Translate this page into:
Expression of nucleic acid oxidation metabolites 8-Oxo-GSn and β-amyloid protein in the urine and cerebral tissues of diabetic rats
⁎Corresponding author. gezhaoming6574@aol.com (Zhaoming Ge)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aim of the study is to analyze the changes of 8-Oxo GSn expression and to explore the possible mechanism of type 2 diabetes mellitus combined with cognitive disorder using Zucker Diabetic Fatty rats (ZDF). Five-month-old ZDF rats and the wild-type control rats were employed in this study. Immunohistochemical (IHC) staining and isotope dilution high performance liquid chromatography - tandem mass spectrometry (ID - LC - MS/MS) method were used for analyzing 8-Oxo GSn in brain tissues and urine. The biochemical markers (Aβ40 and Aβ42) were also analyzed. In the cerebral cortex and hippocampus of ZDF rats, 8-Oxo GSn level was increased significantly than control rats. Aβ40 and Aβ42 levels were significantly increased in the brain of ZDF rats than control animals (P < 0.05). APP expression level was maximum in ZDF rats than that of control rats (P < 0.05). After eight months of 8-Oxo GSn injection into the brain of ZL rats, Aβ expression was considerably increased in ZL rats than control (P < 0.05). Overexpression of 8-Oxo GSn is one of the main reasons for ZL rats combined cognitive disorder.
Keywords
Type 2 diabetes mellitus
8-Oxo GSn
APP expression
Microglia
Cognitive disorder
1 Introduction
Oxidative stress triggered by the chemical and photo-exposure can severely damage cellular biomolecules including RNA through oxidation of nucleotides (Estevez et al., 2021). 7,8-dihydro-8-oxo-deoxyGuanine (8-Oxo G) is one of the important oxidation products of RNA nucleotides. Guanosine is highly vulnerable than other nucleosides to oxidative damage in RNA due to very low redox potential than adenosine and pyrimidines (Kupfer and Leumann, 2014). The level of 8-OxoG on RNA is increased mainly through two different processes. Reactive oxygen species can lead to the formation of 8-oxo-G in DNA molecule, as well as give rise to the oxidation product 8-oxo-dGTP nucleotide pool in the cell. 8-OxoG does not prevent nucleic acid synthesis, but can induce alternate base pair mismatch (van den-Boogaard et al., 2021). 8-OxoG mismatch during RNA synthesis may lead to error in gene expression, because 8-OxoG pairs with adenine and cytosine, insertion of 8-OxoG into mRNA can alter gene expression. These lesions can be improved by base excision repair, nucleotide excision repair and mismatch repair. The 8-OxoG produced by the repair process can be transported by cell membrane and excreted into cerebrospinal fluid, plasma, and urine without further metabolism. Amino acid sequence analysis showed that cells treated with 8-OxoGTP secreted Aβ peptide in addition to a large number of Aβ peptides, and also secreted 28 unidentified Aβ peptides (Aβ1-42) (Dai et al., 2018). The results showed that a large amount of 8-Oxo-G in the body would promote the secretion of Aβ peptide (Condello and Stöehr, 2018).
β-amyloid precursor protein (APP) is a normal metabolite formed by hydrolysis of APP, which has certain neurotoxic property (Montesinos et al., 2020). APP is a type I transmembrane glycoprotein that exists in a variety of tissues and is a regulator of synapse formation and repair (Chen et al., 2017). Human APP can be processed through two different pathways: amyloidosis and non-amyloidosis (Sawikr et al., 2017). The amyloid pathway of APP refers to that APP is sequentially cleaved on the plasma membrane by β-secretase (BACE) and γ-secretase (gamma-secretase) at the N-terminal and C-terminal (Area-Gomez and Schon, 2016), mainly in Aβ40 and Aβ42 forms. Microglia are tissue resident macrophages of the spinal cord and brain, implicated in important homeostatic, developmental, and disease processes and their roles are highly complicated (Goetzl et al., 2018). There is a symbiotic relationship between microglia and neurons, which maintain each other in a non-inflammatory phenotype and activate rapidly when attacked. Microglia initiate and guide the coordination of neuro inflammatory responses (Hoeijmakers et al., 2017). Microglia are parenchymal myeloid cells of the central nervous system, with significant roles in disease, injury, homeostasis and development. A cascade of neurotoxic changes induced by long-term microglia activation and release of pro-inflammatory cytokines is likely to cause Alzheimer's disease (AD) (Yu and Richard, 2015). In this study, isotope dilution high performance liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) was used to detect the level of 8-Oxo-GSN in brain and urine of Zucker Diabetic Fatty (ZDF) obesity model rats.
2 Materials and methods
2.1 Materials
Male ZDF obesity model rats and male Zucker Lean (ZL) rats were obtained from Nanjing Junke Bioengineering Co., LTD. Creatinine and ammonium acetate were ACS grade and methanol (HPLC grade) was obtained from Thermo Fisher Scientific Co., LTD. The other chemicals, including 8-Oxo-GSN were either analytical grade or ACS grade (Shanghai Zhihua Chemical Technology Co., LTD.). A SunFire C18 Column (5 μm, 4.6 × 250 mm) (Waters Technologies (Shanghai) Co., LTD.) was used for the determination of 8-Oxo-GSn.
2.2 Experimental animals and groups
Specific-pathogen-free (SPF) grade 5-month of male ZDF obesity rats (type 2 diabetic rats) and Zucker Lean (ZL) rats (control) were selected (n = 10). These animals were maintained in animal house of Animal Center of Anhui Medical University. Animals were maintained with 12 h light/dark photoperiod at 24 ± 2 °C and supplied with adequate water and food (AIN93 standard diet) for 14 months.
2.3 Samples
After anesthesia, one side of the rat brain tissue was immediately separated on the ice platform. To the brain tissue, physiological saline was added and ground with a glass homogenizer. The homogenized sample was centrifuged at 10000 × g for 15 min at 4 °C. 8-Oxo-GSn was injected into the hippocampus aseptically and the results were observed.
2.4 Pretreatment of urine
Urine sample was collected from the experimental animal. Frozen urine was thawed in water bath at 37 °C for 10 min, centrifuged at 7500 × g for 5 min at 4 °C. The supernatant (20 μL) was mixed with working solution (180 μL) (70% methanol). It was treated with 1% formic acid with 5 mmol/L ammonium acetate. The sample was maintained in a water bath for 10 min and centrifuged at 10000 × g for 15 min. The supernatant was used for ID-LC-MS /MS analysis. 8-Oxo-GSn (10 μL) was used as the internal standard. The creatinine concentration in the urine sample was diluted appropriately before analysis.
2.5 Liquid phase and mass spectrometry
Liquid phase conditions: Water phase (liquid A): To the ammonium acetate solution (5 mmol/L) (1L), 1 mL chromatographic grade formic acid was added. The pH of the solution was adjusted to 3.75. Organic phase (liquid B): Methanol (Fisher Scientific) was used as the organic phase. The injection volume of the sample was 5 μL. The equipment was attached with chromatographic column (Waters SunFire C18; 5 μm, 4.6 × 250 mm) and the column temperature was 35 °C. MS conditions were: ionization-positive ion mode; Multi Reaction Monitoring (MRM); Dry temperature: 200 °C; Dry air flow: 16 L/min; Atomizing gas pressure: 30 psi; Sheath temperature: 400 °C; Sheath gas flow: 12 L/min; Capillary voltage : 2000 V; Nozzle voltage: 0 V; The Fragmentor and CE values of m/ Z from 300 to 168 and 8-Oxo-GSn quantitative ion pairs are 380 V and 14 eV, respectively. The Fragmentor and CE values of qualitative ion pair m/ Z from 300 to 140 of 8-Oxo-GSn are 380 V and 38 eV, respectively. The Fragmentor and CE values of m/ Z 303 → 171 of the internal standard of 8-Oxo-GSn-IS reaction ion pair of 8-Oxo-GSn are 380 V and 12 eV, respectively.
2.6 Observation indicators
Body weight, blood glucose concentration, serum glycosylated hemoglobin, serum insulin were compared between the experimental and the control group. Different age groups were used for the determination of 8-OxoG content in urine, brain and glial Aβ level in brain.
2.7 Statistical analysis
SPSS 18.0 statistical software was used for statistical analysis and processing of the data. The results were expressed as mean ± standard deviation (±S). The mean value of 8-Oxo-GSn/creatinine in the brain and urine of the control group and the experimental group was compared by independent sample T test. Enumeration data were expressed by number (n) or percentage (%) and tested by χ2. The probability value (P < 0.05) indicated statistically significant.
3 Results
3.1 Biochemical analysis of experimental and control group
Blood glucose, serum hba1c and insulin concentrations in the experimental group were significantly increased (P < 0.05). In the experimental group animals, body weight decreased considerably than control rats. Blood glucose level was increased over five-fold in the experimental group animals (Table 1). Note: *Significant different between experimental and control group at P < 0.05 level.
Group
Control group (n = 10)
Experimental group (n = 10)
Weight (g)
451.2 ± 14.4
400.3 ± 20.4*
Blood glucose (mmol/dL)
5.76 ± 0.52
15.82 ± 2.65*
Serum hBA1c (% L)
4.31 ± 0.18
13.21 ± 0.60*
Serum insulin (pmol/ L)
324.6 ± 18.7
549.1 ± 19.6*
3.2 Oxyguanosine content in urine of rats at different age groups
8-Oxo-GSn level in the urine of the experimental and control groups was determined. The level of oxyguanosine and determined isotopes were described in Fig. 1A. Oxidized guanosine in different age groups was detected, and the content of 8-OxoG increased with age, and the experiment was conducted up to 14 months after birth. The oxyguanosine content in urine was maximum in 14-month age group, and there was significant statistical difference (P < 0.01). As described in Fig. 1B, the age-related increase in 8-Oxo-GSn level was found to play a significant role in both experimental and control animals. The numerical differences between ZDF and ZL rats were relatively small in the early stages of growth, but significant differences were observed between ZDF and ZL rats in the later stages (after 10 months). 8-Oxo-GSn level in urine was higher in ZDF rats than in corresponding age group of control ZL rats.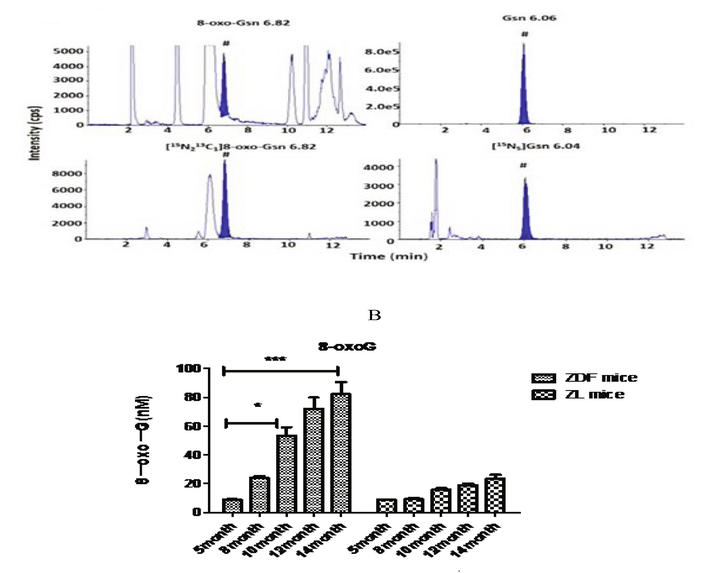
(A) Isotopes of oxyguanosine in experimental animal. (B) Effect of 8-OxoGSn level in urine sample of control and experimental rats.
3.3 Comparison of 8-Oxo GSn levels in brain and urine
It was found that there was statistical difference of 8-OxoGSn level in urine between the 12 month control and experimental group (P < 0.042). The level of 8-oxo GSn was also varied significantly between control and experiment group at 14 months (P < 0.021). After 4 months, 8-OxoGSn level was 8.92 ± 0.51 nM in the urine sample and it increased as 82.11 ± 15.46 nM after 14 months. The amount of 8-OxoGSn ranged between 82.11 ± 15.46 nM (5 months) and 11.16 ± 9.91 nM (14 months) in the brain tissue of control group. Moreover, the synthesized level increased in the brain tissues of the experimental animal. The increase of urine and brain 8-OxoGSn level was related to the increase of age, and significant difference was observed between experimental and control group (ZDF and ZL rats) after 14 months in the brain tissue. ZDF rats in the experimental group had a higher level of 8-OxoGSn in brain tissues (P < 0.05), as shown in Table 2. Note: * Statistical significant at P < 0.05 level.
Group (months)
Sample
Control group (n = 10)
Experimental group (n = 10)
t-value
P-value
5
8.81 ± 0.49
8.92 ± 0.51
0.767
0.772
8
9.33 ± 2.87
23.98 ± 3.14
4.312
0.244
10
Urine
15.84 ± 5.37
53.48 ± 5.62
5.846
0.118
12
18.84 ± 11.23*
71.87 ± 10.19*
3.931
0.042
14
23.53 ± 13.37*
82.11 ± 15.46*
8.931
0.021
5
6.91 ± 0.49
7.10 ± 0.41
0.818
0.567
8
7.20 ± 2.34
10.31 ± 2.14
2.149
0.474
10
Brain
8.12 ± 4.19
11.01 ± 5.34
4.113
0.219
12
8.93 ± 7.28
15.94 ± 8.49
7.185
0.129
14
11.16 ± 9.91*
23.97 ± 9.73*
9.981
0.045
3.4 Comparison of glial Aβ level between experimental and control group
After the blood glucose level of ZDF rats was similar to that of control rats (15.82 ± 2.65 mmol/L and 15.76 ± 0.52 mmol/L), the levels of glial Aβ in the brain of ZDF rats in experimental group and control group were measured and compared. The results showed that the contents of Aβ40 and Aβ42 in glial cells of experimental rats were significantly higher (P < 0.05), as shown in Fig. 2.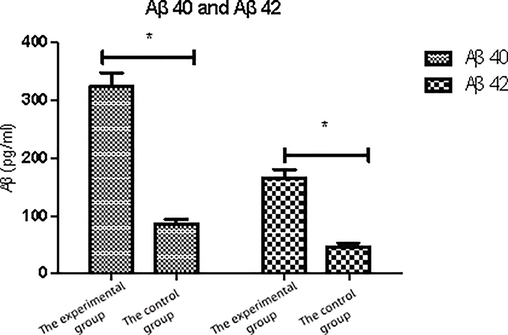
Expression with Aβ 40 and Aβ 42 in each group.
3.5 Comparison of Aβ level in brain of rats after injection
Extracts rich in 8-OxoGSn were injected into the hippocampus region of rats. After 3 months of treatment, Aβ deposition appeared and distributed in the marginal system structure. In addition to the hippocampus, a large number of Aβ deposits were also found in the olfactory bulb, anterior cingulate cortex, motor cortex, somatosensory cortex, frontal cortex, auditory and visual cortex, and highly specific distribution in the limbic system. It was speculated that peripheral or systemic injection of 8-OxoGSn could also cause Aβ amyloid deposition in the brain, and experiments were conducted to determine Aβ amyloid deposition in the brain. The sample containing 8-OxoG can induce amyloidosis rapidly only if they come into direct contact with the brain. However, 8-OxoG cannot be easily spread from the periphery region of the brain, and may require injection of a higher concentration of 8-OxoG extract over a longer period of time. Fig. 3 shows the comparison of Aβ levels before and after 8-OxoGSn injection.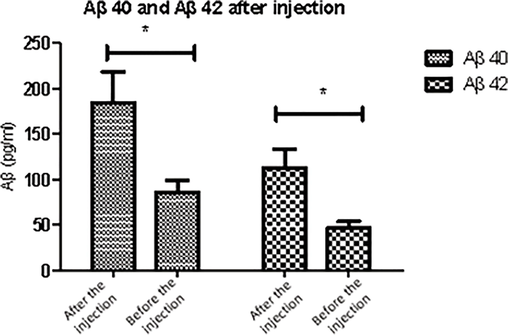
Expression with Aβ 40 and Aβ 42 in each group after 8-OxoGSn injection.
4 Discussion
ROS and superoxide dismutase (SOD) produced as the results of diabetic mitochondrial dysfunction are the main causes of diabetic complications, which are very important for determining and predicting diabetic complications and evaluating oxidative stress in diabetic patients. However, since ROS production in different tissues cannot be directly measured in vivo condition, 8-OxO-GSn extracted from oxidative damaged RNA is the natural choice as a novel biomarker (Sassa and Odagiri, 2020). In Alzheimer's brain, OGG1 and MTH1 maintain a low level of 8-oxoguanine (Oka et al., 2021). 8-OxoG can be used to evaluate the degree of age-related tissue damage and choose the therapeutic opportunity, including important diseases such as diabetes, cognitive dysfunction and Alzheimer's disease (Kolbanovskiy et al., 2021). Therefore, in this study, ZDF obesity model rats were used to establish a diabetic animal model, and the correlation between 8-OxoG and Aβ in urine and brain of rats was detected.
Oxidative stress is a state of imbalance between oxidation and antioxidation in normal cells of human body, which is related to various age-related diseases (Peng-Yuan et al., 2016). In contrast to more stable DNA, RNA is a single-stranded nucleic acid with no histone protection and is more susceptible to oxidation than DNA. There are many oxidative repair mechanisms in the body, so that the oxidative nucleosides in RNA can be repaired and cleared in time. Studies have been found that the free 8-Oxo-GSn is consistent with the plasma level. These studies revealed that we can indirectly determine the oxidative stress level of the body by examining the urine 8-OXo-GSn content. Currently, studies have shown that high glucose content in the serum can up-regulate APP protein level by inhibiting APP protein degradation. In addition, BACE1 also plays a key role in energy metabolism (Plucińska et al., 2016). Other studies have shown that Aβ monomer does not directly affect the function of neurons. The soluble Aβ aggregates generated after monomer hydrolysis have been reported as the key factors in cognitive function in AD patients (Lin et al., 2018; Shrestha et al., 2017). Aβ can also combine with RAGE to destroy the blood-cerebrospinal fluid barrier (Am et al., 2017), weaken its barrier results poor cerebral blood circulation. In addition, the combination of RAGE and Aβ mediated through the blood-cerebrospinal fluid barrier, the increase of Aβ in blood circulation and the decrease of sRAGE led to the decrease of Aβ in the brain. The decreased level of Aβ in the brain results in the deposition of Aβ in brain tissue which damaged central conduction system. This process occurred in chronic complications of diabetes such as cognitive dysfunction (Penke et al., 2017). In addition, abnormal insulin lyase (IDE) activity and levels have also been considered as key factors leading to cognitive dysfunction (Haque and Nazir, 2016). IDE is an enzyme that acts under neutral pH conditions. Oxygen free radicals in the brain may lower the pH value and thus significantly reduce the activity of IDE. IDE acts on mature neurons and involved in the degradation of intracellular soluble Aβ monomer. Therefore, it is of great significance to explore the effect of urinary RNA oxidation product, 8-OxoG on brain glial cells through amyloid Aβ. The results showed that intravenous injection, oral administration, and intraocular/nasal injection could not cause obvious Aβ amyloid deposition in the brain of rats and this study was similar with the results of previous studies (Lin et al., 2018).
In this study, it was found that urine 8-OxoGSn content in ZDF rats was significantly higher than that of control group (P < 0.05). With the increase of age, the levels of 8-OxoGSn in the brain and urine of diabetic rats increased. It is hypothesized that this is because of 8-OxoGTP, and 8-OxoGTP may be degraded to 8-Oxo-GMP in the brain and cells of various tissues. The degraded 8-OxoGMP generally metabolized to 8-Oxo-GSn. Further, 8-Oxo-GSn enters the blood circulation through the cell membrane and is finally discharged through urine. Compared with the control rats, the contents of Aβ40 and Aβ42 in brain of ZDF rats were significantly increased (P < 0.05). The expression of APP in ZDF rats was stronger than that in control rats (P < 0.05). After 8-Oxo GSn injected into the brain of ZL rats in control group, Aβ level increased significantly after five months (P < 0.05). It was also found that microglia produced different Aβ amyloid deposition in different parts of the brain, which was consistent with previous findings (Sharma et al., 2016; Efthymiou and Goate, 2017). In this study, the levels of Aβ amyloid deposition were higher in the hippocampus and temporal lobe than in the frontal lobe, and this revealed impaired functions of different parts of the brain. The temporal lobe and hippocampus are closely related to cognitive memory, while the frontal lobe is related to spatial memory and movement. It is speculated that it may be mainly related to the selection of animal models and the region-specific changes of free radicals in the brain and the region-specific changes of antioxidant damage mechanism. Other antioxidant enzymes found in the brain include superoxide dismutase, cysteinase and glutathione superoxide enzyme as well as glutathione molecules. Studies have been reported that the levels of these antioxidant proteins in different parts of the brain are not significantly different. We found the difference in Aβ amyloid deposition in different parts of the brain and this is the result of differences in nucleic acid oxidation levels in different parts of the brain.
5 Conclusions
Diabetic rats are prone to accumulate 8-Oxo GSn in vivo, which leads to abnormal Aβ generation and degradation as well as abnormal accumulation and deposition of Aβ in brain, resulting in excessive Aβ precipitation in brain glial cells. This can build up over time and lead to cognitive impairment. These results suggest that excessive 8-Oxo GSn may be one of the important causes of type 2 diabetes mellitus complicated with cognitive dysfunction. Analysis on 8-Oxo Gsn will provide great help for the treatment of cognitive impairment in diabetic patients.
Conflict of interest
Declared none by the author.
References
- Imperatorin shows selective antitumor effects in SGC-7901 human gastric adenocarcinoma cells by inducing apoptosis, cell cycle arrest and targeting PI3K/Akt/m-TOR signalling pathway. J. BUON. 2017;22(6):1471-1476.
- [Google Scholar]
- Mitochondria-associated ER membranes and Alzheimer disease. Curr. Opin. Genet. Dev.. 2016;38:90-96.
- [Google Scholar]
- Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sinica. 2017;38(9):1205-1235.
- [Google Scholar]
- Aβ propagation and strains: implications for the phenotypic diversity in Alzheimer's disease. Neurobiol. Dis.. 2018;109:191-200.
- [Google Scholar]
- Transcriptional mutagenesis mediated by 8-oxoG induces translational errors in mammalian cells. Proc. Nat. Acd. Sci.. 2018;115(16):4218-4222.
- [Google Scholar]
- Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegen.. 2017;12(1):1-12.
- [Google Scholar]
- Oxidative damage to RNA is altered by the presence of interacting proteins or modified nucleosides. Front. Mol. Biosci.. 2021;8:631.
- [Google Scholar]
- High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol.. 2018;83(3):544-552.
- [Google Scholar]
- Haque, R., Nazir, A., 2016. Identification and functional characterization of a putative IDE, C28F5. 4 (ceIDE-1), in Caenorhabditis elegans: Implications for Alzheimer's disease. Biochim. Biophy. Acta (BBA)-Gen. Sub. 1860(11), 2454-2462.
- Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model. Brain Behav. Immun.. 2017;63:160-175.
- [Google Scholar]
- Base and nucleotide excision repair pathways in DNA plasmids harboring oxidatively generated guanine lesions. Chem. Res. Toxicol.. 2021;34(1):154-160.
- [Google Scholar]
- Oxidative damage on RNA nucleobases. In: Erdmann V.A., Markiewicz W.T., Barciszewski J., eds. Chemical Biology of Nucleic Acids. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. p. :75-94.
- [CrossRef] [Google Scholar]
- Hepatitis B Virus X protein increases 8-Oxo-7, 8-dihydro-2ʹ-deoxyguanosine (8-Oxodg) level via repressing MTH1/MTH2 expression in hepatocytes. Cell. Physiol. Biochem.. 2018;51(1):80-96.
- [Google Scholar]
- The Alzheimer's disease-associated C99 fragment of APP regulates cellular cholesterol trafficking. EMBO J.. 2020;39(20)
- [CrossRef] [Google Scholar]
- MTH1 and OGG1 maintain a low level of 8-oxoguanine in Alzheimer's brain, and prevent the progression of Alzheimer's pathogenesis. Sci. Rep.. 2021;11(1):1-17.
- [Google Scholar]
- The role and underlying mechanism of differently aggregated components of oligomeric beta-amyloid protein in the progress of Alzheimer's disease. Prog. Biochem. Biophy.. 2016;43(2):109-114.
- [Google Scholar]
- β-Amyloid and the pathomechanisms of Alzheimer’s disease: a comprehensive view. Molecules. 2017;22(10):1692.
- [Google Scholar]
- Neuronal human BACE1 knockin induces systemic diabetes in mice. Diabetologia. 2016;59(7):1513-1523.
- [Google Scholar]
- Understanding the sequence and structural context effects in oxidative DNA damage repair. DNA Rep.. 2020;93:102906.
- [Google Scholar]
- Neuroinflammation in Alzheimer's disease: the preventive and therapeutic potential of polyphenolic nutraceuticals. Adv. Protein Chem. Struct. Biol.. 2017;108:33-57.
- [Google Scholar]
- Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol. Res.. 2016;38(9):838-850.
- [Google Scholar]
- Fungus-derived hydroxyl radicals kill hepatic cells by enhancing nuclear transglutaminase. Sci. Rep.. 2017;7(1)
- [CrossRef] [Google Scholar]
- van den-Boogaard, M.L., Oka, R., Hakkert, A., Schild, L., Ebus, M.E., van Gerven, M.R., Zwijnenburg, D.A., Molenaar, P., Hoyng, L.L., Dolman, M.E.M., Essing, A.H., 2021. Defects in 8-oxo-guanine repair pathway cause high frequency of C> A substitutions in neuroblastoma. Proc. Nat. Acd. Sci. 118(36), e2007898118.
- Microglial Aβ receptors in Alzheimer’s disease. Cell. Mol. Neurobiol.. 2015;35(1):71-83.
- [Google Scholar]







