Translate this page into:
Facile bio-inspired fabrication of AgNPs from Salvia elegans leaf extract and determination of their cytotoxic DNA photocleavage potential
⁎Corresponding authors. vinaybcta@gmail.com (B. Vinay Kumar), shakeeliqubal@gmail.com (S.M.Shakeel Iqubal) shakeeliqubal@ibnsina.edu.sa (S.M.Shakeel Iqubal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
This study introduces a sustainable method for producing silver nanoparticles (AgNPs), focusing on sustainability and environmental protection. The technique includes the use of Salvia elegans aqueous leaf extract as a reducing agent. In addition, the research investigates the dose-dependent degradation of pUC19 DNA by silver nanoparticles, which is facilitated by the generation of singlet oxygen. The presence of S. elegans extract results in significant color changes, going from a colorless state to a dark brown hue, serving as an indication of the synthesis of AgNPs. A range of experimental methods were employed to analyze the biogenic AgNPs, such as UV–visible absorption spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The meticulously produced AgNPs demonstrate a high level of uniformity, featuring a spherical shape and a particle size of 60 nm. DNA photo-cleavage studies show that singlet oxygen plays a crucial role in triggering DNA damage caused by AgNPs, indicating their potential as powerful cytotoxic agents specifically aimed at cancer cells. Additional studies are required to clarify the effectiveness and specificity of AgNPs against various cancer cell types to assess their therapeutic potential. The cytotoxic effects of AgNPs, particularly in relation to DNA photocleavage, are of considerable interest in cancer research. This research holds promise for developing novel and sustainable cancer therapies based on the unique properties of biogenic silver nanoparticles.
Keywords
Silver nanoparticles
Salvia elegans
Cytotoxic
Reducing agent
DNA degradation
XRD
SEM
TEM
1 Introduction
Biomedicine, sensing, catalysis, information technology, electronics, and other fields are only a few of the many applications that nanotechnology has taken up at this time of rapid scientific progress (Tovar-Lopez, 2023, Malik et al., 2023, Hossain et al., 2023, Al-Thaqafy et al., 2023, Shaikh et al., 2022). Various techniques such as electrochemical methods, photochemical reactions, chemical reduction and environmentally friendly approaches, the so-called green chemistry, are used to produce and chemically stabilize metal nanoparticles (Jamkhande et al., 2019, Khan et al., 2019, Sakamoto et al., 2009, Khan and Asiri, 2016). Among these techniques, use of plants for nanoparticle synthesis represents an innovative and environmentally sustainable chemical approach. Biogenically produced silver nanoparticles (AgNPs) are among the inorganic nanomaterials that are most commonly employed because of their special physicochemical characteristics, which come from surface coatings of natural origin in addition to their size and form. This method outperforms traditional chemical and physical techniques in terms of expenditure and environmental impact. Importantly, it is scalable for large-scale production without requiring high pressure, elevated temperatures, or hazardous chemicals (Kharissova et al., 2019, Shankar et al., 2004).
Salvia elegans (S. elegans), a member of the Lamiaceae plant family, possesses caffeic acid groups, making it potentially beneficial for reduction reactions. In a prior study, Albeladi et al. emphasized the efficiency, reliability, and cost-effectiveness of AgNPs synthesized from Salvia officinalis. Phytochemicals present in S. officinalis, particularly rosmarinic acid, are crucial in enhancing the stability of the AgNPS. (Royji Albeladi et al., 2020).
Nanoparticles possess exceptional ability to load drugs and display notable photoluminescent characteristics, which makes them ideal for the specific delivery of chemotheraputic medications and imaging (Sarma and Das, 2021, Bae et al., 2011, Aldabaan et al., 2024, Cheng et al., 2021, Muddapur et al., 2022, Iqbal et al., 2021). This enables the accurate delivery of drug loaded nanomedicines to organs like the brain, which are typically protected by specific barriers. Ongoing advancements in nanomedicine are expected to provide even greater benefits in the future, particularly as researchers focus on enhancing the properties of nanomaterials at the intersection of biology and nanotechnology (Mitchell et al., 2021).
Studies on chemotherapy drugs containing metal compounds have acted as a catalyst for the development of novel cancer drugs. This is primarily attributed to the potential of these drugs to exhibit reduced toxicity while improving antiproliferative properties against malignant tumors (Zhou et al., 2020). Transition metal complexes have gained tremendous research potential in recent decades owing to their remarkable similarity to nucleases in their mode of action. The mechanism behind their activity is driven by the redox properties of the metals, which, when combined with dioxygen, generate reactive oxygen species that lead to DNA damage through base modification or strand breakage (Collin, 2019).
Current experiments in this field have focused on DNA degradation using metal nanoparticles such as platinum and gold nanoparticles. The generation of singlet oxygen using metal nanoparticles is considered advantageous, especially in the company of nanoparticles synthesized from gold and platinum (Jose et al., 2011). The production of singlet oxygen can be enhanced by the presence of metal nanoparticles, as demonstrated in a recent study by Zhang et al (Zhang et al., 2007). Enhanced electromagnetic fields near metal nanoparticles contribute to enhanced penetration. Furthermore, the amount of absorption and the relative augmentation of singlet oxygen generation by photosensitizers can be predicted using a variety of computation techniques (Pucelik et al., 2020). The primary aim of this research was to assess the DNA binding and DNA photocleavage properties of AgNPs synthesized using S. elegans extract. To thoroughly characterize the materials, a range of analytical techniques, including UV–Vis spectrophotometry, TEM, SEM, and XRD were used.
This study supports the trend of harnessing natural resources to create nanomaterials, which is in line with the growing emphasis on the use of nanotechnology in biomedical applications. The synthesis of AgNPs and the assessment of their biological effects place this study at the intersection of materials science and pharmacology. The research aims to develop a facile and eco-friendly approach for synthesizing AgNPs using S. elegans leaf extract while simultaneously assessing their cytotoxic potential through DNA photocleavage assays. This study not only contributes to the field of green nanotechnology but also opens avenues for the development of innovative therapeutic agents against cancer, enhancing our understanding of the interplay between nanomaterials and biological systems.
2 Experimental
2.1 Identification and Extraction of the plant
Fresh leaves of S. elegans (Tangerine Sage) were collected from the Thurahalli forest area in Bengaluru, Karnataka, India (Fig. 1). The leaves were carefully gathered, washed, and sun-dried, repeating this process four to five times to eliminate any dust and impurities. A total of 25 g of the leaves were cut into small pieces and placed in a 250 mL flask containing demineralized water (100 mL). The mixture was heated at 100 °C for 45 min, it was allowed to cool. The leaf extracts were then filtered using Whatman filter paper, and the resulting extracts were utilized for synthesizing silver nanoparticles.
Photograph of S. elegans plant.
2.2 Synthesis of AgNPs
S. elegans extract and silver nitrate (AgNO3) were used to produce silver nanoparticles. A noticeable hue shift occurred at 50–60 °C as a result of the slow addition of 10 mL of S. elegans extract to 50 mL of 0.1 mM aqueous AgNO3 solution in order to decrease Ag + ions. The UV–Vis spectra of the solution was used to monitor the decrease of Ag + ions.
2.3 UV–visible spectroscopy based analysis
The reduction of silver ions in the colloidal solution was characterized and verified using UV–Visible spectroscopy (Hitachi, U-3310 Tokyo, Japan). For analysis, 1 mL sample was put into a quartz cuvette. Absorbance was conducted from 200-700 nm using distilled water as the reference standard. Baseline readings were also taken of 1 mM solutions of AgNO3 and HAuCl2 to isolate the spectral signals of each metal ion. The UV–vis spectrophotometer measured light attenuation across the wavelength range, revealing where the metal ions were most reduced through formation of gold and silver nanoparticles based on peaks in absorbance. This helped identify the composition of the synthesized colloid and verify the ion-to-metal reduction process.
2.4 SEM based analysis
The sample was prepared by applying a drop of AgNPs onto a gold-coated copper grid for 5–6 min and then allowing it to air dry before transferring it to the microscope, which was operated at an accelerated voltage of 10 kV (Model: HITACHI S-3400 N).
2.5 XRD based analysis
The phase nature, lattice parameters, and crystalline size and structure of the green-produced AgNPs were ascertained by XRD analysis using the Shimadzu XRD-6000/6100 model. Utilizing Cu-Kα radiation, the scattering 2θ range was 20◦–80◦. 30 kv and 30 mA of current were used to operate the instrument.
2.6 TEM based analysis
Ten milliliters of AgNPs solution were put on a Formvar/Carbon lacey 3-mm copper grid and dried at room temperature. After drying, the grid was placed in a transmission electron microscope equipped with a microanalysis holder. The analysis was performed in TEM mode with an accelerating voltage of 100 kV. Silver nanoparticles were analyzed using a Hitachi HT7700 Exalens transmission electron microscope.
2.7 DNA binding studies
Employing the UV–visible absorption method, we examined the DNA binding properties of AgNPs. Calf thymus DNA (CT-DNA) was selected for this study in order to ensure that no proteins were present. Using a pH 7.2 buffer solution with 5 mM Tris-HCl and 50 mM NaCl, each experiment was conducted in accordance with the instructions given in the literature (Shahida Parveen et al., 2014).
2.8 Photonuclease studies of pUC19 DNA
Pre-incubated samples containing pUC19 DNA (100 µM), nucleotide phosphate (10 µL), and the proper dosage of medications were used in the investigation. The quartz tube in which these samples were placed had an inner diameter of 3 mm. Tris-HCl buffer, pH 8.0, was utilized as the medium in the AgNP instance. After that, UV light was emitted. Following irradiation, the quartz tube holding the irradiated sample was filled with 2 μL of sample buffer. A 0.8 % (w/v) agarose gel was then instantaneously loaded with the contents in their entirety. In this experiment, the gel was subjected to electrophoresis for four hours at a voltage of 40 V and a pH of 8.0 in a conventional TBE buffer. After that, staining of the gel was done with a staining solution that contained 0.5 μg/ml of ethidium bromide for 30 min. Through a series of control tests with various quenchers, the precise reactive oxygen species causing DNA damage was determined. The dioxygen was removed using nitrogen gas, and the 1O2 was quenched using 10 mmol of NaN3. Furthermore, 200 mmol (mmol) of dimethyl sulfoxide (DMSO) were employed as a hydroxyl radical (•OH) scavenger.
3 Results and Discussion
This study describes a practical method for the synthesis of AgNPs using S. elegans extract. The reduction of silver ions to AgNPs is probably due to the phytochemicals, especially caffeic acid and phenolic compounds. During the synthesis reaction, the color of the solution was found to remain constant, indicating depletion of all silver salt in the solution. The subsequent analysis of the AgNPs confirmed the exceptional efficiency of the product. The effectiveness of the reaction was finally assessed by examining the absorption spectra collected at regular intervals. A recent study successfully illustrated the use of a natural plant extract as a catalyst for the formation of C = N bonds, thereby facilitating an organic process. The data points provided in the text are expressed as a range of numbers, in particular (Vidyavathi et al., 2021, Vidyavathi et al., 2022).
Additionally, an additional research study was conducted using natural catalysts such as caffeic acid (extracted from S. elegans) for the synthesis. This study revealed significant production of AgNPs within a period of 5 to 15 min when a stoichiometric amount of Salvia elegans extract was introduced.
3.1 UV–vis spectroscopy
The synthesis of metallic nanoparticles by reduction in an aqueous solution was observed using the UV–visible spectroscopy method following interaction with S. elegans extract. Fig. 2 shows the absorption spectra of AgNPs using the UV–Vis method. Throughout the reaction, the AgNPs solution consistently exhibited a surface plasmon band at approximately 424 nm (Pattanayak et al., 2017). The nanoparticles were evenly distributed throughout the aqueous solution, as evidenced by the absence of aggregation in the UV–visible absorption spectra.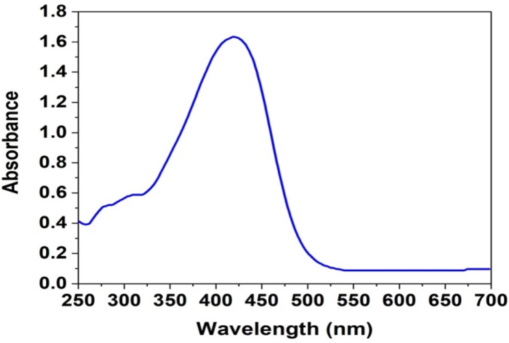
UV–Vis absorption spectra of AgNPs.
3.2 SEM analysis
SEM was employed to estimate the morphology of the material and reveal the presence of nanostructured AgNPs. The nanoparticles exhibit a configuration characterized by both stochasticity and nonuniformity, as shown in Fig. 3. This observation is consistent with findings from previous studies (Mukherji et al., 2019), which found that these nanoparticles typically adopt a spherical shape.
SEM image of the AgNPs.
3.3 TEM analysis
A detailed examination of the transmission electron microscopy (TEM) images in Fig. 4 predicted the sizes of the AgNPs, which were found to be 60 nm. The characteristic dot pattern observed in the images indicates the presence of single-crystal particles. The use of extracts from the S. elegans plant leads to the synthesis of crystalline nanoparticles characterized by uniform particle size and minimal coagulation. This phenomenon facilitates the homogeneous distribution of the particles and thus ensures a uniform size (Mukherji et al., 2019).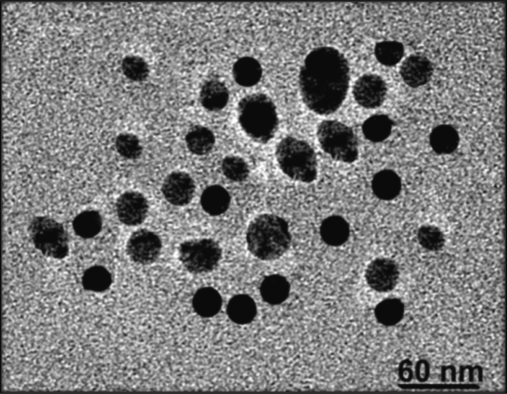
TEM image of the AgNPs.
3.4 XRD analysis
The AgNPs were characterized using the XRD method, as depicted in Fig. 5. The confirmation of the crystalline structure of the AgNPs occurred subsequent to the identification of five peaks [38.063 (111), 44.1392 (200), 64.4001 (220), 77.3686 (311), and 81.558 (222)], aligning with cubic silver nanoparticles (Fig. 5), and corresponding precisely with data from JCPDS file no. 89–3722. Particle size was determined using the Debye-Scherrer formula, analyzing the width of the first peak.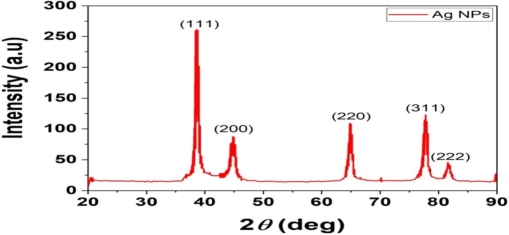
XRD analysis of the AgNPs.
3.5 DNA interaction studies
3.5.1 Electronic absorption studies
Studying the interaction between the AgNPs and CT-DNA can be carried out using UV–Vis absorption bands. When AgNPs were introduced into CT-DNA at a steady concentration, a significant reduction in the intensity of their UV–Vis absorption bands, known as hypochromism, was observed. In addition, a slight shift toward longer wavelengths, particularly a red shift of 3 to 4 nm, was evident, as shown in Fig. 6. The data shows that the AgNPs penetrate the DNA effectively.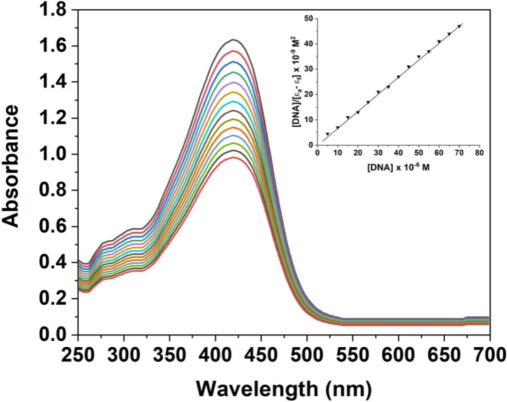
UV–Vis spectrum of the synthesized AgNPs.
The intrinsic binding constant (Kb) for AgNPs is determined to be 2.4 × 104 M−1. This measure is largely consistent with the results of a previous study (Das et al., 2021), indicating a high level of agreement. The binding affinity values obtained from electronic spectrum analysis suggest that AgNPs have a higher degree of intercalation with nuclear DNA compared to commercially available drugs.
3.6 DNA photocleavage studies
To analyze the influence of AgNPs on pUC19 DNA, a series of experiments were performed. DNA samples were subjected to sequential treatment with increasing concentrations of AgNPs ranging from 50 to 200 μM. This treatment lasted 100 min at a 37 °C in phosphate buffered saline (PBS) diluted sufficiently to achieve a pH of 7.4. The treated DNA samples were then processed for further analysis. Following incubation, the DNA was was subjected to electrophoresis on an agarose gel. A significant association was found between the amount of DNA degradation caused by AgNPs and the concentration of AgNPs applied (Fig. 7, lanes 5–8).
Image of the gel electrophoresis for the control trials with SC DNA (0.5 µg) and additional additives at 365 nm after an hour of exposure. Lane 1 represents DNA Control + supernatants; lane 2 represents DNA + DMSO (4 µl) + AgNPs; lane 3 represents DNA + NaN3 (38 µM) + AgNPs; lane 4 represents DNA + Tris (14 µl) + AgNPs; lane 5 to 8 represents DNA + 50, 100, 150, 200 µM AgNPs, respectively.
Throughout the DNA incubation process, control samples were prepared using solutions containing S. elegans extract and the supernatant of the AgNP dispersion coupled with silver nitrate. However, none of these components had the ability to break down DNA. This discovery lends support to the theory that the presence of AgNPs is the primary cause of DNA degradation. Chemical scavengers were used to locate and remove the active species from the reaction mixture in order to more thoroughly understand the molecular mechanism of the degradation process (Vadivel et al., 2021; Vinay Kumar et al., 2021). The molecules used in this study included dimethyl sulfoxide, which acts as a scavenger for hydroxyl free radicals, as well as sodium azide and tris-[hydroxylmethyl]aminomethane, both of which act as scavengers for singlet oxygen (Bin-Jumah et al., 2020).
The experimental data depicted that the inclusion of sodium azide (0.2 M) and tris-[hydroxylmethyl]-aminomethane (0.2 M) resulted in complete suppression of DNA degradation facilitated by AgNPs, as shown in Fig. 7, lanes 3 and 4 shown. Conversely, the effectiveness of dimethyl sulfoxide (0.2 M) in attenuating complete DNA degradation was observed to be limited (Fig. 7, lanes 2). The data obtained from the study provide convincing evidence that DNA degradation caused by AgNPs is facilitated by the involvement of singlet oxygen.
4 Conclusion
This research investigated the synthesis of silver nanoparticles (AgNPs) from Salvia elegans leaf extract and assessed their cytotoxic potential through DNA photocleavage. Characterization revealed that the AgNPs had an average diameter of 60 nm. Notably, these nanoparticles significantly induced DNA degradation at 37 °C in a dose-dependent manner, highlighting the pivotal role of AgNPs in this process. The study also confirmed the involvement of activated oxygen species in DNA damage, supported by previous findings that sodium azide and tris-[hydroxylmethyl]-aminomethane effectively inhibited such damage. These results provide empirical evidence for the capacity of AgNPs to induce DNA damage via singlet oxygen production. Consequently, AgNPs demonstrate considerable promise as cytotoxic agents, with potential applications in targeting cancer cells in therapeutic settings.
Future Implications: The implications of this research extend beyond the immediate findings, offering avenues for developing novel cancer therapies and enhancing our understanding of the interactions between nanomaterials and biological systems. Continued exploration in this field could lead to breakthroughs in cancer treatment and a more sustainable approach to nanotechnology. Several cancer cell lines are particularly relevant for evaluating the cytotoxic effects of AgNPs synthesized from S. elegans. Cervical cancer cells (HeLa cell line), widely used in cancer research, serve as a baseline for studying cytotoxicity. MCF-7 cells, which are estrogen receptor-positive breast cancer cells, are useful for investigating hormone-responsive tumors. A549 cells, a human lung adenocarcinoma line, are relevant for lung cancer therapies, while HCT116 cells aid in assessing the efficacy of AgNPs in gastrointestinal malignancies. Lastly, U87MG cells, representing glioblastoma, are crucial for exploring treatments for aggressive brain tumors.
CRediT authorship contribution statement
Mufarreh Asmari: Data curation, Conceptualization. A.H. Shridhar: Formal analysis. Joy H. Hoskeri: Methodology. B. Vinay Kumar: Writing – review & editing, Software, Project administration, Methodology. Nayef Abdulaziz Aldabaan: Validation, Methodology. Ibrahim Ahmed Shaikh: Writing – original draft, Validation. Abdulaziz Hassan Alhasaniah: Visualization. Mater H. Mahnashi: Visualization, Resources, Funding acquisition. Arun Shettar: Formal analysis. Basheerahmed Abdulaziz Mannasaheb: Validation, Methodology. Aejaz Abdullatif Khan: Validation, Formal analysis. Amal Bahafi: Methodology, Formal analysis. Uday M. Muddapur: Writing – review & editing, Methodology. S.M.Shakeel Iqubal: Writing – review & editing, Writing – original draft.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King of Khalid University for funding this work through Small Groups Project under grant number RGP1/17/45. The author would like to express sincere gratitude to AlMaarefa University, Riyadh, Saudi Arabia, for supporting this research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of antimicrobial, anticancer, antidiabetic, antioxidant activities and silver nanoparticles synthesized from Indian Clove-Syzygium aromaticum leaf extract. Journal of King Saud University-Science. 2024;36
- [Google Scholar]
- Physicochemical investigation and fluorescence quenching of biologically active pyrrole-containing push-pull chromophore by Ag nanoparticles. J. Mol. Struct.. 2023;1274:134421
- [CrossRef] [Google Scholar]
- Nanomaterials for cancer therapy and imaging. Mol. Cells. 2011;31:295-302.
- [CrossRef] [Google Scholar]
- Effects of green silver nanoparticles on apoptosis and oxidative stress in normal and cancerous human hepatic cells in vitro. Int. J. Nanomedicine. 2020;15:1537-1548.
- [CrossRef] [Google Scholar]
- Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol. Oncol.. 2021;14:85.
- [CrossRef] [Google Scholar]
- Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci.. 2019;20:2407.
- [CrossRef] [Google Scholar]
- Biocompatible silver nanoparticles: An investigation into their protein binding efficacies, anti-bacterial effects and cell cytotoxicity studies. J. Pharm. Anal.. 2021;11:422-434.
- [CrossRef] [Google Scholar]
- Advances and significances of nanoparticles in semiconductor applications-A review. Results Engineering. 2023;19
- [Google Scholar]
- Effect of CTABr (surfactant) on the kinetics of formation of silver nanoparticles by Amla extract. J. Mol. Liq.. 2021;329:115537
- [CrossRef] [Google Scholar]
- Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol.. 2019;53:101174
- [CrossRef] [Google Scholar]
- Singlet oxygen mediated DNA degradation by copper nanoparticles: potential towards cytotoxic effect on cancer cells. J. Nanobiotechnology. 2011;9:9.
- [CrossRef] [Google Scholar]
- Fluorescence quenching of environmentally benign highly fluorescence donor (D)-π-acceptor (A)-π-donor (D) quinoline dye by silver nanoparticles and anionic surfactant in liquid stage. J. Mol. Liq.. 2016;221:381-385.
- [CrossRef] [Google Scholar]
- Nanoparticles: Properties, applications and toxicities. Arab. J. Chem.. 2019;12:908-931.
- [CrossRef] [Google Scholar]
- Greener synthesis of chemical compounds and materials. R. Soc. Open Sci.. 2019;6:191378
- [CrossRef] [Google Scholar]
- Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov.. 2021;20:101-124.
- [CrossRef] [Google Scholar]
- Plant-based synthesis of gold nanoparticles and theranostic applications: A review. Molecules. 2022;27:1391.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev.. 2019;4
- [CrossRef] [Google Scholar]
- Butea monosperma bark extract mediated green synthesis of silver nanoparticles: Characterization and biomedical applications. J. Saudi Chem. Soc.. 2017;21:673-684.
- [CrossRef] [Google Scholar]
- Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: properties, mechanisms, and applications. Coord. Chem. Rev.. 2020;416:213340
- [CrossRef] [Google Scholar]
- Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol.. 2020;9:10031-10044.
- [CrossRef] [Google Scholar]
- Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. c: Photochem. Rev.. 2009;10:33-56.
- [CrossRef] [Google Scholar]
- Multifunctional nanoparticles—cost versus benefit of adding targeting and imaging capabilities. Multifunctional Theranostic Nanomedicines in Cancer. Elsevier 2021:367-387.
- [Google Scholar]
- Synthesis, characterization, and DNA binding studies of nanoplumbagin. J. Nanomater.. 2014;2014
- [CrossRef] [Google Scholar]
- Characterization of bioactive compounds from acacia concinna and citrus limon, silver nanoparticles’ production by a. concinna extract, and their biological properties. Molecules. 2022;27:2715.
- [CrossRef] [Google Scholar]
- Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci.. 2004;275:496-502.
- [CrossRef] [Google Scholar]
- Recent progress in micro- and nanotechnology-enabled sensors for biomedical and environmental challenges. Sensors (basel). 2023;23:5406.
- [CrossRef] [Google Scholar]
- DNA binding activity of novel discotic phenathridine derivative. J. Mol. Liq.. 2021;332:115798
- [CrossRef] [Google Scholar]
- Cashew nutshell liquid catalyzed green chemistry approach for synthesis of a Schiff base and its divalent metal complexes: molecular docking and DNA reactivity. Nucleosides Nucleotides Nucleic Acids. 2021;40:264-287.
- [CrossRef] [Google Scholar]
- Punica granatum pericarp extract catalyzed green chemistry approach for synthesizing novel ligand and its metal(II) complexes: Molecular docking/DNA interactions. J. Mol. Struct.. 2022;1249:131656
- [CrossRef] [Google Scholar]
- Advancement in specific strand scission of DNA and evaluation of in-vitro biological assessment by pharmacologically significant tetraaza macrocyclic metal complexes constrained by triazole. Nucleosides Nucleotides Nucleic Acids. 2021;40:896-913.
- [CrossRef] [Google Scholar]
- Metal-enhanced singlet oxygen generation: A consequence of plasmon enhanced triplet yields. J. Fluoresc.. 2007;17:345-349.
- [CrossRef] [Google Scholar]
- The drug-resistance mechanisms of five platinum-based antitumor agents. Front. Pharmacol.. 2020;11
- [CrossRef] [Google Scholar]







