Translate this page into:
Facile green synthesis of semiconductive ZnO nanoparticles for photocatalytic degradation of dyes from the textile industry: A kinetic approach
⁎Corresponding authors. shubhapranesh@gmail.com (Jayachamarajapura Pranesh Shubha), sfadil@ksu.edu.sa (Syed Farooq Adil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
One-pot, facile and green synthesis of zinc oxide nanoparticles are synthesized using cow dung as fuel by combustion procedure. The synthesized material is characterized by using various techniques such as XRD, FTIR, UV, FESEM, and EDX. To assess the photocatalytic efficacy of the as-synthesized material, harmful cationic and anionic dyes such as methylene blue (MB) and alizarin red S (AZ) dyes, respectively, are selected as benchmark dyes. The influence of light source, dye concentration, photocatalyst dosage, and pH value on the efficiency of photocatalyst and kinetics of photodegradation are systematically studied. The photodegradation results revealed that the synthesized ZnO NPs exhibited removal efficiency of MB and AZ dyes upon irradiation with UV light. Concisely, the removal efficacy of the ZnO NPs under UV light irradiation exhibited an MB and AZ degradation of 99.9% and 96.8%, respectively. A reasonable photo-catalytic mechanism for the high photodegradation efficacy of MB and AZ dyes by the prepared photocatalyst is also proposed. The green fabricated photocatalyst is promising material and could be applied for waste-water remediation and other ecological applications.
Keywords
ZnO
Methylene blue
Alizarin red S
Degradation
Photocatalysis
1 Introduction
In recent years, the harmful organic pigments and their wastewater products generated from various industries such as textile, plastic, paper, pesticides, leather, and petrochemical industries were severely contaminating aquatic life and poisoning human beings (Maruthupandy et al., 2020). These toxic organic dyes cause severe environmental pollution and approximately 10–15% of the dyes are produced in the wastewater which is a fundamental source of environmental pollution (Koutavarapu et al., 2020). The organic dyes existent in the effluents produced from the above-mentioned industries are difficult to degrade, owing to their high chemical stabilities. Many physicochemical and biological methods including chemical oxidation, adsorption, precipitation, ion-exchange, biological, reverse osmosis, and photocatalytic processes were applied to degrade the harmful organic dye (Mydeen et al., 2020). However, most of these aforementioned procedures suffer from many limitations such as high cost and inefficacious (Alharthi et al., 2020). Besides, these techniques need a costly operation and recurring expenses, therefore these processes are not suitable for small-scale industry. Hence, effective, low cost and environmentally-friendly alternative procedures are required to reduce the aforementioned disadvantages for the decomposition of dyes present in effluents (Soto-Robles et al., 2021; Subramanian et al., 2022).
Among the processes suggested so far, Advanced-Oxidation-Process (AOP), which is considered as one of the most effective strategies for the decomposition of noxious organic pollutants in water with the use of semiconductor-based photocatalysts, attributed to its high efficacies, environmentally friendliness, and inexpensive (Pouretedal et al., 2009; Alharthi et al., 2020). This promising method includes the decomposition of deleterious organic dyes through a mechanism of oxidation by the production of highly reactive free radical ions (OH•) due to the presence of a photo-catalyst under the influence of light source which could be UV light, visible light, or natural solar light irradiation. Semiconductor materials can decompose the organic and synthetic pigments into harmless and eco-friendly compounds, such as water and carbon dioxide (Natarajan et al., 2018b). The semiconductor-based photocatalysts are highly proficient in decreasing charge reunion which leads to high UV and Vis. light performance owing to their narrow bandgap energy (Natarajan et al., 2018a). Various photocatalysts have been explored worldwide, among the different types of photocatalysts, metal oxides that possess outstanding bandgap energy of 3.32 eV exhibit superb potential in industrial applications (López et al., 2019; Barglik-Chory et al., 2004).
The photocatalytic degradation of dyes includes the generation of positively charged holes (h+) in the valence band (VB) and negatively charged electrons (e−) in the conduction band (CB) in presence of light sources such as from UV light or solar irradiation. The photon energy has to be larger than the band-gap of the semiconductor materials utilized as a photocatalyst to excite the electrons from the valence band to the conduction band (Hernández-Carrillo et al., 2018). These photo-induced charge carriers (electrons and holes) are further used for oxidation and reduction of different types of pollutants existent in the wastewater, for example, the MB and AZ dyes (Bhushan et al., 2020). The semiconductor-based photocatalysts including ZnO, CeO2, ZrO2, TiO2, etc., are extensively employed as photocatalysts for the decomposition of organic contaminants (Suresh and Sivasamy 2020). Among the different types of semiconductors, ZnO is a premium photocatalyst and possesses numerous applications in several fields including energy storage devices, cancer therapy, supercapacitors, solar cells, and ecological pollution abatement owing to its unique properties such as non-toxicity, low cost, wide adsorption spectrum, abundant availability, high electron mobility and high thermal/chemical/physical stability (Wetchakun et al., 2019). These aforementioned merits make ZnO an idealistic choice for the photocatalytic degradation of dyes. Despite these advantages, ZnO nanoparticles also have drawbacks including broad band-gap, the photo-induced charge carriers recombination at a rapid rate and hence limits the usage of ZnO (Hernández-Carrillo et al., 2018; Look, 2001).
In this research, an attempt is made to synthesize ZnO NPs as photocatalyst in ecologically friendly and inexpensive combustion methodology using the cow dung as fuel to achieve high photo-decomposition activity over two organic chronic dyes (MB and AZ dyes) as demonstrated in Scheme 1. The prepared ZnO NPs are employed as photocatalyst for the removal of dyes in an aqueous solution in presence of various light sources. Additionally, the effect of several catalytic factors such as irradiation time, dye concentration (ppm), photocatalyst amount, and pH value on the photocatalytic decomposition of MB and AZ dyes are investigated. The photocatalytic data disclosed that the ZnO NPs photocatalyst exhibited superior photocatalytic efficiency for the degrading of MB and AZ dyes under UV irradiation, and a 99.9% and 96.8% of MB and AZ dyes are decomposed within 100 min, respectively, under the optimal conditions.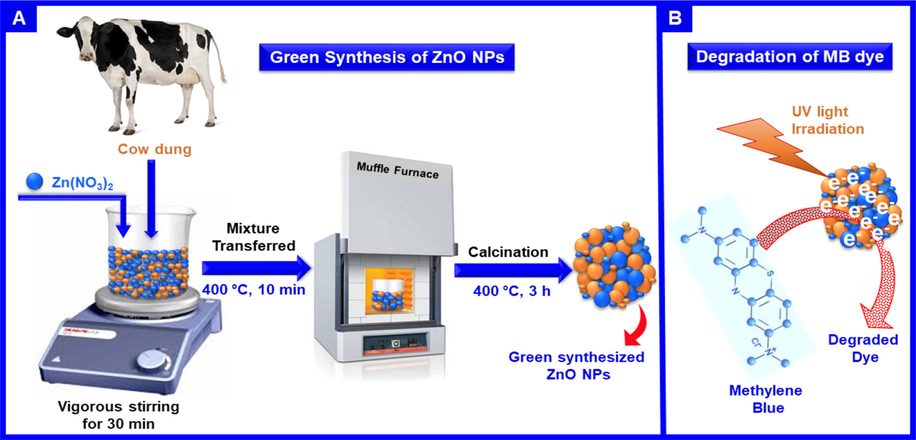
(A) Scheme illustrating the fabrication of ZnO NPs and (B) Schematic representation of the photodegradation of MB dye.
2 Materials and methods
2.1 Materials
Analytical grade zinc nitrate hexahydrate, Zn(NO3)2·6H2O (Sigma-Aldrich) is utilized without further purification. Cow dung is collected and dehydrated under sunlight for 15 days and used as fuel.
2.2 Synthesis of ZnO NPs photocatalyst
The calculated amounts of zinc nitrate hexahydrate is dissolved in 10 mL of distilled water and 1.0 g of fresh cow dung with constant vigorous stirring for 30 min. Subsequently, the obtained mixture are kept in a muffle furnace at 400 °C. A pale yellow powder is obtained after 10 min, which is calcined at the same temperature for 3 h.
2.3 Characterizations of ZnO NPs photocatalyst
The specifics related to the characterization instruments are mentioned in the supplementary file.
3 Results and discussion
3.1 Characterization of the ZnO NPs photocatalyst
The UV–visible absorption spectrum of as-fabricated ZnO NPs are presented in Fig. 1(a) with an inset figure of the Tauc plot. The absorption maximum was obtained at 368 nm wavelength indicating the formation of hexagonal ZnO NPs. This is attributed to the fundamental band-gap absorption occurring by the electronic transition between the valence band of the oxygen (2p orbitals) to the conduction band of Zn atoms (3d orbitals) (O2p → Zn3d) as previously reported (Zak et al., 2011). The direct bandgap energy of as-fabricated ZnO NPs is calculated as 3.19 eV by extrapolating the straight line in the Tauc plot.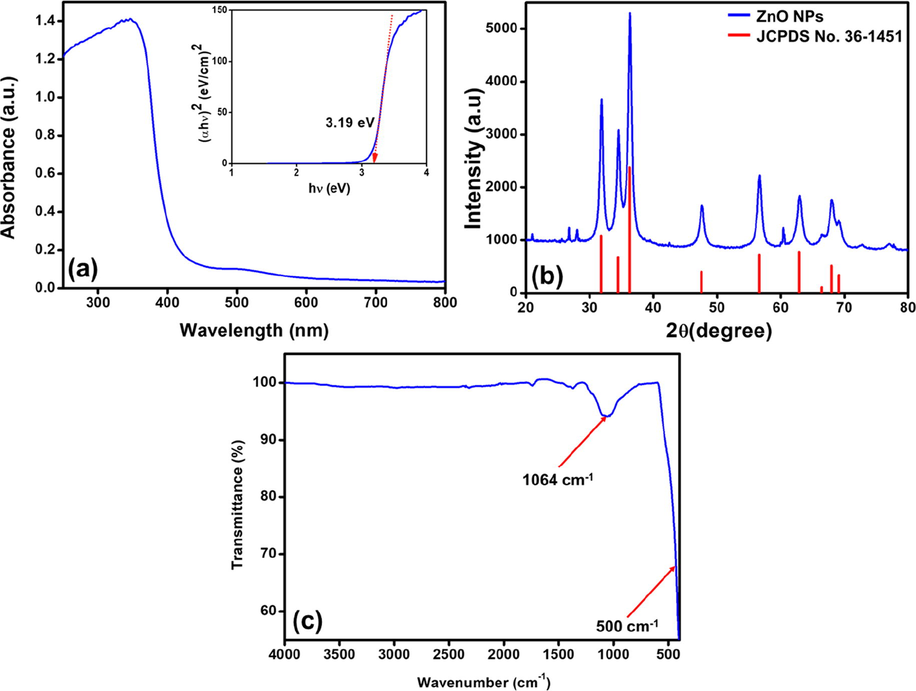
(a) UV–Vis analysis and bandgap spectra (inset), (b) XRD diffractogram pattern, and (c) FTIR spectra of the fabricated ZnO NPs.
To comprehend the crystal structure of the as-fabricated ZnO NPs, the powder XRD spectroscopic analysis is employed. Fig. 1(b) illustrates the XRD diffractogram for the ZnO NPs obtained by eco-friendly combustion of the cow dung procedure. The ZnO NPs exhibited a series of diffracted characteristic peaks at 31.8°, 34.4°, 36.3°, 47.5°, 56.6°, 62.8°, 66.5°, 67.9°, and 69.1° which can be assigned to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), and (2 0 1) planes, respectively. All these characteristic reflections are indexed to wurtzite ZnO hexagonal crystalline phase which is well-matched with their standard (JCPDS file no. 36-1451 with space-group P63mc, a = 3.243 Å and c = 5.201 Å) (Kumar et al., 2012). The mean crystalline size for the crystal facet of (1 0 1) of synthesized ZnO NPs is found to be ∼10 nm and the lattice spacing of crystals between the adjacent (1 0 1) facets is found to be ∼0.25 nm, which are calculated by utilizing the Debye-Scherrer equation and Bragg's law, respectively. Accordingly, the obtained XRD results show the high crystallinity of the prepared material and that no other impurities are detected.
The as-fabricated ZnO NPs is subjected to FTIR spectroscopy analysis Fig. 1(c). The spectral results emphasize the formation of ZnO NPs with an intensive peak below the wavenumber of 500 cm−1 belonging to the (Zn–O) stretching vibrations, as previously reported in the literature (Zhu et al., 2010). Nevertheless, the existence of other absorption peaks such as the wide-ranging peak situated at 1064 cm−1, which is attributed to the presence of the absorbed CO2 probably from the residues of the combustion of cow dung during the synthesis of ZnO NPs (Garba et al., 2019).
The topographic properties of prepared ZnO NPs are examined using FE-SEM. Fig. 2A and B represents the two FE-SEM micrographs for the ZnO NPs in low and high magnifications, respectively. The obtained images of the fabricated ZnO NPs distinctly show the presence of porous on the surface with irregular morphology. The elemental analyses of the fabricated ZnO NPs is conducted using EDX spectroscopy and the element percentage obtained results is displayed in Fig. 2C. It discloses that the fabricated NPs consists of the desired elements composition i.e. Zn and O, and the elemental composition percentage are illustrated in the internal table.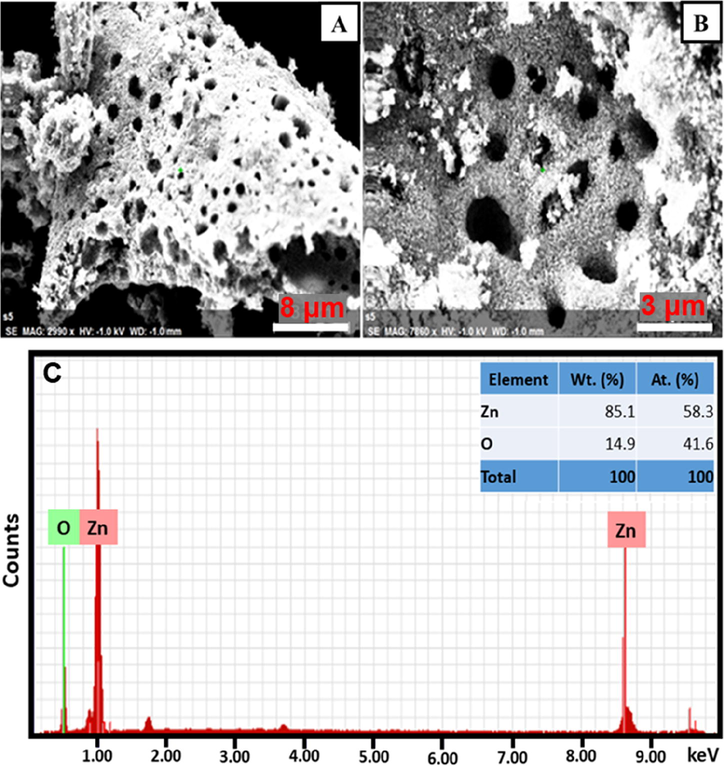
(A) and (B) FE-SEM micrographs of the fabricated ZnO NPs at different magnification and (C) EDX spectra of the prepared ZnO NPs.
3.2 Photocatalytic performance results of MB and AZ dyes over ZnO NPs photocatalyst
The main target of this work is to utilize the green synthesized ZnO NPs as photocatalyst for the decomposition of MB and AZ dye molecules, hazardous chemicals existent in the aqueous effluents from the textile industry. The photocatalytic decomposition efficacy of the fabricated ZnO NPs are tested by changing the various experimental conditions. The theory of semiconductor containing photocatalysts showed that the surface area, bandgap, crystallinity, particle size, morphology, and amounts of free OH• radicals on the photo-catalyst surface determine its photocatalytic activity (Zhang et al., 2018). The theory explained the production of electrons and holes on the photocatalyst surface by the absorption of light and the as-produced electrons and holes will engage in the reaction or undergo recombination. If the extra surface is provided for the electrons and holes, they would relocate where the electrons would be caught by photocatalyst while the holes would be trapped by hydroxyl radicals and generate OH• and HO2•. For metal oxide nanoparticles, more surfaces are available for relocation of photoproduced charge carriers and thus the generated hydroxyl radicals are used effectively to photodegradation of dyes. From the obtained observations from the UV–Vis spectra, it is distinct that the fabricated nanoparticles are active in the UV domain. Additionally, the band-gap is calculated to be Eg = 3.19 eV. The photocatalytic factors including a light source, photocatalyst quantity, concentration of dye, pH value, and irradiation time are systematically examined while MB and AZ are selected as the standard pollutants for photo-catalytic degradation in the report. The difference in absorption intensity of the peak recorded at 663 nm (MB λmax) is monitored to conclude the attained results.
From the absorbance, UV–Vis spectra, Ci and Cf are initial and final dye concentrations of the solution in the photocatalytic system, respectively and the calculation for the degradation activity of the MB and AZ dyes are carried out utilizing the following Eq. (1):
3.2.1 Influence of light source on dye decomposition
The initial studies are carried out to emphasize the light source that gave the maximum degradation activity of the synthesized ZnO NPs. Therefore, the photocatalytic degradation studies of MB and AZ dyes in the presence of ZnO NPs are conducted in three different conditions i.e. dark, UV light, and visible light. The photocatalytic data obtained are presented in Fig. 3. When the degradation experiment is conducted in the dark, the decomposition of both dyes is insignificant and can be ignored. Besides, when the degradation data obtained from the experiments performed in presence of visible light and UV light are compared, the obtained data revealed that the photodecomposition of MB and AZ dyes under UV light irradiation is considerably higher than the decomposition obtained when visible light is used. For UV light, the prepared ZnO NPs effectively degraded 99.7% of the MB dye which is much higher than visible light, which gave only a 22.0% degradation at a similar irradiation time (100 mins) as displayed in Fig. 3a. Similarly, AZ dye exhibits considerably higher photodegradation activity of 75.6% within 100 mins under UV light compared to visible light, which yielded only 19% degradation of AZ under the same conditions (Fig. 3b). The UV–Vis spectra of the fabricated ZnO NPs supported the results obtained for the photo-decomposition of MB and AZ in presence of UV light. From the above-mentioned observations, it could be stated that the photo-degradation performance of MB and AZ dyes using UV light is better than visible light. Consequently, it could be deduced that the synthesized ZnO NPs is efficient under UV light and all the further optimized studies are conducted under UV light.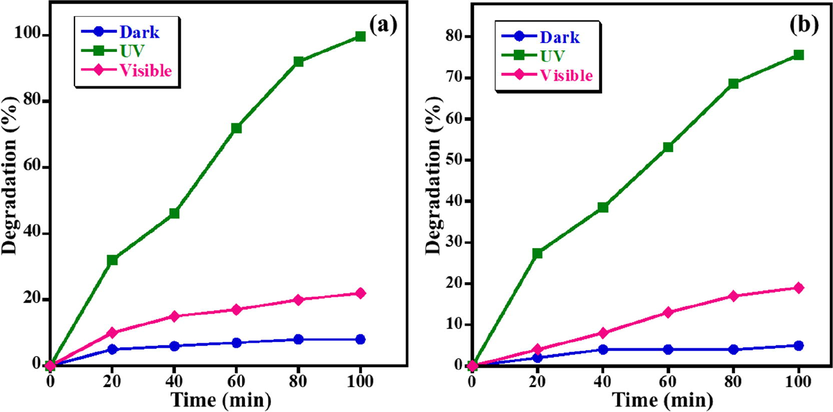
Effect of light source on photo-catalytic degradation of (a) MB and (b) AZ (Conditions: dye concentration = 5 ppm, photocatalyst amount = 15 mg, and pH of solution = 7).
3.2.2 Influence of amount of ZnO NPs photocatalyst on dye decomposition
In addition, the optimal photocatalyst dosage for the photodecomposition percentage of MB and AZ dyes is also assessed by altering the quantity of ZnO NPs from 5.0 mg to18.0 mg under UV light irradiation while a 5 ppm dye concentration and pH 7 remained unaltered. The results disclosed that the removal efficacy of both dyes is considerably influenced by the photocatalyst amount (Fig. 4). In the case of MB dye, it is observed that only 65.3% of MB dye degradation when 5.0 mg of photocatalyst is utilized. As expected, when the photocatalyst dose increased to 10.0 mg and 15 mg, 82.6% and 99.9% degradation of MB has detected, respectively. Notwithstanding, further increasing the amount of photocatalyst to 18.0 mg yielded a lower degradation activity and an 88.4% removal percentage of MB is obtained. An identical trend is noticed for the photodecomposition of AZ dye, the degradation activity of AZ dye is also increased linearly with the increase of ZnO NPs photocatalyst. It is obvious that by increasing the catalyst quantity from 5.0 mg to 18.0 mg, a photodegradation of AZ improved from 46.5% to 87.5% at the same photocatalytic conditions (Fig. 4b). This enhancement in the degradation is directly attributed to the introduction of more active sites in the medium that can generate more radical ions. When the photocatalyst dose exceeded a critical boundary there will not be sufficient space for the nanoparticles to disperse in the solution and the particles can stick to each other and become aggregated, owing to the particle’s surface energy. Therefore, most of the photocatalytic active sites are blocked or covered up and the degradation efficacy of the system decreased (Nguyen et al., 2018). As illustrated in Fig. 4(a,b), MB shows the best degradation performance at a photocatalyst amount of 15.0 mg, whilst, the highest decomposition of AZ dye at 18.0 mg, nevertheless, the variance of photocatalyst quantity is trivial for MB and AZ dyes. Therefore, 15.0 mg has chosen as the optimal photocatalyst dosage and is utilized for the rest of the experiments to optimize other factors.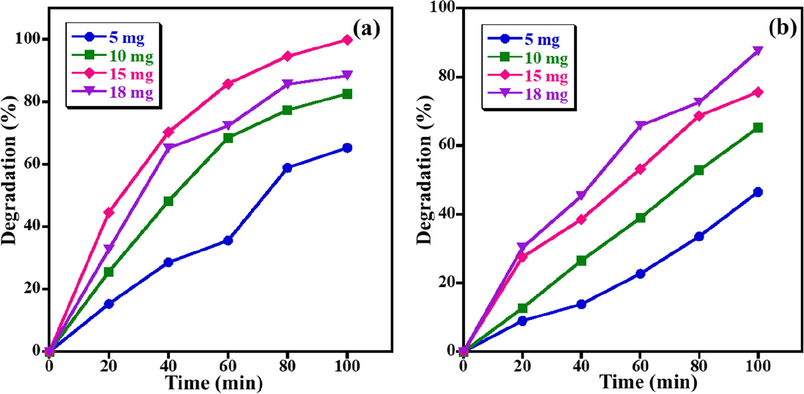
Impact of quantity of photocatalyst on photo-catalytic decomposition of (a) MB and (b) AZ (Conditions: dye concentration = 5 ppm, pH of solution = 7, and UV light irradiation).
3.2.3 Influence of dye concentration on dye decomposition
The impact of dye concentrations on the decomposition performance of the MB and AZ dyes is also assessed by changing the dye concentration from 5.0 ppm to 30.0 ppm using UV light irradiation while maintaining the photocatalyst amount at 15.0 mg and pH 7 as demonstrated in Fig. 5. The obtained results disclosed that the photocatalytic performance of the ZnO NPs is inversely proportional to the concentration of MB and AZ dyes under similar circumstances, i.e. maximum removal efficacy is observed at a minimum concentration (5 ppm). For MB dye, when the MB concentration raised from 5.0 to 30.0 ppm, the MB photo-degradation gradually reduced from ∼ 99.9% to ∼ 87.6%. A similar trend takes place for the degradation of AZ dye, in which the photo-degradation activity of AZ decreased from 75.6% to 39.3% when the AZ concentration increased from 5.0 to 20.0 ppm (Fig. 5b). This abatement owing to the decreased light absorption on the photocatalyst surface by raising the dye concentration, which in turn reduces the production of OH• radical ions which play a vital role in the photodegradation process (Nguyen et al., 2018). Therefore, it is indispensable to keep the ratio of concentration of photocatalysts to dyes alike. As a result, the maximal photodecomposition performance is obtained at the concentration of MB and AZ dyes of 5 ppm, therefore, 5.0 ppm dye concentration has selected in the next optimization studies.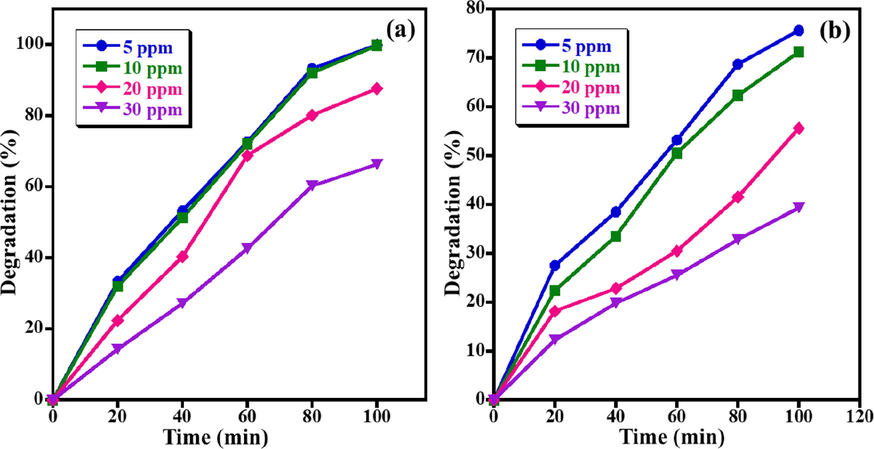
Effect of initial concentration on photo-catalytic decomposition of (a) MB and (b) AZ. (Conditions: photocatalyst amount = 15 mg, pH of solution = 7, and UV light irradiation).
3.2.4 Influence of pH value on dye decomposition
Indeed, the pH value of solution is one of the most important variables in the photocatalytic decomposition of hazardous organic dyes and the photo-catalytic efficacy of the catalyst is commonly linked to the existence of OH• radicals in the reaction medium, which improves the photodegradation of dye numerous folds in basic aqueous solutions. Fig. 6(a,b) discloses the influence of pH solution on photodegradation of MB and AZ dyes in presence of ZnO NPs. The effect of pH of solution on photo-degradation of MB and AZ is studied at four various pH values of 4.0, 5.2, 7.0 and 10.0 with maintaining other variables unaltered (i.e., 5.0 ppm concentration of dye, 15.0 mg catalyst amount and UV light irradiation). The lowest decomposition efficacy is observed at the lowest pH value (i.e., pH 4.0) with 34.3% and 30.6% of MB and AZ being decomposed at 100 min, respectively. Nevertheless, when the pH of the solution raised, the ZnO NPs photocatalyst showed higher degradation activity and 57.3%, 99.7%, and 99.8% degradation of MB are obtained at pH 5.2, 7.0, and 10 (Fig. 6a). This is possibly owing to the higher pH values leading to formation of negative charges on the photocatalyst surface. Since MB is a cationic dye that possesses a positive charge and the dyes have adsorbed on the surface. Additionally, higher amounts of OH• radical ions are adsorbed on the photocatalyst surface and the photodegradation process is carried out more efficiently in higher pH values (Farzana and Meenakshi, 2014). Likewise, the maximal AZ dye degradation activity of 96.8% is achieved at higher pH of 10 under similar conditions as displayed in Fig. 6b. The aforementioned results illustrated that photocatalytic degradation of dye in alkaline medium is considerably higher than acidic medium, which probably ascribed to the enhanced production of OH• radical ions (strong oxidizing species) (Saeed et al., 2016). Similar results were reported by Giacco et al. (2017), which reported that the obtained highest photodegradation of AZ dye at higher pH value due to the oxidation of dye by hydroxyl radicals and positive hole generated in the alkaline medium.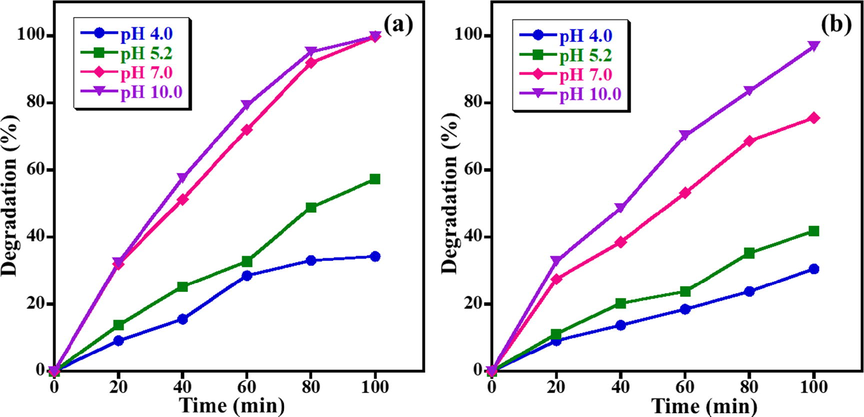
Effect of pH of solution on photo-catalytic degradation of (a) MB and (b) AZ (Conditions: dye concentration = 5 ppm, photocatalyst amount = 15 mg, and UV light irradiation).
The kinetic studies of photocatalytic degradation of MB and AZ dyes over the synthesized ZnO NPs photocatalyst distinctly displayed that the photocatalytic degradation process occurs through the Langmuir-Hinshelwopseudo-1st-order reaction according to the following Eq. (2):
In the above equation, t (min) is the irradiation time, Ci is the initial dye concentration at t = 0 min, Cf is the final dye concentration at time t, and k is the 1st-order rate constant. The MB photodegradation rate constants of 0.00455, 0.00847, 0.05146, and 0.05675 min−1 have accomplished for pH 4.0, 5.2, 7.0, and 10.0, respectively (Fig. 7a). Whilst, the MO photodegradation rate constants of 0.00344, 0.00529, 0.01407, and 0.03139 min−1 have calculated for pH 4.0, 5.2, 7.0, and 10.0, respectively (Fig. 7b).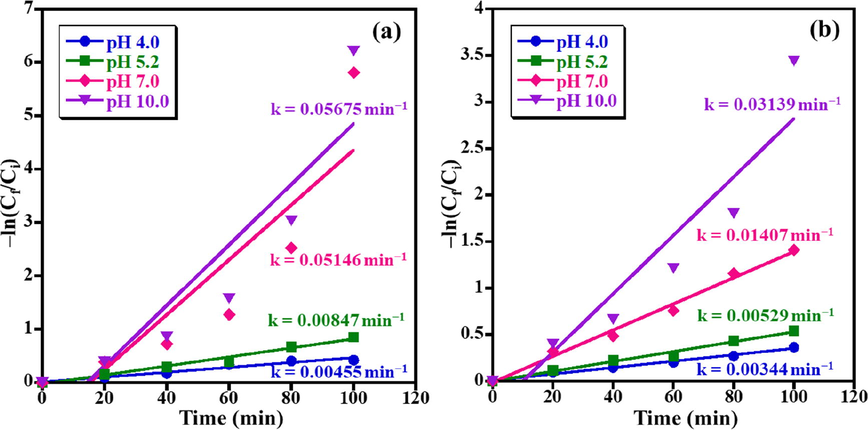
1st-order kinetics results for photo-catalytic removal of (a) MB and (b) AZ dyes in presence of ZnO NPs photocatalyst.
A comparison of the photo-decomposition performance of the synthesized ZnO NPs photocatalyst with many formerly photocatalytic systems containing ZnO for removal of MB and AZ, as displayed in Table 1. The prepared ZnO NPs photocatalyst in this study has comparatively superior photo-degradation performance for MB and AZ dyes in using UV light irradiation.
MB Dye Degradation
Photocatalyst
Light source
Time (min)
[Dye]
Catalyst dose
Degradation (%)
Ref.
ZnO NPs
UV-lamp
100
5 ppm
15 mg
99.8
Herein
ZnO
Hg-lamp 10 W
120
15 ppm
100 mg
90.0
(Soto-Robles et al., 2021)
Mn-ZnO
UV Lamp
90
10 ppm
24 mg
60.0
(Barick et al., 2010)
ZnO/AC
UV-lamp
45
2 × 10−5 M
0.25 g/L
92.2
(Mydeen et al., 2020)
Ag-ZnO
Solar radiation
30
10 ppm
100 mg
98.5
(Stanley et al., 2021)
Ag-ZnO@GO
Xe-lamp 20 W
180
15 ppm
20 mg
85.0
(Tran Thi et al., 2019)
ZnO/CuO
Solar radiation
120
20 ppm
0.5 g/L
93.0
(Cahino et al., 2019)
AZ Dye Degradation
ZnO NPs
UV-lamp
100
5 ppm
15 mg
96.8
Herein
ZnO/PNA
UV-lamp 250 W
120
250 ppm
200 mg
85.0
(Riaz et al., 2016)
Cd-ZnS
Solar radiation
120
5 × 10−5 M
30 mg
96.7
(Jabeen, Shah, and Khan 2017)
CuO/ZnO/TiO2
UV-light 6 W
180
10 ppm
100 mg
100
(Nasief and Abd 2019)
ZnO/GO
Xe-lamp 300 W
90
25 µg/mL
10 µg/mL
95.6
(Anjum et al., 2021)
ZnO SCs
LED lamp 23 W
780
20 ppm
0.1 g
58.0
(Kaur et al., 2020)
30%ZnO/Nylon
UV-light 15 w
300
200 ppm
0.01 g
58.3
(Saeed et al., 2021)
3.3 Photocatalytic mechanism
Scheme 2 illustrates the possible scheme presentation for photocatalytic decomposition of AZ dye on ZnO NPs surface. Under UV light irradiation, the electron-hole pairs are produced between the valence band (VB) and conduction band (CB) of ZnO NPs (Kansal et al., 2013). Therefore, the photo-produced electrons (e−) generated in the CB of ZnO is scavenged by O2 to form anion radical (O2•−) which on protonation (H+) gives HOO•. Nevertheless, the photo-induced holes (h+) which are generated in the VB of ZnO will react with –OH or H2O to form very active species of •OH radical ions. Therefore, the produced •OH radical ions are responsible for the full mineralization of dye, which in turn produces lower hazardous product such as CO2 as well as H2O. Accordingly, the photo-produced •OH and h+ active species are responsible for the decomposition of harmful pollutant (MB and AZ) dyes, and possible mechanisms can be summed up as follows (Rahman et al., 2013):
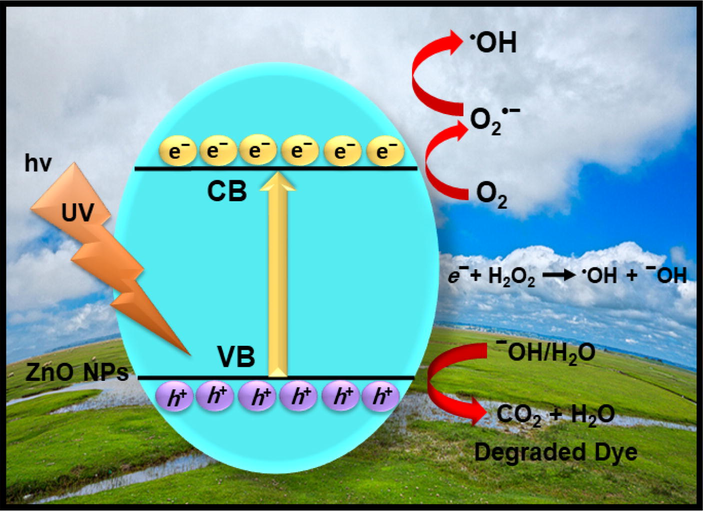
Possible photo-catalytic mechanism for charge transfer and photodecomposition of MB and AZ over ZnO NPs under UV light irradiation.
According to the aforementioned equations, the photo-decomposition of dye is accomplished over the ZnO NPs photocatalyst.
4 Conclusions
In brief, we report herein the efficacious synthesis of ZnO NPs, using an ecofriendly, facile, and one-pot combustion of cow dung method. The characterizations techniques confirmed the well-crystallinity wurtzite hexagonal ZnO NPs phase. The as-prepared sample is examined as a photo-catalyst for the photodecomposition of MB and AZ dyes, deleterious industrial effluents. The ZnO NPs are used for the photodecomposition of MB and AZ dyes under UV light irradiation, we have found that the fabricated ZnO NPs is efficient and the degradation performance is influenced by the variations in the light source, initial dye concentration, photocatalyst dosage, irradiation time, and pH value of the medium. The highest photocatalytic removal efficacy of MB (99.9%) and AZ (96.8%) was achieved at100 min under the optimal experimental conditions. Hence, it is can be stated that the ZnO NPs act as a highly effective catalyst for the decomposition of MB and AZ dyes which could be ascribed to electron-hole separation produced on the ZnO NPs surface in presence of UV irradiation. Henceforth, the green synthesized ZnO NPs could be employed as a promising candidate for remediation of wastewater and related photo-catalytic applications.
Acknowledgements
The authors would like to acknowledge the Researchers supporting project number (RSP-2021/222), King Saud University, Riyadh, Saudi Arabia. This work was supported by Vision Group on Science and Technology (SMYSR-2016; GRD 506) Govt. of Karnataka, India, Authors are thankful to the Principal and Management of Don Bosco Institute of Technology for their constant support and Technical Research Centre–Microscopy lab at JNCASR for providing microscopy facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alharthi, Fahad A., Nabil Al-Zaqri, Adel El marghany, Abdulaziz Ali Alghamdi, Ali Q. Alorabi, Neazar Baghdadi, H. S. Al-Shehri, Rizwan Wahab, and Naushad Ahmad. 2020. 'Synthesis of nanocauliflower ZnO photocatalyst by potato waste and its photocatalytic efficiency against dye', J. Mater. Sci.: Mater. Electron., 31: 11538–11547.
- Photo-degradation, thermodynamic and kinetic study of carcinogenic dyes via zinc oxide/graphene oxide nanocomposites. J. Mater. Res. Technol.. 2021;15:3171-3191.
- [Google Scholar]
- Adjustment of the band gap energies of biostabilized CdS nanoparticles by application of statistical design of experiments. J. Phys. Chem. B. 2004;108:7637-7640.
- [Google Scholar]
- Porosity and photocatalytic studies of transition metal doped ZnO nanoclusters. Microporous Mesoporous Mater.. 2010;134:195-202.
- [Google Scholar]
- Photodegradation of methylene blue dye by powders of Ni–ZnO floweret consisting of petals of ZnO nanorod around Ni-rich core. Mater. Chem. Phys.. 2020;253:123394
- [Google Scholar]
- Characterization and evaluation of ZnO/CuO catalyst in the degradation of methylene blue using solar radiation. Ceram. Int.. 2019;45:13628-13636.
- [Google Scholar]
- Counterion effect of cationic surfactants on the oxidative degradation of Alizarin Red-S photocatalysed by TiO2 in aqueous dispersion. J. Photochem. Photobiol., A. 2017;332:546-553.
- [Google Scholar]
- Synergistic effect of chitosan and titanium dioxide on the removal of toxic dyes by the photodegradation technique. Ind. Eng. Chem. Res.. 2014;53:55-63.
- [Google Scholar]
- Evaluation of adsorptive characteristics of cow dung and rice husk ash for removal of aqueous glyphosate and aminomethylphoshonic acid. Sci. Rep.. 2019;9:1-10.
- [Google Scholar]
- Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2018
- [Google Scholar]
- Photo catalytic degradation of Alizarin red S using ZnS and cadmium doped ZnS nanoparticles under unfiltered sunlight. Surf. Interfaces. 2017;6:40-49.
- [Google Scholar]
- Photocatalytic degradation of Alizarin Red S using simply synthesized ZnO nanoparticles. Mater. Lett.. 2013;106:385-439.
- [Google Scholar]
- Synthesis, characterization, and visible-light-induced photocatalytic activity of powdered semiconductor oxides of bismuth and zinc toward degradation of Alizarin Red S. Water Environ. Res.. 2020;92:1376-1387.
- [Google Scholar]
- A novel one-pot approach of ZnWO 4 nanorods decorated onto gC 3 N 4 nanosheets: 1D/2D heterojunction for enhanced solar-light-driven photocatalytic activity. J. Mater. Sci.. 2020;55:1170-1183.
- [Google Scholar]
- Investigations on structural, optical and second harmonic generation in solvothermally synthesized pure and Cr-doped ZnO nanoparticles. CrystEngComm. 2012;14:1653-2168.
- [Google Scholar]
- Recent advances in ZnO materials and devices. Mater. Sci. Eng., B. 2001;80:383-437.
- [Google Scholar]
- López, Uribe, Alvarez Lemus, MC Hidalgo, R López González, P Quintana Owen, S Oros-Ruiz, SA Uribe López, and J Acosta. 2019. 'Synthesis and characterization of ZnO-ZrO2 nanocomposites for photocatalytic degradation and mineralization of phenol', J. Nanomater., 2019.
- Graphene-zinc oxide nanocomposites (G-ZnO NCs): synthesis, characterization and their photocatalytic degradation of dye molecules. Mater. Sci. Eng., B. 2020;254:114516
- [Google Scholar]
- Facile synthesis of ZnO/AC nanocomposites using Prosopis Juliflora for enhanced photocatalytic degradation of methylene blue and antibacterial activity. Optik. 2020;224:165426
- [Google Scholar]
- Synthesis of nanocomposite and studying the degradation of alizarin dye. J. Biochem. Technol.. 2019;10:69.
- [Google Scholar]
- Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci.. 2018;65:201-222.
- [Google Scholar]
- Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B. 2018;227:296-311.
- [Google Scholar]
- Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: efficiency and degradation pathways. J. Cleaner Prod.. 2018;202:413-427.
- [Google Scholar]
- Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater.. 2009;162:674-681.
- [Google Scholar]
- Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett.. 2013;91:170-214.
- [Google Scholar]
- Comparative studies of the photocatalytic and microwave–assisted degradation of alizarin red using ZnO/poly (1-naphthylamine) nanohybrids. J. Mol. Liq.. 2016;216:259-267.
- [Google Scholar]
- Preparation of ZnO/Nylon 6/6 nanocomposites, their characterization and application in dye decolorization. Appl. Water Sci.. 2021;11:1-10.
- [Google Scholar]
- Synthesis of graphene-supported bimetallic nanoparticles for the sunlight photodegradation of Basic Green 5 dye in aqueous medium. Sep. Sci. Technol.. 2016;51:1421-2146.
- [Google Scholar]
- Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J. Mol. Struct.. 2021;1225:129101
- [Google Scholar]
- Excellent Photocatalytic degradation of Methylene Blue, Rhodamine B and Methyl Orange dyes by Ag-ZnO nanocomposite under natural sunlight irradiation. Optik 2021:166518.
- [Google Scholar]
- Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts. RSC Adv.. 2022;12:985-997.
- [Google Scholar]
- Fabrication of graphene nanosheets decorated by nitrogen-doped ZnO nanoparticles with enhanced visible photocatalytic activity for the degradation of Methylene Blue dye. J. Mol. Liq.. 2020;317
- [Google Scholar]
- Tran Thi, Viet Ha, Tri Nhut Pham, Tien Thanh Pham, and Manh Cuong Le. 2019. 'Synergistic Adsorption and Photocatalytic Activity under Visible Irradiation Using Ag-ZnO/GO Nanoparticles Derived at Low Temperature', J. Chem., 2019.
- An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2-and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem.. 2019;71:19-49.
- [Google Scholar]
- Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int.. 2011;37(1):393-398.
- [Google Scholar]
- Facile one-step synthesis of TiO 2/Ag/SnO 2 ternary heterostructures with enhanced visible light photocatalytic activity. Sci. Rep.. 2018;8:1-11.
- [Google Scholar]
- Monodispersed hollow microsphere of ZnO mesoporous nanopieces: preparation, growth mechanism and photocatalytic performance. Mater. Res. Bull.. 2010;45:2024-2030.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102047.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







