Translate this page into:
Field performance assessment of formulated Pseudomonas fluorescens for enhancing plant growth and inducing resistance against rice blast disease
⁎Corresponding author. atiq.ppath@bau.edu.bd (Md. Atiqur Rahman Khokon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
To control blast disease in the rice field under natural conditions, three antagonistic isolates of rhizospheric P. fluorescens were formulated in talc, kaolinite, PVP, and vegetable oil using an RCBD with three replications. At various phases of the rice plants' growth, all of the products markedly accelerated plant growth and yield-contributing characteristics. Pf-8, compounded in talc (5 %) and vegetable oil (2 %) among the three isolates of P. fluorescens, significantly elevated vegetative and yield parameters with higher (2.11 and 2.08) benefit-cost ratios, respectively. At 90 days after transplanting (DAT), T11 (5 % Pf-8 Talc) and T13 (2 % Pf-8 Vegetable oil) showed a significant reduction in blast incidence (76.81 %, 75.45 %) and severity (71.57 %, 69.82 %), with the largest populations of P. fluorescens (9.60 × 1010 and 9.51 × 1010) in the rhizosphere. Moreover, a significantly increased level of phenol and hydrogen peroxide (H2O2) was found in Pseudomonas-treated leaves (Pf-8) at 30, 60, and 90 DAT indicating a strong relationship with rice blast disease reduction. Further, blast incidence and severity showed a negative correlation with vegetative parameters, yield parameters, phenol, and H2O2. Thus, it may be claimed that using formulated P. fluorescens (Pf-8) to manage rice blast in the field could be an alternate strategy to using chemicals.
Keywords
Disease incidence and severity
Formulation
H2O2
Phenol
Pseudomonas fluorescens
- PGPR
-
Plant growth-promoting rhizobacteria
- BAU
-
Bangladesh Agricultural University
- RCBD
-
Randomized Complete Block Design
- BRRI
-
Bangladesh Rice Research Institute
- CFU
-
Colony Forming Unit
- BCR
-
Benefit-cost ratio
- IAA
-
Indole Acetic Acid
- PVP
-
Polyvinylpyrrolidone
- DMRT
-
Duncan's Multiple Range Tests
- ANOVA
-
Analysis of Variance
- CMC
-
Carboxy Methyl Cellulose
- ROS
-
Reactive Oxygen Species
Abbreviations
1 Introduction
In Bangladesh, various biotic & abiotic factors impede rice yield. One of the most deadly and common diseases affecting irrigated rice in temperate and sub-tropical regions of East Asia is the blast of rice (Magnaporthe oryzae). Under extreme infection circumstances, the projected production loss in the farmer's field due to blast disease is 56.9 % and 65.4 % in rainfed and irrigated ecological systems, respectively (Hossain et al., 2017). Surprisingly, BRRIDhan 28, the most popular rice variety which covers about 40 % of the Boro season in Bangladesh has been reported to suffer notable production losses as a result of the rice blast (Mahmud and Hossain, 2018).
The most efficient way to control blast disease is still to apply chemical fungicides such as tebuconazole, azoxystrobin + difenoconazole, trifloxystrobin + tebuconazole, and tricyclazole (Mohiddin et al., 2021). However, reckless application of these fungicides results in the fungicide's resilient development, which may eventually drive up the agricultural production cost and destroy the biodiversity of aquatic and soil systems. Moreover, the adverse consequences of fungicides on non-target species (carcinogenicity, high residual toxicity, and severe toxic qualities) and the possible risk of polluting the environment restrict the recurrent and sole use of fungicides (Xin et al., 2020; Xu et al., 2021).
In comparison to chemical fungicides, the use of PGPR as a natural component ensures several benefits, including increased and precise disease suppressive efficiency, a decreased likelihood of pathogen resistance, and the ability to manage disease both directly through their antagonistic behavior against pathogens and indirectly through inducing systemic resistance (ISR) that poses the minimal risks to non-target organisms and environment (Ons et al., 2020). P. fluorescens have shown enormous potential to develop resistance against various plant-associated pathogens (Choudhary et al., 2007; De Vleesschauwer et al., 2008). Upregulation of different enzyme activities related to disease resistance like SOD, CAT, PO, dehydroxyascobate reductase, S-transferase, & glutathione has been documented by Prabhukarthikeyan et al. (2018). P. fluorescens also has been documented as the most effective PGPR against a range of infectious pathogens like Fusarium spp., Rhizoctonia spp., Magnaporthe oryzae, Sarocladium oryzae, etc. (Reddy et al., 2009; David et al., 2018). For creating a favorable microclimate and supporting long-term microbial survivability, PGPR cells are designed in forms that can be used in future applications. Numerous liquids and solid formulations using PGPR were developed to enhance crop productivity (Prabhukarthikeyan et al., 2018; Lobo et al., 2019). These formulations contain carrier materials and additives/adjuvants that are inexpensive, safe, and simple to produce inoculants (Kumaresan and Reetha, 2011; Suryadi et al., 2013; Lee et al., 2016). Kaolinite and talc are good carrier materials because of their fineness, lightweight, and good capacity to absorb bacterially-laden liquids. Water-, oil-, or polymer-based ingredients are employed in liquid formulations to improve adhesiveness, durability, emulsifier & dispersion properties. PVP is an affordable, safe polymer and has been reported to be employed in the production of inoculants due to their thermal stability, and adhesive qualities that might improve the adherence of bacterial cells to plant parts, and viscous nature, which could slow down the bioinoculants' drying process.
In the present study, an investigation on the efficacy of P. fluorescens formulated in talc, kaolinite, vegetable oil, and PVP was carried out to manage rice blast disease in the field under a natural epiphytotic environment.
2 Methodology
2.1 Study location and treatment combinations
In 2021, during the boro season, the study was conducted in the experimental field of BAU Farming System using an RCBD with three replications. In the present research, a blast-susceptible rice cultivar (BRRIDhan 28) was employed. The study used 10 plants per replication, with each experimental plot of 10 m2 (4 m × 2.5 m) and maintaining line-to-line & plant-to-plant spacing of 25 cm. All of the treatments received the necessary amounts of potassium, phosphorus, and nitrogen fertilizers from the BRRI. The supplementary table 1 contains a list of treatment combinations employed in this trial.
2.2 Selection of bacterial isolates and developing formulation
Previously isolated and characterized rice rhizospheric P. fluorescens were assessed for their capacity to suppress the mycelial growth of M. oryzae by the dual culture method as described by Chakraborty et al. (2021). Isolates, Pf-6 (MN256392.1), Pf-7 (MN256393.1), and Pf-8 (MN256394.1) which previously exhibited complete growth inhibition of M. oryzae, were utilized for developing new formulations (supplementary table 2) and field trial for blast disease control. Formulations were prepared following the method of Vidhyasekaran and Muthamilan (1995) & Suryadi et al. (2013) with slight modification and stored in an ambient room condition (23 ± 2 ℃).
2.3 Preliminary evaluation of formulated products by germination assay
A series of concentrations of the formulation were selected for primary evaluation. To assess the percentage of germination, the formulated P. fluorescens (1 %, 3 %, and 5 %-solid and 0.5 %, 1 %, and 2 %-liquid formulations) and distilled water-treated rice seeds were evenly distributed over moistened sterile blotter discs in Petri dishes (ISTA, 2005). The treatments were kept at 25 ± 2 °C for fourteen days in an incubator, and the germination percentage of the seeds was estimated using the following formula:
2.4 Evaluation of bacterial formulations under natural epiphytotic conditions
In this study, the formulations were applied during the transplantation of seedlings as well as at different growth phases of rice plants. The roots of 20 days seedlings were immersed overnight into mineral clay-based formulation (5 %) & 3 h in oil, polymer formulation, and bacterial suspension (2 %) (selected from the preliminary evaluation of different concentrations of formulations), and subsequently, the seedlings were planted in the field. Each treatment was applied to the foliage 4 times viz. 20, 35, 50, and 65 days after transplanting before the anticipated time of infection for panicle attack (the panicle formation stage) (Prathuangwong et al., 2013).
The viability of bacteria in the formulations was assessed at various time points after storage by serial dilution of 1 g or 1 mL of product with double distilled water and 0.1 mL suspension was dispersed over the King’s B medium and incubated for 2 days at 28℃. The following formula was utilized for computing CFU: CFU/mL = (No. of colonies × Dilution factor)/Volume of the culture plate
Biochemical analysis of treated plants was assayed using leaves. The measurement of phenol and H2O2 was conducted following the methods Ding et al. (2019), Alexieva et al. (2001), & Heath and Packer (1968), respectively. Data on disease incidence (%), severity (%), vegetative, & yield attributes were recorded at different growth stages. Percent disease incidence and severity (leaf and panicle blast), grain yield, and BCR were estimated following James (1974), Rais et al. (2018), and Chakraborty et al. (2021), respectively.
2.5 Data analyses
Minitab 18 software was used to assess the statistical analysis of the data and computing correlation coefficient. DMRT was utilized to evaluate the treatment differences that were deemed significant after a one-way ANOVA revealed such differences.
3 Results and discussions
3.1 Effect of formulated P. fluorescens on germination percentages in laboratory condition
The highest germination percentage was observed in seeds bio-primed with 5 % solid and 2 % liquid formulations of Pf-6, Pf-7, and Pf-8 ranging from 90 to 100 %, while the control (water) recorded the least with 34 % germination (supplementary table 3). In comparison to the control, treating seeds with bacterial strains enhanced seed germination and seedling height because the bacterial strains stimulated the production of phytohormones such as cytokinins auxins, & gibberellins. PGPR like P. aeruginosa actively colonizes around plant roots, produces IAA, salicylic acid, and siderophore, and thus increases plant growth and yield (Hariprasad et al., 2014).
3.2 Effect of formulated P. fluorescens on vegetative and yield contributing parameters of rice under natural epiphytotic conditions in the field
The efficacy of formulated P. fluorescens on vegetative & yield attributes of rice cv. BRRIdhan 28 in the field under natural epiphytotic conditions were evaluated. All the formulations showed statistically significant variation in different vegetative growth parameters like no. of leaves, no. of tillers/hill, no. of panicles/hill, plant height, and yield contributing characters such as no. of healthy grains/panicle, total grains/panicle, panicle length compared to untreated control plots (Table 1, 2, 3 and 4). Among them, Pf-8 formulated in talc and vegetable oil performed better than the others. This finding confirms that the application of formulated P. fluorescens has an impact on the physiological processes of rice at various vegetative stages. The application of bio-agents like P. fluorescens increases vegetative growth and yield through the production of amino acids, vitamins, and phytohormones, including IAA, cytokinins, and gibberellins, and nutrient solubilization/uptake. Moreover, bacterial solubilization of insoluble phosphate in the soil may be the cause of the yield increase in treated plots compared to control. These bacteria demonstrated a useful function in plant growth promotion and P absorption through the breakdown of inorganic insoluble phosphate. Yield increases in a variety of crops have been documented since P. fluorescens isolates are recognized as plant growth promoters (Yasmin et al., 2016; Mishra et al., 2023). Moreover, statistically significant and the highest yield of rice was also recorded in T11 (5.68 t/ha) and T13 (5.63 t/ha) with the highest benefit-cost ratio (2.11 and 2.08) respectively. The direct growth promotion with increased hormones and mineral uptake by PGPR could improve the efficacy of disease control and then achieve a significant increase in yield. The higher selling prices and lower manufacturing costs in the case of plants treated with formulated products are thought to be the reason for the higher BCR. An experiment by Adhikari (2009) also revealed a higher BCR in the organic carrot production system (1.52) than in the inorganic one (1.44) due to the higher profit and lower production cost in the organic production system. ** = 1 % level of significance, CV = Co-efficient of variation ** = 1 % level of significance, CV = Co-efficient of variation ** = 1 % level of significance, CV = Co-efficient of variation ** = 1 % level of significance, CV = Co-efficient of variation
Treatments
Plant height (cm)
No. of tillers/hill
No. of leaves/hill
T0
31.67 d
2.66 f
10.67 f
T1
40.67 a
4.00 b-e
12.00 d-f
T2
40.00 ab
5.00 ab
15.00 a-c
T3
34.67 b-d
3.00 ef
13.33 c-e
T4
32.33 d
3.33 d-f
11.33 ef
T5
32.00 d
3.33 d-f
11.67 d-f
T6
40.57 a
5.33 a
14.67 a-c
T7
40.00 ab
4.66 a-c
14.00 b-d
T8
34.00 cd
3.33 d-f
11.33 ef
T9
32.00 d
4.33 a-d
13.00 c-f
T10
31.67 d
3.66 c-f
12.00 d-f
T11
42.53 a
5.33 a
16.70 a
T12
41.33 a
4.33 a-d
15.33 a-c
T13
42.00 a
5.00 a
16.33 ab
T14
40.67 ab
5.00 ab
15.00 a-c
T15
32.00 d
3.33 d-f
11.00 ef
Level of significance
**
**
**
CV (%)
9.64
16.79
9.55
Treatments
Plant
height (cm)
No. of
tillers/hill
No. of panicles
/hill
No. of infected panicles
/hill
No. of
leaves/hill
No. of infected leaves/hill
T0
53.17 c
10.67 c
10.00 f
12.00 a
60.00 c
36.00 a
T1
76.67 b
18.00 ab
12.67 e
7.66cde
71.00 c
26.00 de
T2
74.00 b
19.33 ab
14.00 c-e
7.00 de
73.00 bc
25.00 de
T3
58.67 c
14.67 a-c
12.33 e
10.67 ab
73.33bc
27.00 cd
T4
54.00 c
14.00bc
13.67 de
8.00c-e
70.00 c
27.00 cd
T5
55.00 c
14.33a-c
13.33 e
8.66 cd
71.67bc
27.00 cd
T6
76.67 b
19.33 ab
16.67 a-c
6.00 e
90.00 ab
31.00 b
T7
70.67 b
18.33 ab
16.00 b-d
7.33 de
90.00 ab
30.00 bc
T8
56.00 c
14.33a-c
13.00 e
12.00 a
71.00 c
29.67b-d
T9
54.33 c
14.67a-c
14.33c-e
12.67 a
73.30bc
30.33bc
T10
55.33 c
14.67a-c
13.67 de
11.33 ab
73.33bc
31.00 b
T11
88.67 a
20.00 a
17.33 ab
6.00 e
100.0 a
22.00 f
T12
76.00b
19.33 ab
18.67 a
7.33 de
96.67 a
25.00 de
T13
87.00 a
20.00 a
17.67 ab
6.00 e
97.00 a
20.67f
T14
77.03 b
19.00 ab
18.67 a
7.00 de
90.00 ab
26.00 de
T15
56.00 c
14.67a-c
14.00 de
9.66bc
73.33 bc
34.00 a
Level of significance
**
**
**
**
**
**
CV (%)
8.68
17.45
8.99
12.85
12.48
8.42
Treatments
Plantheight
(cm)No. of
tillers/hillNo. of panicles/hill
No. of infected panicles/hill
No. of
leaves/hillNo. of infected leaves/hill
T0
87.17 d
15.67 c
13.00 c
13.67 a
83.34 d
45.67 a
T1
119.3 b
24.50 a
20.33 b
13.00 a
89.03 c
43.33 a
T2
118.7 b
24.00 a
19.00 b
13.05 a
88.00 c
42.34 a
T3
98.67 c
19.67 b
18.67 b
12.00 ab
98.33 b
30.00 bc
T4
94.00 c
19.00 b
18.00 b
11.33 ab
95.00 b
30.00 bc
T5
95.00 c
19.67 b
18.67 b
12.67 ab
98.33 b
29.00 bc
T6
118.7 b
24.33 a
17.33 b
8.00 cd
87.07 c
34.30 b
T7
119.7 b
25.65 a
24.67 a
8.00 cd
88.03 c
35.35 b
T8
96.00 c
19.33 b
18.33 b
13.00 ab
96.67 b
35.00 b
T9
94.33 c
19.67 b
18.67 b
12.00 ab
98.33 b
32.00 bc
T10
93.67 c
19.67 b
18.67 b
12.67 ab
98.33 b
34.00 b
T11
125.0 a
25.00 a
24.00 a
7.00 d
121.7 a
22.00 d
T12
121.7 ab
24.67 a
18.67 b
8.67 cd
97.07 b
29.30 bc
T13
125.0 a
25.00 a
25.00 a
7.00 d
125.0 a
21.00 d
T14
117.3 b
24.00 a
18.00 b
12.00 ab
98.03 b
27.33 c
T15
95.67 c
19.67 b
18.57 b
10.33 bc
98.33 b
28.00 c
Level of significance
**
**
**
**
**
**
CV (%)
8.27
11.48
12.21
14.84
10.03
9.56
Treatments
No. of panicles/hill
Total grains/panicle
No. of healthy grains/panicle
No. of diseased grains/panicle
Panicle length (cm)
Yield (t/ha)
BenefitCost Ratio
T0
15.00 b
185.33 e
117.00 d
76.33 a
18.00 d
0.67 g
0.57 h
T1
24.33 a
198.35 bc
165.33 a
39.00 e
23.00 b
4.64 ce
2.02 b
T2
23.33 a
200.00 b
164.67 a
39.05 e
22.57 b
4.74 be
1.96 bc
T3
18.67 b
194.00 d
185.00 bc
40.00 e
22.67 b
4.63 ce
1.73 e
T4
18.00 b
187.00 e
139.33 c
47.67 bc
18.00 d
4.73 be
1.68 e
T5
18.67 b
187.34 e
136.67 c
50.67 b
18.00 d
3.50 f
1.53 f
T6
23.33 a
185.68 e
153.00 b
52.67 b
27.00 a
5.40 ac
1.90 c
T7
24.67 a
185.00 e
152.00 b
33.00 f
27.00 a
5.23 ae
1.96 bc
T8
18.33 b
192.35 d
151.33 b
41.00 de
22.33 bc
4.50 e
1.12 g
T9
18.67 b
186.00 e
140.00 c
46.00 b-d
19.67 b-d
4.53 de
1.14 g
T10
18.67 b
191.00 d
143.33 bc
47.67 bc
18. 67 cd
4.51 de
1.10 g
T11
24.00 a
205.00 a
168.33 a
32.00 f
27.67 a
5.68 a
2.11 a
T12
23.67 a
200.00 b
166.65 a
33.33 f
27.33 a
5.33 ac
2.00 b
T13
25.00 a
205.00 a
167.68 a
32.33 f
28.33 a
5.63 a
2.08 a
T14
24.67 a
199.03 bc
165.70 a
33.33 f
27.00 a
5.30 ad
1.82 d
T15
18.67 b
190.00 d
145.00 bc
45.00 c-e
22.00 bc
3.53 f
1.68 e
Level of significance
**
**
**
**
**
**
**
CV (%)
12.21
11.24
11.5
9.94
8.40
6.24
1.47
3.3 Effect of formulated P. fluorescens on blast disease incidence and severity of rice under natural conditions in the field
Besides growth promotion and yield increment, the prevalence and severity of blast disease varied significantly at different growth stages after formulated P. fluorescens was applied to foliage (Fig. 1). The severity ranged from 19.00 to 67.00 %, and the blast incidence from 16.00 to 73.33 %. In comparison to the control (spraying with water), all P. fluorescens formulations showed a significant decrease in incidence and severity at all phases of growth. However, among all the treatments, T11, T12, and T13 showed promising responses in reducing the percent incidence & severity of rice blast. The incidence and severity of the blast were more effectively controlled at 90 DAT (ripening stage). It is hypothesized that the reduction in the percent rice blast incidence & severity may be the result of utilizing the beneficial traits of P. fluorescens for controlling plant diseases that include competing for nutrients and space; antibiosis through the production of antibiotics, namely, pyrrolnitrin, pyocyanin and production of siderophores (pyoverdin), which restricts the iron availability necessary for the pathogenic growth. In addition, PGPR such as Bacillus spp. and Pseudomonas spp. can also diminish blast symptoms by interfering with the spore tip mucus and extrinsic matrix's adhesion from the leaf surface, which obstructs the effective adhering of M. oryzae. The functional ability of formulated products is described in Supplementary figure 4. The outcomes of the study are in line with the report of Amruta et al. (2018) who also noticed the reduction of blast and enhanced plant growth in rice for the spraying of P. fluorescens and B. subtilis.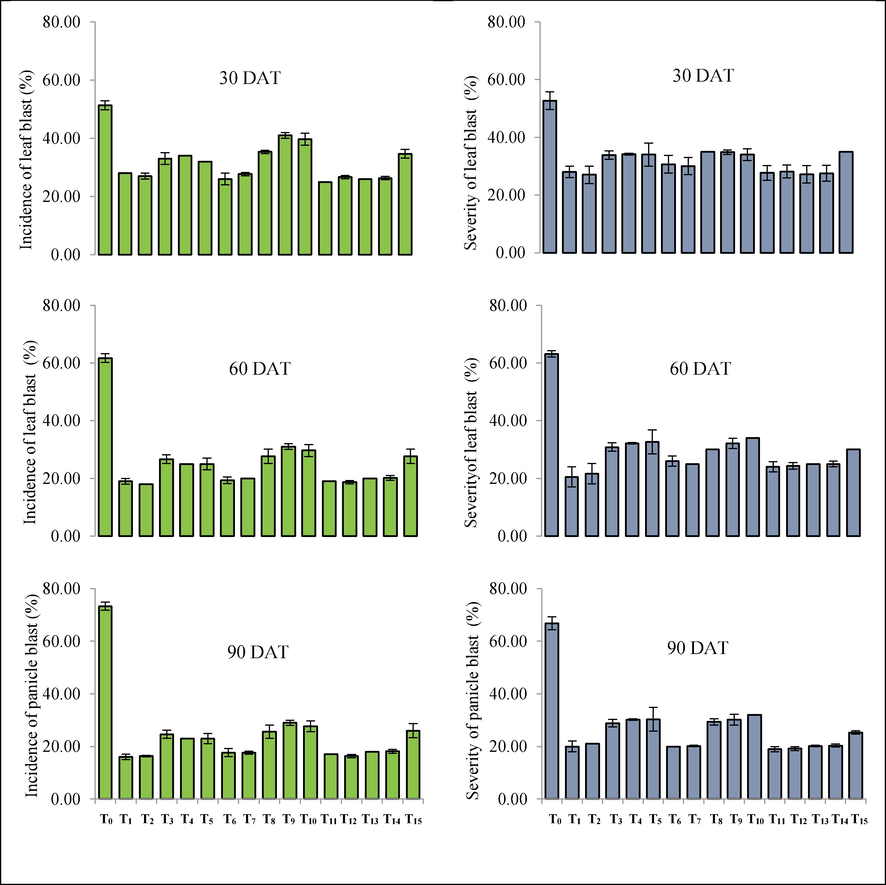
Effect of different treatments on the incidence and severity of blast of rice at different growth stages.
3.4 Shelf life of P. fluorescens in different formulations
Three P. fluorescens isolates (Pf-6, Pf-7, and Pf-8) were tested for survival after being stored for up to ten months at intervals of thirty days (supplementary table 4). The CFU number was the highest in all formulations for the first three months. The highest CFU of Pf-8 was recorded in Talc formulation (8.51 × 108) after fresh preparation at 1st month after storage which was found 9.60 × 1010 CFU at 3 months after storage. The prepared products are capable of maintaining the highest level for up to four months, as seen by the slow drop in CFU numbers after that time. After 10 months of storage, the lowest CFUs were found. P. fluorescens can survive well throughout the study period. It can be concluded that solid and liquid formulations that had additives (CMC, CaCO3, tritonX, etc.) supplemented with glycerol enhanced the shelf life and performance of the bacteria in the formulations as they provide nutrition, protect bacteria from drying out and also enhance the effectiveness of biocontrol efficacy. It is reported that the use of oil, additives like CMC, tryptone, glycerol, etc. with talc in the formulation maximizes the population of both P. fluorescens, T. harzianum, T. viride, Bacillus spp. (Rangeshwaran et al., 2010; Kala et al., 2013; Archana et al., 2015).
3.5 Effect of formulated P. fluorescens on total phenol and H2O2 contents in plants
To investigate the potential of P. fluorescens formulations to influence the defense system of rice plants, the total phenol content, and hydrogen peroxide (H2O2) levels were quantified in the treated plants (Table 5). Total phenol and H2O2 were measured at 30, 60, and 90 DAT to understand the longevity of the increased levels of these components. Total phenol content increased significantly with time in all treatments when compared to the constitutive level T0 (control) at all periods. But at 90 DAT, the highest (403.5 µg g−1) total phenol content was found in T11 (5 % Pf-8 Talc) followed by 401.6 µg g−1 in T13 (2 % Pf-8 Vegetable oil) compared to control (294.5 µg g−1). A comparable pattern was recorded in case of H2O2 content. The highest H2O2 was estimated in T11 (85.17 nmol g−1 FW) and T13 (82.09 nmol g−1 FW) at 90 DAT. The findings revealed that both total phenol and ROS gradually increased and remained elevated up to the ripening stage which may increase tolerance against rice blast disease. Consequently, the application of formulated P. fluorescens reduces the occurrence and severity of blast disease by accumulating phenolics and ROS that enhance the plant's tolerance to the disease, ultimately leading to improved yield-contributing factors and overall rice yield. Several researches revealed that ROS & phenolic compounds produced by plants in reaction to pathogen infection are linked to the host's resilience (Xia et al., 2015; Zaynab et al., 2018). ** = 1 % level of significance, CV = Co-efficient of variation
Treatments
Phenol (μg g−1)
H2O2 (nmol/g FW)
30 DAT
60 DAT
90 DAT
30 DAT
60 DAT
90 DAT
T0
286.3 i
293.6 p
294.5 n
25.17 l
46.26 l
55.18 i
T1
292.5 fg
330.6 e
390.6 e
54.27 c
73.13 c
77.17 c
T2
293.4 ef
320.6 h
385.5 f
56.10 bc
75.30 b
81.00 b
T3
290.6 gh
297.4 n
310.4 j
39.07 gh
55.09 i
61.00 fg
T4
288.4 hi
311.4 j
315.4 i
36.03 i
59.13 g
65.08 e
T5
291.0 gh
309.4 k
321.0 h
34.10 ij
53.19 j
57.10 hi
T6
295.4 de
325.4 g
393.5 d
41.13 g
63.19 f
69.27 d
T7
292.4 fg
326.5 f
391.0 e
44.20 f
60 21 g
66.17 e
T8
297.6 d
301 m
307.5 k
38.07 h
52.09 j
58.21 gh
T9
291.0 gh
295.5 o
300.4 m
33.21 j
49.23 k
59.57 gh
T10
296.4 d
302.4 l
305.7 l
31.0 k
57.17 h
63.23 ef
T11
305.5 a
358.6 b
403.5 a
59.08 a
78.17 a
85.17 a
T12
301.0 c
355.5 c
396.4 c
51 06 d
70.07 d
76.31 c
T13
304.6 ab
359.5 a
401.6 b
57.08 b
76.12 b
82.09 b
T14
302.6 bc
351.0 d
390.6 e
41.11 g
65.13 e
71.10 d
T15
295.5 de
312.6 i
364.6 g
46.20 e
60.18 g
66.17 e
Level of significance
**
**
**
**
**
**
CV (%)
0.43
0.16
1.00
2.80
1.56
2.46
3.6 Correlation matrices analyses
A correlation matrix was developed to analyze whether vegetative attributes, yield attributes, phenol, and H2O2 accumulation have any relation with disease incidence and severity (Supplementary figures 1, 2, and 3). The correlation analysis revealed a significant negative correlation among vegetative attributes, yield contributing attributes, phenol, and H2O2 content with the percent incidence & severity of blast disease during tillering, booting, and ripening stages. This result indicated the change in rice physiology affects vegetative growth and reduction in incidence and severity due to phenol and H2O2 accumulation by the application of formulated P. fluorescens treatments which ultimately enhance rice yield in the field. This outcome is in line with the findings of Chakraborty et al. (2021).
4 Conclusion
The locally isolated and formulated rhizobacteria from rice fields (Pf-8) can effectively suppress the incidence and severity percentage of rice blast disease and promote plant growth by modulating the total phenol and H2O2 levels. It is therefore concluded that bacterial isolate (Pf-8) has immense potential for commercialization. However, further research should be focused on overcoming extreme environmental conditions like salinity and drought. The application of nanotechnology in the formulation could enhance the efficacy.
Funding statement
The Peoples' Republic of Bangladesh's Ministry of Education (MoE) provided a research fund (Project no. 2018/504/MoE − LS 2017357), and the Ministry of Science and Technology (MoST) awarded the first author a National Science & Technology (NST) fellowship.
CRediT authorship contribution statement
Shila Chakraborty: Writing – original draft, Validation, Formal analysis, Data curation. Md. Morshedul Islam: Validation, Investigation, Data curation. Md. Atiqur Rahman Khokon: Writing – review & editing, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Economics of organic vs inorganic carrot production in Nepal. J. Agric. Environ.. 2009;10:23-28.
- [CrossRef] [Google Scholar]
- The effect of drought and ultraviolet radiation on growth and stress markers in peas and wheat. Plant Cell Environ.. 2001;24(12):1337-1344.
- [CrossRef] [Google Scholar]
- Exploring the potentiality of novel rhizospheric bacterial strains against the rice blast fungus Magnaportheoryzae. Plant Pathol. J.. 2018;34(2):126.
- [CrossRef] [Google Scholar]
- Development and standardization of invert emulsion formulation based bacterial endophyte of Pseudomonas fluorescens EPO 15. Trends Biosci.. 2015;8(15):3836-3841.
- [Google Scholar]
- Development of formulation of fluorescent pseudomonads and its evaluation on bio-management of blast of rice. Arch. Phytopathol. Pflanzenschutz.. 2021;54(3–4):208-229.
- [CrossRef] [Google Scholar]
- Induced systemic resistance (ISR) in plants: mechanism of action. Indian J. Microbiol.. 2007;47:289-297.
- [CrossRef] [Google Scholar]
- Pseudomonas fluorescens: a plant-growth-promoting rhizobacterium (PGPR) with potential role in biocontrol of pests of crops. In: Crop Improvement through Microbial Biotechnology. Elsevier; 2018. p. :221-243.
- [CrossRef] [Google Scholar]
- Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol.. 2008;148(4):1996-2012.
- [CrossRef] [Google Scholar]
- Distribution and quantitative analysis of phenolic compounds in fractions of Japonica and Indica rice. Food Chem.. 2019;274:384-391.
- [CrossRef] [Google Scholar]
- Mechanisms of plant growth promotion and disease suppression by Pseudomonas aeruginosa strain 2apa. J. Basic Microbiol.. 2014;54(8):792-801.
- [CrossRef] [Google Scholar]
- Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 1968;125(1):189-198.
- [CrossRef] [Google Scholar]
- Occurrence of Blast Disease in Rice in Bangladesh. American J. Agri. Sci.. 2017;4(4):74-80.
- [Google Scholar]
- ISTA, 2005. International Seed Testing Association. Proceedings of the International Seed Testing Association. International Rules of Seed Testing. Seed Sci. Technol.15, 1–9.
- James, W. C., 1974. Assessment of plant diseases and losses. Annu. Rev. Phytopathol. 1227–1248. https://doi.org/10.1146/annurev.py.12.090174.000331.
- Studies on shelf life of Trichoderma spp. and Pseudomonas fluorescens in different formulating materials. Plant Disease Research. 2013;28(1):53-57.
- [Google Scholar]
- Survival of Azospirillum brasilense in liquid formulation amended with different chemical additives. J. Phytology. 2011;3(10)
- [Google Scholar]
- Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth-promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl. Microbiol. Biotechnol.. 2016;100(18):7977-7987.
- [CrossRef] [Google Scholar]
- Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res.. 2019;219:12-25.
- [CrossRef] [Google Scholar]
- Efficacy of BAU-Biofungicide, chemical fungicides and plant extracts on rice (Oryza sativa L.) diseases and yield. J Plant Physiol. Pathol.. 2018;6(2):1-8.
- [CrossRef] [Google Scholar]
- Isolation and characterization of halotolerant plant growth promoting rhizobacteria from mangrove region of Sundarbans, India for enhanced crop productivity. Front. Plant Sci.. 2023;14:1122347.
- [CrossRef] [Google Scholar]
- Combination of strobilurin and triazole chemicals for the management of blast disease in mushkbudji-aromatic rice. J. Fungi.. 2021;7(12):1060.
- [CrossRef] [Google Scholar]
- Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms. 2020;8(12):1930.
- [CrossRef] [Google Scholar]
- Antibiotic-producing Pseudomonas fluorescens mediates rhizome rot disease resistance and promotes plant growth in turmeric plants. Microbiol. Res.. 2018;210:65-73.
- [CrossRef] [Google Scholar]
- Bioformulation Pseudomonas fluorescens SP007s against dirty panicle disease of rice. Afr. J. Microbiol. Res.. 2013;7(47):5274-5283.
- [CrossRef] [Google Scholar]
- Antagonistic Bacillus spp. reduce blast incidence on rice and increase grain yield under field conditions. Microbiol. Res.. 2018;208:54-62.
- [CrossRef] [Google Scholar]
- Additives in Powder-Based Formulation for Enhanced Shelf Life of Pseudomonas fluorescens and Bacillus sp. J. Biol. Control. 2010:158-163.
- [Google Scholar]
- Characterization of antifungal metabolites of Pseudomonas fluorescens and their effect on mycelial growth of Magnaporthe grisea and Rhizoctonia solani. Int. J. Pharm. Technol Res. 2009;1:1490-1493.
- [Google Scholar]
- Management of rice blast disease (Pyriculariaoryzae) using formulated bacterial consortium. Emir. J. Food Agric. 2013:349-357.
- [CrossRef] [Google Scholar]
- Development of formulations of Pseudomonas fluorescens for control of chickpea wilt. Plant Dis.. 1995;79:782-786.
- [CrossRef] [Google Scholar]
- Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot.. 2015;66(10):2839-2856.
- [CrossRef] [Google Scholar]
- In vitro fungicidal activity and in planta control efficacy of coumoxystrobin against Magnaporthe oryzae. Pestic. Biochem. Physiol.. 2020;162:78-85.
- [CrossRef] [Google Scholar]
- Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol.. 2021;12:689527.
- [CrossRef] [Google Scholar]
- Plant growth promotion and suppression of bacterial leaf blight in rice by inoculated bacteria. PLoS One. 2016;11(8):e0160688.
- [CrossRef] [Google Scholar]
- Role of secondary metabolites in plant defense against pathogens. Microb. Pathog.. 2018;124:198-202.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103228.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







