bacterial infection. Brain heart infusion (BHI) agar and thiosulphate citrate bile-salt sucrose (TCBS) agar were used for plating the samples.
Molecular Diagnosis, Samples of gills, heart, liver, spleen, kidney, and intestine were collected for both electron microscopy and for extracting DNA and PCR-based detection of the Iridovirus. Gill wet mounts were observed under the microscope to detect marine ich, and for virus detection, following primers (Table 1) were used. DNA isolation was performed using Qiagen blood/tissue DNA extraction kit following the kit protocols. Following the amplification of specific DNA templates, the amplicons were sent to a service provider for amplicon sequencing and sequence analysis (Chromous Biotech, Bengaluru, India). Diagnostic procedures used in the present investigation were according to the Iridovirus diagnosis protocols of the World Organization of Animal Health diagnostics (WOAH, 2023) (https//www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access, accessed on 8th July 2024).
Ribonuclease reductase small sub-unit (RNRS) gene /ISKNV and MCP genes of Iridovirus family
Amplicon Size (bp)
References
RNRS gene
V1 F 5′- CACGTGTTGGCTTTCTTCGC-3′
V4 R 5′- AGACAGGCAAAGTCACAGTG-3′
434
V5 R 5′ – GAGCATCAAGCAGGCGAT-3′
622
Oshima et al., 1998
MCP-K1
F 5′- AAATGGCTCTTTGGAGTGTC-3′
Jeong et al., 2003,2005
R 5′- AATCCATCGGTATTATG-3′
939
Light and Electron Microscopy, Wet mounts of gills were observed under a compound microscope for recording the parasite. Spleen imprints, The fish were thoroughly bled, spleen was dissected into a sterile Petri plate, and cut into two halves one of the halves was used to get tissue imprints onto the slide, and the remaining portion was used for electron microscopy. The slide was fixed using methanol, stained with Geimsa, and observed under the microscope. The gills, heart, liver, spleen, kidney, and intestine samples were fixed in 0.3 % glutaraldehyde, critical point dried, and processed according to standard protocols for transmission electron microscopy. Thin sections were marked for observations under the transmission electron microscope (TEM). The EM facility of the Kuwait University, Health Science Centre, Jabriya, Kuwait was used for this purpose.
3 Results and discussions
The diagnostic procedure and infectivity confirmations were derived based on a stepwise protocol for FFT in our study (Fig. 1).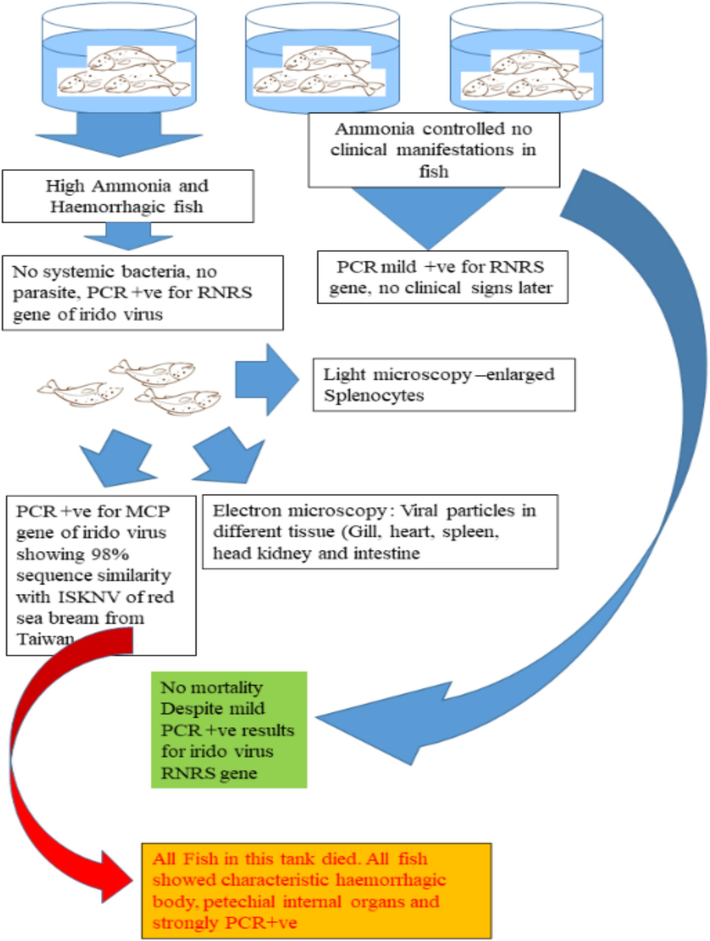
Step-wise protocol of ISKNV diagnosis followed for FFT in Kuwait.
Clinical Manifestations started appearing in all three tanks in the semi flow-through system, which recorded elevated ammonia levels of 0.15- to 0.2 ppm. The fish showed reduced appetite, irritability, and reddening. The storage tank water was completely replenished following the symptoms. Ammonia levels returned to less than 0.1 ppm following replenishment. Despite these efforts, one tank continued to have elevated levels of ammonia (>0.1 ppm). Clinical symptoms in this tank became more intense with hemorrhagic snout, fin bases, and distal body surface (Fig. 2). While. clinical manifestations of the fish in the other two tanks became normal as the ammonia levels were consistently lower than 0.1 ppm. All the fish in the severely affected tank died within 4–5 days despite efforts to keep the ammonia levels low and preventive measures (oxytetracycline bath 40 ppm daily) to control opportunistic vibriosis.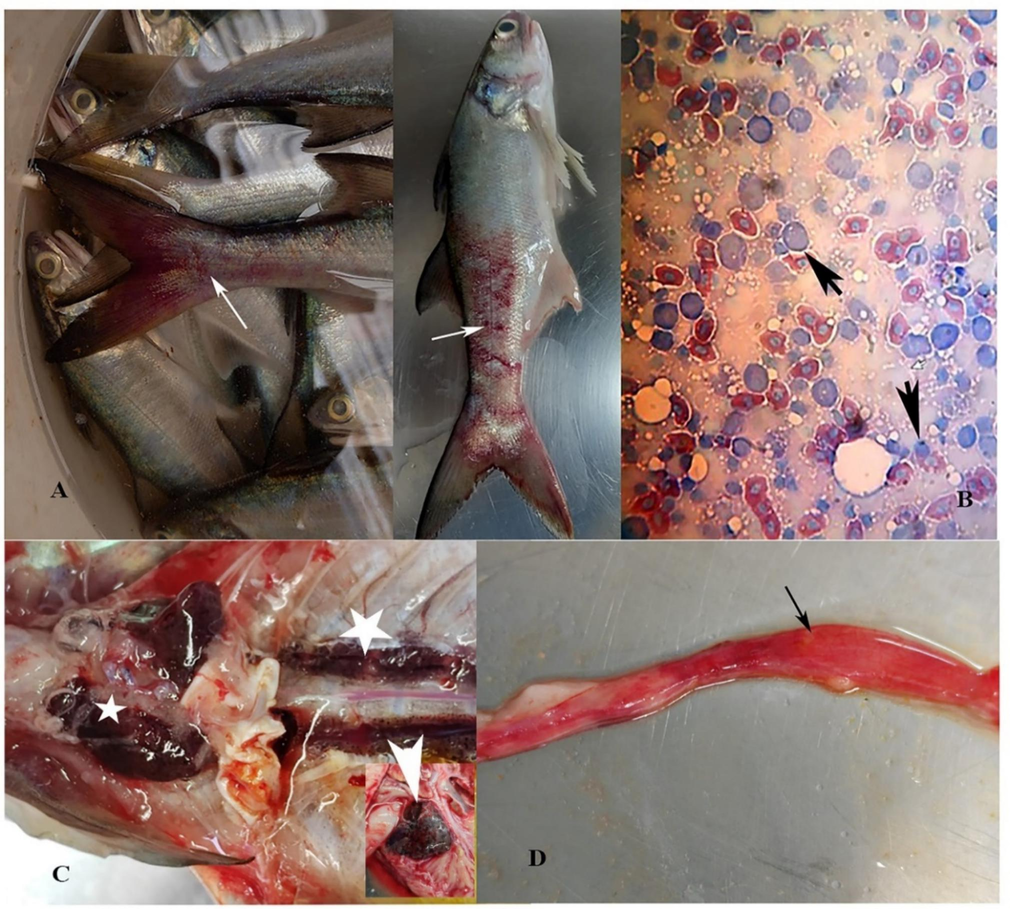
Clinical manifestations in FFT with ISKNV infections. A, Gross appearance showing extensive body hemorrhage (arrows). B, Hypertrophied splenocytes (short arrows) in spleen tissue imprints (Geimsa stained). C, Enlarged head kidney (small star), necrotic mid kidney (large star), and inset showing enlarged spleen (arrowhead). D, Mid and hindgut with bloody exudates (arrow).
Hemorrhagic body surface, fin bases, and snout were the consistent clinical manifestations in all the fish. Internal organs showed petechial hemorrhages, pale liver, enlarged spleen, and empty gut with the hemorrhagic midgut. The spleen tissue imprint showed basophilic inclusion bodies (Fig. 2). Clinical manifestations in FFT, as observed in the present case, match the manifestations reported for several fish species (Kurita, 2012: Subramaniam et al., 2012: Dong et al., 2017: OIE, 2019). Spleen and kidney enlargement seen in FFT are similar to the observations made by He and his team (He et al., 2000 and 2002). Mandarin fish and several other marine fish, under an experimental infection model, showed spleen, kidney, and endocardial hypertrophy as common ISKNV-associated manifestations. Total heterotrophic bacterial counts in the affected tank water were 1.2 ± 6.3 x 103 CFU/ml and the total vibrio counts were < 100 CFU/ml. Neither systemic bacterial infection nor parasitic infestation was recorded from the infected fish. There was no significant difference between the bacterial counts of different tanks. In our case study, we see a relation between the elevated water ammonia levels and the outbreak of ISKNV. However, in the tanks where ammonia levels triggered clinical manifestations and the virus was detected in the FFT, improvement in the water quality was probably responsible for preventing the seriousness of the infection, and no mortality was recorded from those tanks. It is well known that the environment influences the pathogen invasiveness (Engering et al., 2013). Such an environment-triggering effect was demonstrated in an experiment where dissolved oxygen levels influenced ISKNV infectivity (Yu et al., 2022). Exposure to stress and endogenous ammonia adversely affects the ability of the fish to maintain homeostasis (Randall and Tsui, 2002). They observed that apart from the metabolic stressors, hyper-ammonia condition was a predisposing factor in the carp edema virus (CEV) infections in carp. Similarly, metabolic disturbances were also reported to be one of the pre-disposing factors in CEV (Pikula et al., 2021).
Fourfinger threadfin is gaining importance as a food fish following its controlled seed production and larval rearing efforts (World Aquaculture Society, 2024). Aquaculture production of FFT in Kuwait is in its early days thanks to the Government initiative. This species is known to be a stress-sensitive fish as we noticed ammonia-mediated necrotic lesions in this fish during the early development period. Similar stress-induced immune compromises have been reported from Atlantic salmon (Tamsyn et al, 2021). Molecular diagnostics using primers from published and related work (Oshima et al. 1998) produced positive amplification for the iridovirus (Fig. 3). The sequence analysis of the 622 bp amplicon produced 99 % sequence similarity with the angelfish iridovirus RNRS gene (MK689685.1). Hence another set of primers (Jeong et al., 2003) targeting the major capsid protein gene of ISKNV was used for specific diagnostics. These reactions produced 879 bp amplicon (Fig. 2) and a 98 % sequence similarity with MCP gene sequence of red seabream ISKNV from Taiwan (KT781098.1). The 879 bp MCP gene sequence of the ISKNV in FFT from Kuwait was submitted to the NCBI (OR136938.1).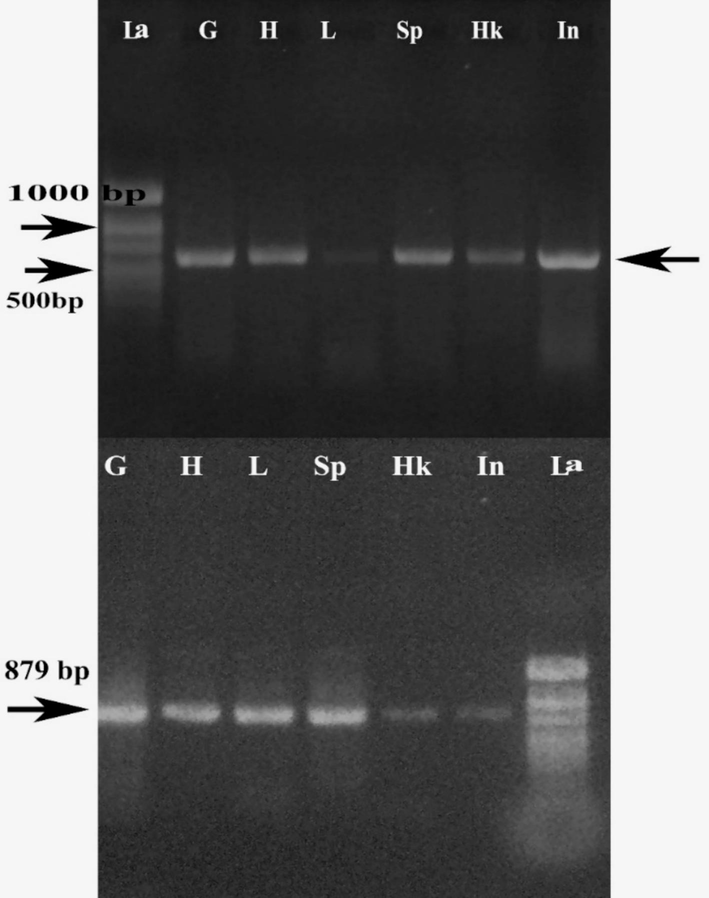
PCR amplification of RNRS gene (622 bp) and ISKNV-specific MCP gene (879 bp) of infected FFT. G-gills, H-heart, L-liver, Sp –spleen, Hk-head kidney, In- intestine, La-ladder.
The distance tree is depicted in Fig. 4. The gene sequence corresponded to the gene sequence of the laminin-type epidermal growth factor-like protein (LTEGFP). The MCP of the megalocytivirus, to which the ISKNV belongs, contains 40 % of the proteins with more than 36 additional polypeptides. These polypeptides are known to take part in viral particle formation (Robin et al., 1986). The MCP gene sequence of ISKNV of FFT, corresponding to the LTEGFP gene sequence, indicates a possible role of this protein in the infectivity of the virus. The involvement of the LTEGFP in providing a mock basement membrane that facilitates viral attachment to lymphatic endothelial cells has been demonstrated earlier (Xu et al 2010).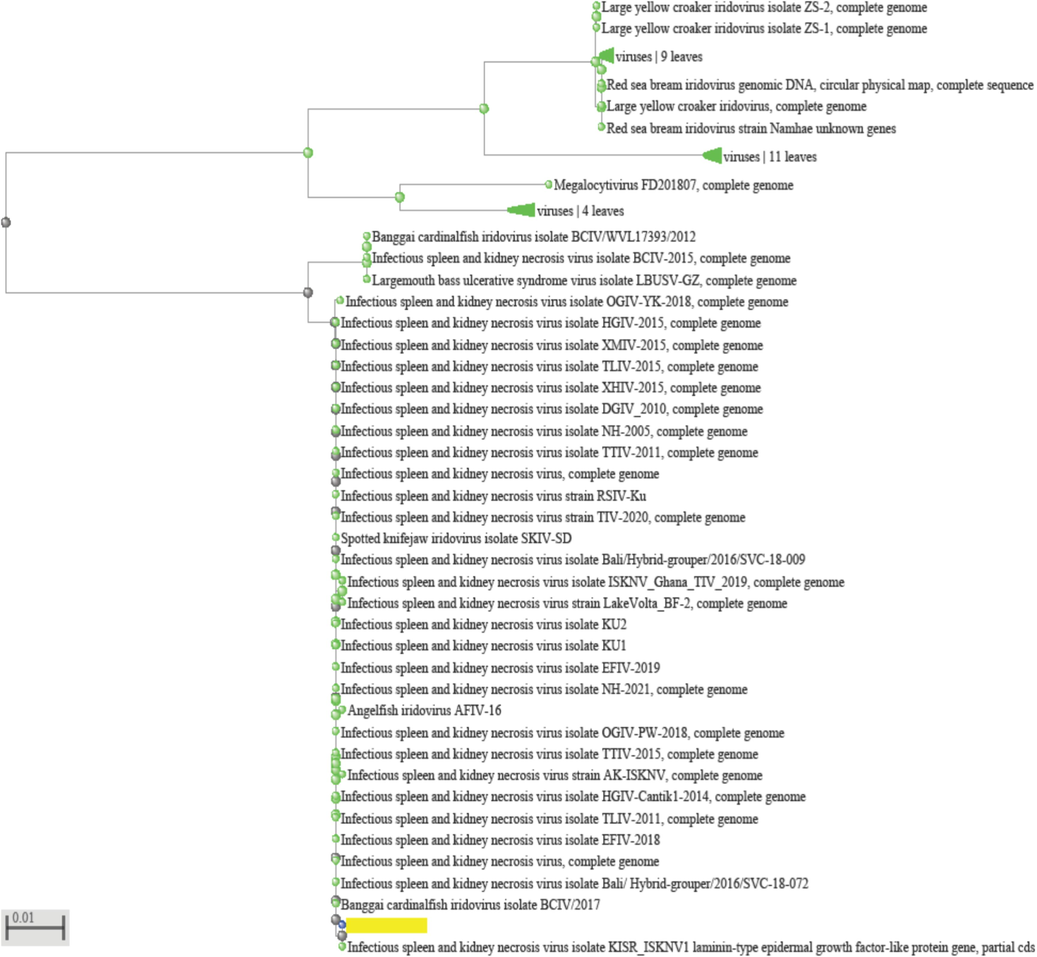
Distance tree of ISKNV of FFT from Kuwait.
Electron Microscopy of different tissues of FFT (Fig. 5) revealed the presence of viral particles associated primarily with the epithelial cells of gills, myocardial interstitial spaces, hepatic acini, splenocytes, pronephric lymphoid tissue, intestinal epithelium, and muscle-epidermal basement. The viral particles were associated with the inclusion bodies within various cells of the gills, heart, liver, spleen, kidney, intestine, and muscle. It will be interesting to see if this LTEGPF gene corresponds to the VP23R protein (Xu et al., 2010) reported to have an ISKNV infection-related role in ISKNV infectivity. There are no reports of ISKNV from cultured FFT. However, this species has been listed as susceptible to ISKNV by the WOAH in its report (WOAH, 2023). The first report of FFT susceptibility to viral infections from cultured fish indicated RNA viruses cause pathological manifestations in FFT from Singapore (Chang et al., 2002). The list of species susceptible to ISKNV (WOAH, 2023) reflects the possibility of infections in natural environments and culture conditions. The carrier state of the cultured organisms, either through the environment or via parental transfer, is one of the potential sources of the disease. Limitations of detection specificity and sensitivity of ISKNV, in stockable fish, could also contribute to the disease situation when culture conditions become stressful to fish. The possible role of the integument and the gill epithelium in becoming portals of entry for the ISKNV cannot be ruled out as the viral particles were seen associated with the basement membranes of the skin and epithelial lining of the gills in our study.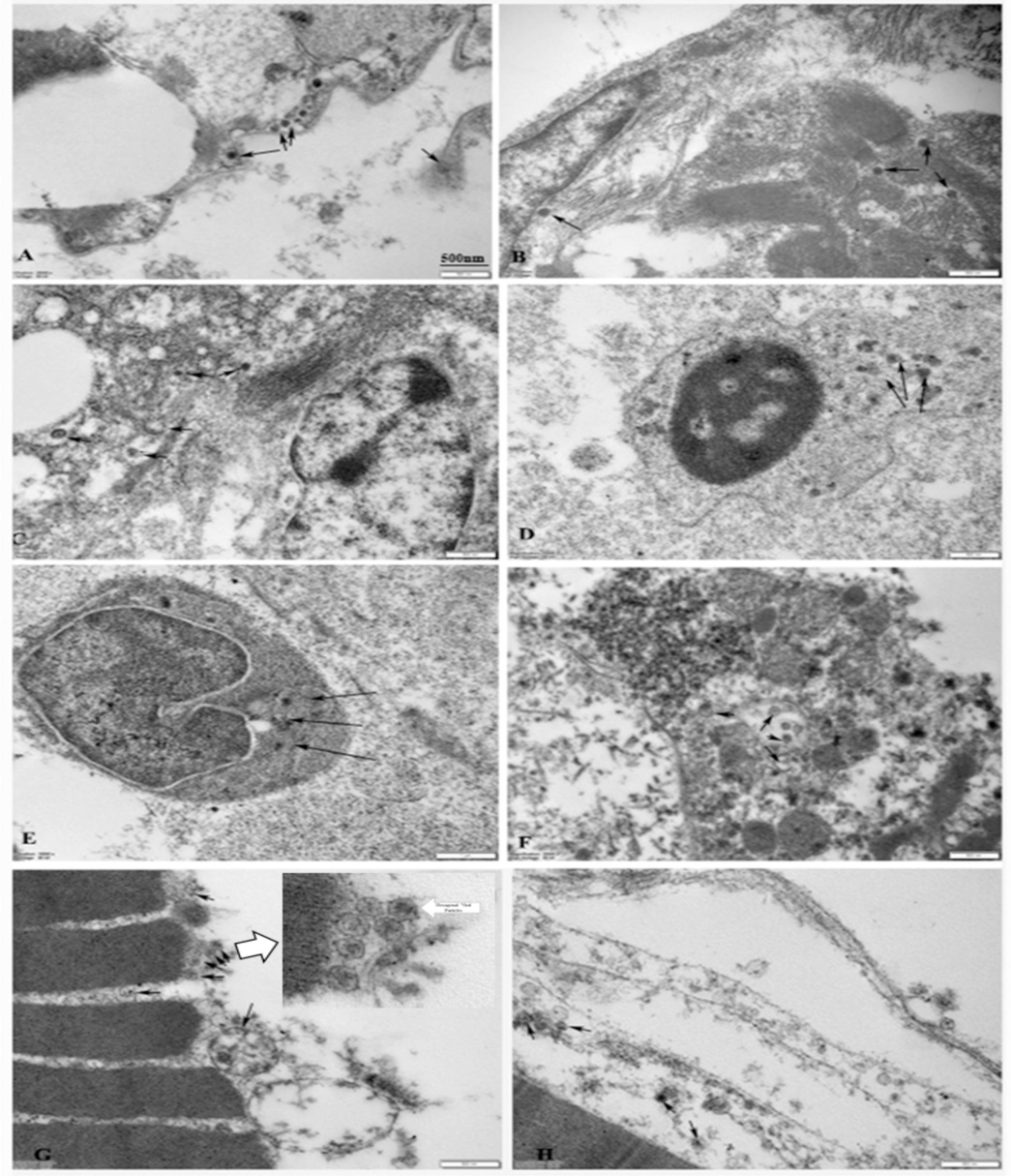
Viral infections as seen under the transmission electron microscope. A-Gill epithelium containing viral particles (arrows). B −Myocardium showing viral particles in the myofiber interstitium (arrow). C −Liver tissue with viral particles (arrows). D −Splenocyte showing intracytoplasmic localization of viral particles (arrows). E −pronephric lymphopoietic tissue with viral inclusion (arrows). F- Intestinal epithelium with viral particles (arrows). G −Epidermis and basement membrane with viral particles (arrows).
4 Conclusion
Fourfinger threadfin is sensitive to handling and water quality parameters. The aim and the purpose of the present investigation of deciphering the cause of illness and mortality were achieved. A greater purpose was also met by sequencing the major capsid protein of the virus for future work on vaccine development. Infectivity and resultant moralities could occur due to ISKNV in this fish. Hence, water quality management and preventive vaccination procedures must be applied in commercial aquaculture of this species.
CRediT authorship contribution statement
I.S. Azad: Validation, Supervision, Methodology, Investigation, Data curation, Conceptualization. A. Al-Yaqout: Supervision, Methodology, Funding acquisition. S. El-Dakour: Supervision, Methodology. S. Kawahara: Validation, Supervision, Investigation. M. Al-Roumi: Supervision, Resources.
Acknowledgment
We thank Ms. Jessy and the Head of the Electron Microscopy Unit of Kuwait University, Health Science Centre, Jabriya, Kuwait for extending their facilities for microscopic investigations. The authors are thankful to Kuwait Foundation for Advancement of Science (KFAS) for partial funding support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Growth and survival of Indian Salmon Eleutheronema tetradactylum (Shaw, 1804) in brackish water pond. J. Fish. Aquat. Sci.. 2011;6:479-484.
- [Google Scholar]
- Initial investigations into two viruses isolated from marine food fish in Singapore. Vet. Rec.. 2002;150:15-16.
- [Google Scholar]
- Infectious spleen and kidney necrosis disease (ISKND) outbreaks in farmed barramundi (Lates calcarifer) in Vietnam. Fish Shellfish Immunol.. 2017;68:65-73.
- [CrossRef] [Google Scholar]
- Pathogen-Host- Environment interplay and disease emergence. Emerg. Microbes Infect. 2013;2(2) e5
- [CrossRef] [Google Scholar]
- Systemic disease caused by an iridovirus-like agent in cultured mandarinfish, Siniperca chuatsi (Basillewsky), in China. J. Fish Dis.. 2000;23:219-222.
- [CrossRef] [Google Scholar]
- Experimental transmission, pathogenicity and physical-chemical properties of infectious spleen and kidney necrosis virus (ISKNV) Aquaculture. 2002;204:11-24.
- [Google Scholar]
- Jeong, J.B., Jun,L.J., Park,K.H., Kim,K.H., Chung, J.K., Komisar,J.L., Jeong, H.D. 2005. Asymptomatic iridovirus infection in various marine fishes detected by a 2-step PCR method, Aquaculture, 255, 1–4, 30-38. 10.1016/j.aquaculture.12.019.
- Characterization of the DNA nucleotide sequences in the genome of red sea bream iridoviruses isolated in Korea. Aquaculture. 2003;220:119-133.
- [Google Scholar]
- Megalocytiviruses. Viruses.. 2012;4:521-538.
- OIE. 2019. Red Sea Bream Iridoviral Diseases, Manual of Diagnostic Tests for Aquatic Animals –accessed on 14/11/2019.
- Rapid diagnosis of red sea bream iridovirus infection using the polymerase chain reaction. Dis. Aquatic Organ.. 1998;32:87-90.
- [CrossRef] [Google Scholar]
- Carp edema virus infection is associated with severe metabolic disturbance in fish. Front. Vet. Sci.. 2021;8
- [CrossRef] [Google Scholar]
- Megalocytivirus and other members of the family Iridoviridae in finfish, a review of the etiology, epidemiology, diagnosis, prevention and control. Viruses. 2023;15:1359.
- [CrossRef] [Google Scholar]
- Identification of the glycoproteins of lymphocystis disease virus (LDV) of fish. Arch. Virol.. 1986;87:297-305.
- [Google Scholar]
- Early life stress causes persistent impacts on the microbiome of Atlantic salmon. Comp. Biochem. Physiol. D: Genomics Proteomics. 2021;40:100888 10.1016/j.cbd
- [Google Scholar]
- WOAH (https//www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access. accessed on 8th July 2024).
- World Aquaculture Society, https//www.was.org/articles/The-Singapore-Aquaculture-Industry-Contributing-to-Singapores-Food-Security.aspx (Accessed in Feb 2024).
- VP23R of Infectious Spleen and Kidney Necrosis Virus mediates formation of virus-mock basement membrane to provide attaching sites for lymphatic endothelial cells. J. Virol.. 2010;84(22):11866-11875.
- [Google Scholar]
- Hypoxia triggers the outbreak of infectious spleen and kidney necrosis virus disease through viral hypoxia response elements. Virulence. 2022;13(1):714-726.
- [CrossRef] [Google Scholar]
Further Reading
- OECD/FAO (2023), ''OECD-FAO Agricultural Outlook'' OECD Agriculture statistics (database), http,//dx.doi.org/10.1787/agr-outl-dataen.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103393.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







