Translate this page into:
Exploring the uncharted: Zinc and phosphate solubilization in Zn-P isolates from wheat rhizosphere inceptisols
⁎Corresponding author. renugupta2781975@gmail.com (Renu Gupta) renuguptaju96@gmail.com (Renu Gupta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This research delves into the untapped potential of phosphorous and zinc solubilizing rhizobacteria, known as Zn-P isolates, from wheat rhizosphere inceptisols. A total of thirty rhizosphere soil samples were collected, resulting in recovery of forty unique bacterial isolates. After initial screening, out of forty isolates, recovered on the basis of halozone formation on the nutrient agar medium. four were subjected to biochemical and further molecular identification. Four isolates, identified as Bacillus subtilis (Zn-P-1), Pseudomonas aureginosa (Zn-P-2), Staphylococcus aureus (Zn-P-3), and Methylobacterium organophyllum (Zn-P-4) through a set of 16S rRNA primers, forward (5́- GGATGAGCCCGCGGCCTA-3́) and reverse (5́- CGGTGTGTACAAGGCCCGG-3́), exhibited superior solubilization efficiency of phosphorous and zinc These strains were evaluated via in vitro and pot culture assays. The study found that Zn-P-1 demonstrated the highest zinc solubilization (134.87 mg/l) when ZnO was used as the zinc source as compared to ZnCO3 and Zn-EDTA as also highest at 72.32 mg/l in CaH2PO4 and lowest at 14.44 mg/l with KH2PO4 using P sources, thus highlighting the role of Bacillus in zinc and phosphorous activity with substrate type. The inoculation of tri-calcium phosphate (TCP) and ZnCO3, along with Bacillus and Methylobacterium, led to increased phosphorous and zinc solubilization, uptake, and use efficiency, marking these rhizobacteria as potentially beneficial for nutrient enhancement and PGPR activities in wheat crops grown in inceptisols.
Keywords
Wheat
PGPR
Inceptisols
Phosphorous
Zinc
Rhizobacteria
Solubilization
1 Introduction
Next to nitrogen, phosphorus (P) stands out as the second most crucial macronutrient for promoting plant development. It plays a pivotal role in supplying, transferring, and storing energy for all biochemical processes within plants, as highlighted by Khan et al. (2009). Despite the pressing need for increased crop yields, a mere 0.1 percent of total soil phosphorus is soluble and accessible to plants. This scarcity stems from phosphate fixation and the limited solubility of phosphorus in soil, as elucidated by Pereira and Castro (2014). Indeed, only soluble ionic phosphate (Pi) proves efficient as mineral nutrition for plants. Microbial-mediated phosphorus management plays a crucial role in various biological processes, including the transformation of unavailable and insoluble soil nutrients, as highlighted by Babalola and Glick (2012). The ability of phosphate-solubilizing rhizobacteria (PSRB) to convert insoluble forms of phosphorus into accessible forms, such as orthophosphate, stands as a key characteristic in enhancing crop plant development and output. This process facilitates the utilization of phosphorus by plants, contributing significantly to their growth and productivity.
The ability of phosphate-solubilizing bacteria to convert insoluble phosphorus (P) into soluble P through mechanisms such as the release of organic acids, chelation, and ion exchange contributes to soil fertility enhancement (Sharma et al., 2013). A lot of bacterial strains have been found to be involved in the process of phosphate solubilization. These belong to a diverse range of genera, including Mesorhizobium, Arthrobacter, Chryseobacterium, Bacillus, Gordonia, Delftia, Enterobacter, Mycobacterium, Pantoea, Klebsiella, Micrococcus, Flavobacterium, Phyllobacterium, Rhizobium, Serratia, Sinorhizobium and Pseudomonas, as highlighted by Chen et al. (2006).Tricalcium phosphate (TCP) is a mineral apatite known to be more easily degradable compared to single superphosphate (SSP). Thus, its use alongside phosphate-solubilizing rhizobacteria (PSRB) has been found to enhance phosphate solubilization by releasing organic acids, thereby rendering native and added phosphorus soluble. influences plant growth positively, as demonstrated by Kshetri et al. (2018).
Zinc (Zn) serves as a critical trace element essential for plant growth and reproduction, participating in numerous biological processes. Low solubility of zinc rather than its scarcity is the main reason for zinc deficiency.
Despite being required in trace amounts, zinc deficiency remains common in wheat during different crop growth phases. The zinc availability is influenced by several factors, including soil texture, pH, phosphorus content, soil type, and prevailing weather conditions. The complex interplay of these factors affects the solubility and accessibility of zinc in the soil, consequently impacting its uptake by plants and their overall health and development.
Soil moisture and pH levels influence the solubility of zinc which is why semi-arid and arid parts of Indian agro-ecosystems often experience deficiency of zinc. Addressing this deficiency usually requires the external application of soluble zinc sources like ZnCO3, ZnO, and ZnSO4. However, it's crucial to acknowledge that only approximately 20 % of the administered zinc is readily accessible for plant absorption, with the rest transforming into different inaccessible forms.
Certain bacteria, including Burkholderia cenocapacia, Bacillus sp. and Pseudomonas sp. have been found to play a crucial role in converting zinc to soluble forms, thereby making zinc available for uptake by plants. This transformative process has been documented by studies conducted by Khande et al. (2017).
Zinc-solubilizing bacteria utilize diverse mechanisms, including acidification, siderophore production, and oxidoreductive systems on cell membranes, to solubilize zinc. They release organic acids into the soil, which sequester zinc cations and can also chelate zinc, thus improving zinc availability for plant uptake. The activities of these bacteria play a crucial role in mitigating zinc deficiency and fostering robust plant growth in agricultural environments.
Furthermore, PSRB (phosphate-solubilizing rhizobacteria) and ZnSRB (zinc-solubilizing rhizobacteria) isolates obtained from indigenous wheat crops offer a promising alternative technique to address soluble phosphorus, zinc, and macronutrient deficiencies in the wheat rhizosphere, as highlighted by Khan et al. (2006). These isolates encompass a diverse range of unrelated bacteria, suggesting their potential to address multiple nutrient deficiencies simultaneously.
Hence, research on zinc and phosphorus solubilization by bacteria carries significant importance in enriching zinc nutrition in plants and tackling nutrient deficiencies in agricultural systems. It offers valuable insights into the potential of microbial interventions to enhance nutrient availability, soil fertility, and ultimately crop productivity. With this background the present study was conducted to untap the potential of phosphorous and zinc solubilizing bacterial isolates from wheat rhizosphere from inceptisols of two wheat growing districts of Jammu province of J&K (India).
2 Materials and methods
2.1 Isolation of bacteria
In Udhampur and Jammu districts of Jammu and Kashmir, India, thirty rhizosphere soil samples were collected from the wheat rhizosphere using GPS coordinates. These samples were subjected to analysis, resulting in the recovery of forty bacterial isolates based on formation of halozone of solublization on nutrient agar medium. To commence the isolation process, 0.5 mm of the diluted sample suspension from a 10–5 dilution was evenly spread on nutrient agar plates followed by their incubation for a week at 28±2 °C in a biochemical oxygen demand (BOD) incubator. Following the incubation period, isolates showing halozone formation were identified and selected for further analysis.
Subsequently, these selected isolates were subcultured on modified Pikovskaya medium (PVK medium) (Pikovskaya, 1948) and zinc-solubilizing medium. This step allowed for the characterization and evaluation of the isolates' abilities to solubilize zinc and phosphorus, respectively, two very essential plant growth and development nutrients. The subculturing process served to isolate and identify bacterial strains with potential applications in agriculture, particularly in enhancing nutrient availability in the soil for improved crop productivity.
2.2 Measurement of pH
The pH levels of the Zn-P culture filtrates and the uninoculated samples were checked by filtering the culture using an Elico pH meter.
2.3 Zn-Solubilization (plate assay)
For zinc assay, all isolates were inoculated into zinc agar medium containing 0.1 % insoluble zinc compounds viz. ZnO, ZnCO3 and Zn-EDTA followed by their incubation for 48 h at 30 °C. Further the clearing zone diameters around each colony was measured. The screening of isolates was done on the basis of solubilization potential and the solubilization criteria was taken as a preliminary tool for assessing potential of Zn-P isolates. The solubilization criteria was studied as benchmark test for further biochemical (using chemical kit-KB002 R-Himedia) and molecular identification, and its evaluation under pot culture assays.
2.4 Isolation of genomic DNA methodology
Genomic DNA of the screened isolates was isolated following (Wilson, 2001).
2.5 Electrophoresis of genomic DNA
A 0.8 % agarose gel was prepared by dissolving agarose powder in 1 × TAE (Tris-Acetate-EDTA) buffer. Ethidium bromide, a fluorescent dye, was added to the agarose gel to enable visualization of DNA bands under UV (ultraviolet) light. The DNA samples, along with a DNA ladder marker ranging from 100 to 1500 base pairs (bp), were carefully loaded into the wells of the agarose gel. The gel was submerged in a tank containing 1 × TAE buffer. An electric current is applied across the gel, causing the negatively charged DNA molecules to migrate towards the positive electrode. The gel is typically run at a constant voltage, such as 100 V and it was was visualized on a UV transilluminator.
2.6 Amplification of genomic DNA
The amplification of genomic DNA was conducted through the polymerase chain reaction (PCR) using using a set of forward (5́-GGATGAGCCCGCGGCCTA-3́) and reverse primers (5́-CGGTGTGTACAAGGCCCGG-3́) for 16S rRNA. The PCR reaction mixture, totalling 25 µl, comprised 50 ng of genomic DNA, along with the following components: 15 mM Tris/HCl pH 8.5, 10 mM KCl, 0.1 % (v/v) Triton X, 3 mM MgCl2, 0.25 mM each deoxyribonucleotide triphosphate (dNTP), 2 units of Taq DNA polymerase, and 0.5 µM each forward and reverse primer. The amplification process was conducted in a thermal cycler, with a total of 35 cycles. The cycling conditions involved an initial denaturation step at 94 °C for 5 min, followed by 33 cycles of denaturation at 94 °C for 40 s, annealing at 55 °C for 45s, and extension at 72 °C for 45 s. After amplification, the PCR products were subjected to electrophoresis in a 1.0 % agarose gel. The gel, stained with a DNA-intercalating dye such as ethidium bromide, was then placed under UV transillumination to visualize the amplified DNA bands. The DNA bands were captured using a gel documentation system, allowing for further analysis and documentation of the PCR results.
2.7 DNA sequencing and sequence analysis
The gel aliquots with amplified products of biochemically screened isolates were subjected to sequencing and further analysis and phylogenetic tree was constructed following procedure as outlined by Williams et al. (2000).The result of most potent Zn-P-1 solubilizer (Bacillus subtilis) is presented in Fig. 5a.
2.8 Effect of P and Zn sources on P-solubilisation and Zn –solubilisation (broth assay)
2.8.1 P-solubilization
The effect of different soluble and insoluble phosphorous sources was studied with the addition of 1 % of respective phosphorous salts viz. TCP, CaH2PO4 and KH2PO4 and zinc sources, by 1 % ZnO, 2 % ZnCO3 and 1 % Zn EDTA in respective PVK medium and zinc solubilizing medium inoculated with selected microbial culture of each strain and incubated and added with 25 ml Barton’s reagent, final volume being 50 ml each and respective OD was measured using spectrophotometer.
The bacterial isolates were individually inoculated into PVK medium supplemented with0.1 % CaH2PO4 and KH2PO4, as well as zinc solubilizing medium containing 1 % ZnO, 2 % ZnCO3, and 1 %Zn-EDTA. Each flask was inoculated with 1 ml suspension of the test culture, and centrifuged to remove cellular debris and cells and subsequently, analysed using Atomic Absorption Spectrophotometry.
2.8.2 Zn −solubilisation
2.8.2.1 Zinc solubilization in basal (PVK and ZnS) medium
The experimental procedure involved inoculating bacterial isolates into basal medium for comparison at 7th and 10th days in PVK medium, and the 10th and 13th days in the zinc medium. The assessment of available zinc content was based on a turbidity test, which provides a qualitative measure of the solubilization process, using Atomic Absorption Spectrophotometry (AAS). The results of zinc solubilization were tabulated on the 13th day, coinciding with the point of maximum growth observed in the bacterial cultures.
2.8.2.2 Zinc tolerance, solubilization and screening of Zn-P isolates
The ability of the selected isolate to tolerate solubilized zinc was assessed under in vitro conditions using nutrient broth supplemented with different concentrations of soluble zinc (ZnSO4). Initially, the nutrient broth was prepared and divided into 10 ml aliquots in test tubes to facilitate the growth assessment of the zinc-phosphate (Zn-P) isolates. To evaluate zinc tolerance, the isolated phosphate-solubilizing rhizobacteria (PSRB) were streaked onto nutrient agar supplemented with 1 mg/l zinc sulphate heptahydrate were then incubated to allow for bacterial growth.at 37 ◦C. Subsequently, (MIC) of the isolates was determined using the agar plate dilution method, as described by Luli et al. (1983). and concentrations of zinc sulphate heptahydrate, ranged from 1 mM to 15 mM. Following the incubation period, the MIC of the bacterial isolates was determined by observing their growth on after they failed to develop on the plates.
2.8.2.3 Determination of interaction of PGPR activities in wheat
The pH of the soil was determined using the method outlined by Jackson et al. (1973).
For estimating available phosphorus, Olsen's extractant method was employed. Available zinc in the soil was determined using the DTPA extraction method (Lindsay and Norvell, 1978).
Soil Microbial biomass carbon (SMBC) was quantified using the chloroform fumigation extraction method developed by Vance (1987). Phosphatase activity was determined by colorimetric estimation of p-nitrophenol released by phosphomonoesterase activity, following the method described by Tabatabai and Bremner (1969). Phytic acid content was assessed using the method outlined by Haung and Lantzsch (1983). Various efficiencies were calculated using specific formulas. Agronomic efficiency (AE) was computed using the formulas provided by Fageria and Baligar (2003) and Ladha et al. (2005). Recovery efficiency (RE) was determined using the formulas by Doberman (2005) and Ladha et al. (2005). Physiological efficiency (PE) calculations were based on formulas provided by Fageria and Baligar (2003) and Doberman (2005). The Zinc Harvest Index (ZHI) was calculated using the formula provided by Fageria and Baligar (2003). Total phosphorus (Grain + straw) from plant samples was estimated using the Vandomolybdate yellow color method from diacid extract, measured using a spectrophotometer following the method by Piper (1966). The zinc content in the dry matter of wheat grain and straw was determined using an Atomic Absorption Spectrophotometer. Grain and straw yield were estimated at harvest to complete the analysis.
3 Results and discussion
and identified as Bacillus subtilis strain (Zn-P-1), Pseudomonas aureginosa (Zn-P-2), Staphylococcus aureus (Zn-P-3) and Methylobacterium organophyllum (Zn-P-4). The nomenclature was assigned to the isolates on the basis of P and Zn solubilization, first three isolates were P- solubilizers and also exhibited Zn- solubilization ability, whereas fourth isolate was Zn- solubilizer and exhibited P- solubilization ability.
3.1 Solubilization capability of isolates
The solubilization capability of the isolates was assessed by observing the clear zones formed around the wells after 7th and 9th days of incubation on PVK agar and ZnS agar at 28 °C. All bacterial isolates exhibited clear zones on ZnS agar, with four selected isolates showing maximum clearance zones of varying diameters. The halo zone diameters ranged from 0.12 to 0.55 cm among the four isolates. The Solubilization Index (SI) of Zn-P-4 was found to be the highest, reaching 0.35 and 0.55 at 10th and 13th days, respectively, among all the isolates. The phosphate solubilizing efficiency (SE %) was calculated for the selected isolates, ranging from 112 to 155, with the highest value of 155 observed for Zn-P-4. Maximum solubilization was observed on the 13th day (Fig. 1). The notable SI and SE (%) observed with the Zn-P-4 isolate may be attributed to the release of IAA and gluconic acid, as suggested by Potshangbam et al. (2018). Zn-P-1 isolate exhibited the highest values of SI and SE (%) following Zn-P-4 isolate. The observed variation in solubilization ability with different zinc sources could be attributed to the metabolic activity of the given strain, aligning with the findings of Rehman et al. (2021).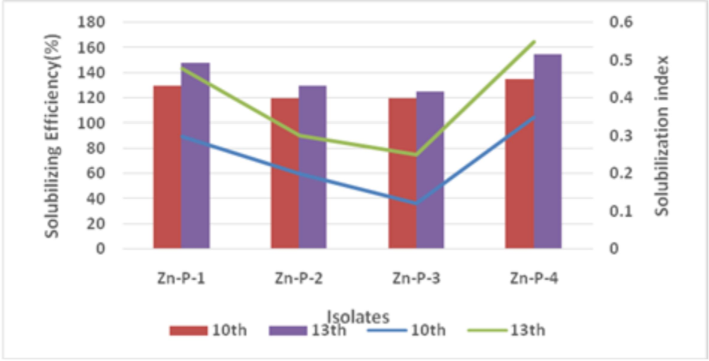
Solubilization index and efficiency of Zn-P isolates.
3.2 Effect of P and Zn source on P solubilisation
The solubilization potential was evaluated through qualitative and quantitative methods under in vitro conditions, revealing that Zn-P-1 and Zn-P-4 were among the most potent solubilizers.
3.3 Phosphate solubilisation
3.3.1 Plate assay
The halo zone of solubilization ranged from 11.00 mm to 15.5 mm, with Zn-P-1 exhibiting the maximum zone of solubilization. A correlation was observed between the incubation time and the size of the zone, indicating that an increase in the incubation period led to larger zones for each isolate. The percent solubilization efficiency ranged from 112 % to 155 % on the 10th and 13th day of the incubation period, with the SE% increasing over time. The solubilization index, based on colony diameter and halo zone for each PSB isolate, varied from 0.12 to 0.55 among the four efficient isolates, with the index enhancing with the incubation period. The diameter of the clear halo zone formed by the bacterial isolates directly correlated with phosphate solubilization efficiency, and an increase in the incubation time led to larger zones for each isolate as reported by Lavakush et al. (2012) using Pikovskaya’s media, and are consistent with the findings of Cavite et al. (2018), who suggested that the zone formation could be attributed to the activity of phosphate enzymes by Zn-P-1 and Zn-P-4 isolates. A high PSI index (2.82) recorded by the Zn-P-1 isolate indicates that this strain isolated from the wheat rhizosphere is an effective phosphate solubilizer, which is in consistency with the findings of Aarab et al. (2019).
3.3.2 Broth assay
All the isolated strains effectively solubilized the insoluble zinc (Zn) sources, including ZnCO3, ZnO, and Zn-EDTA, as well as the phosphorus (P) sources, namely KH2PO4 and CaH2PO4, under the broth assay conditions. The release of phosphorus was comparatively low in the CaH2PO4-amended medium compared to the KH2PO4 medium among the P sources, whereas the release was lowest for ZnO compared to ZnCO3 among the Zn sources, and these differences were statistically significant (Table 1). Among the P sources, P release ranged from 8.39 to 10.12 µg/ml in CaH2PO4, with the lowest release for Staphylococcus (Zn-P-3) and the highest for Bacillus (Zn-P-1), and from 4.86 to 11.24 µg/ml in KH2PO4-amended medium. Among the Zn sources, P release ranged from 5.02 to 8.34 µg/50 ml in ZnO (Zn-P-3), with the lowest for Staphylococcus and the highest for Bacillus (Zn-P-1), and from 4.00 to 15.17 µg/50 ml in Zn-EDTA, with the lowest for Pseudomonas (Zn-P-2) and the highest for Bacillus (Zn-P-1). In ZnCO3-amended medium, P release ranged from 5.31 to17.20 µg/ ml, with the lowest for Methylobacterium and the highest for Bacillus (Zn-P-1). Phosphorus release varied from 3.08 to 17.20 regardless of the wavelengths measured at 660 nm and 420 nm, with the highest values noted at the 660 nm wavelength on the 13th day (Table 1). The greatest phosphorus solubilization, specifically 0.684 mg/ mm, was observed for Zn-P-1 (Bacillus) on day 13th, followed by a subsequent decline in solubilization. The highest phosphorus release was recorded for Bacillus (Zn-P-1), which could be attributed to various mechanisms of phosphate solubilization by all three strains, with solubilization notably decreasing alongside a decline in pH, reaching its lowest point on the 13th day (Fig. 2). Similar findings were reported by Eramma et al. (2020).
Isolate
ZnCO3
ZnEDTA
ZnO
KH2PO4
CaH2PO4
AtWavelength420nm
SEm(±)
0.012
0.012
0.047
0.046
0.046
CD at
5 %0.037
0.037
0.142
0.138
0.138
AtWavelength660nm
SEm(±)
0.05
0.042
0.047
0.042
0.012
CD at5%
0.15
0.140
0.140
0.142
0.037
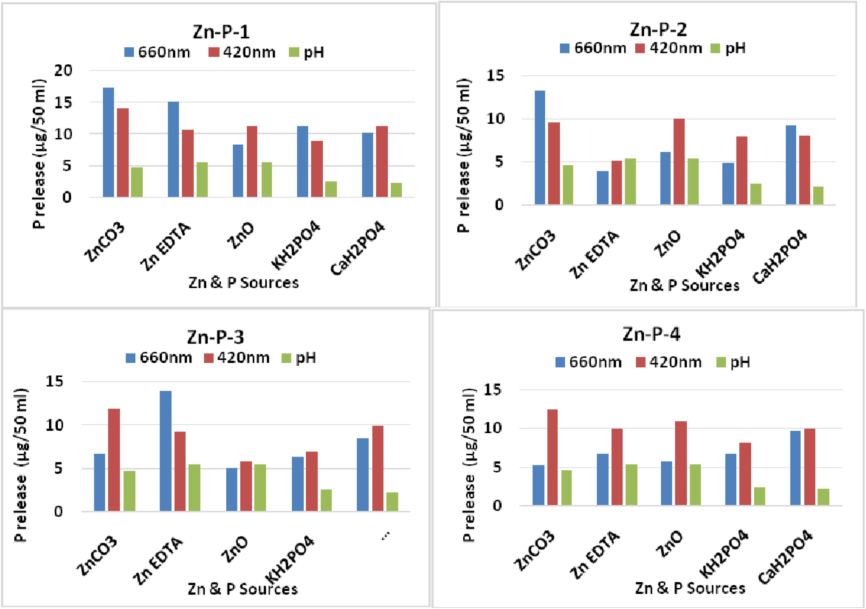
P-solubilisation (µg /50 ml-Barton’s) of Zn-P isolates at 13th day of incubation.
3.4 Zinc solubilization (release)
3.4.1 Plate assay
All the isolated strains effectively solubilized the insoluble zinc (Zn) sources used, namely ZnCO3, ZnO, and Zn-EDTA, as well as the phosphorus (P) sources, namely KH2PO4 and CaH2PO4, under the plate assay conditions. The zone of solubilization was comparatively higher in ZnO-amended medium compared to ZnCO3 and Zn-EDTA in the plate assay. These results align with earlier studies that reported improved solubilization of Zn in ZnO-enhanced medium (Rehman et al., 2021). The size of the zone of solubilization ranged from 12.5 mm to 14.0 mm in ZnCO3 and from 13.00 mm to 15.5 mm in ZnO-incorporated medium, and from 13.5 mm to 14.5 mm in Zn-EDTA. Furthermore, the zone of solubilization was higher in CaH2PO4-amended medium compared to KH2PO4 medium. The size of the solubilization zone ranged from 8.0 mm to 9.50 mm in CaH2PO4 and from 6.5 mm to 8.0 mm in KH2PO4-incorporated medium. It could be presumed that solubilization of Zn by the isolates might be attributed to organic acid production of Bapiri et al. (2012).
3.4.2 Broth assay (selective medium)
The quantitative estimation of Zinc content in PVK broth was measured through AAS at 660 nm wavelength. The highest zinc solubilization was found to be 10.5 mg/l in Zn-P-1 isolate and lowest 2.50 mg/l in Zn-P-3, whereas highest zinc solubilization was found to be 11.80 mg/l in Zn-P-1 isolate and lowest 3.90 mg/l in Zn-P-3in PVK medium, at 7th and 10th days respectively (Table 2). A significant difference was observed as compared to control at 7th and 10th days in PVK medium. The estimation of Zinc content in zinc solubilizing broth was determined through AAS at 660 nm wavelength and ranged from 37.75 in Zn-P-3 to 43.00 mg/l in Zn-P-1 isolate and also 39.87 mg/ml in Zn-P-3 to 44.25 mg/l in Zn-P-1 Bacillus isolate at 10th and 13th day respectively. A significant difference was estimated as compared to control at 10th and 13th day in ZnS medium. This might be due to the fact that Bacillus sp. exhibited more solubilization as compared to other strains and activity is pronounced with substrate type. Similar results were reported by (Ahmad et al., 2021). Zn-P-1, Bacillus, Zn-P-2- Pseudomonas, Zn-P- 3-Staphylococcus, Zn-P- 4- Methylobacterium.
S. No.
Isolate
PvK broth (mg/l)
ZnS broth (mg/l)
Day 7
Day 10
Day 10
Day 13
1.
Zn-P-1
10.5
11.80
42.87
43.25
2.
Zn-P-2
8.25
9.50
40.75
41.75
3.
Zn-P-3
2.50
3.90
37.75
39.87
4.
Zn-P-4
4.50
5.75
43.00
44.25
5.
Control
1.50
2.85
13.50
14.10
SEm(±)
0.24
0.47
0.50
0.53
CD at 5 %
0.76
1.57
1.67
1.69
3.4.3 Broth assay (P and Zn-sources)
All the isolated strains effectively solubilized the insoluble zinc (Zn) sources used, namely ZnCO3, ZnO, and Zn-EDTA, as well as the phosphorus (P) sources, namely KH2PO4 and CaH2PO4, under the broth assay conditions (Table 3, Fig. 3). Zn-P-4 (Methylobacterium) was among the most potent solubilizers to release zinc when using ZnCO3 and Zn-EDTA, whereas Zn-P-1 released the highest zinc when using ZnO as zinc sources. Zinc release was highest at 149.4 mg/l in Zn-EDTA and lowest at 6.64 mg/l in ZnCO3 in the selective medium using Zn sources. as also reported by Borah et al. (2018). The modified ZnO media provided instant Zn release compared to other Zn sources used for Zn-P-4 organisms as zinc sources (Gandhi and Muralidharan, 2016). The pH ranged from 1.9 to 4.7 using P sources and 4.2 to 6.9 using Zn sources and was statistically significant. The lowest pH value was observed with CaH2PO4 and the highest was observed in ZnO and Zn-EDTA at the 13th day of the incubation period.The least pH value (4.8) was shown by Zn-P-1 culture on the 13th day after inoculation, indicating higher acidity due to growth (Fig. 3b). The decrease in pH of the medium containing the insoluble salt confirms the solubilization effect, which may be attributed to the production of organic acids, as reported by Rehman et al. (2021). The pH of isolates was observed on successive days in PVK and ZnS broth against control media and ranged from 2.9 to 4.6. The lowest pH value (3.0) in isolate Zn-P-3 at day 13th and the highest pH was observed as 4.7 in isolate Zn-P-4.9. The remarkable decline in pH was observed till day 13, after which pH attained a constant value. Keeping the solubilization criteria as a preliminary tool for assessing the potential of Zn-P isolates, the evaluation of isolates and its interaction effect on PGPR activities and P & Zn solubilization in wheat rhizosphere were studied under pot culture assays. Following initial screening, twelve bacterial isolates were obtained on nutrient agar supplemented with a 1 mM concentration of zinc, out of which four were selected based on their solubilization abilities. Nutrient agar containing varying concentrations of zinc sulfate heptahydrate (ranging from 1 to 15 mM) was employed to determine the minimum inhibitory concentration (MIC) for zinc sulfhate heptahydrate on the 6th, 10th, and 24th days, respectively. The minimum inhibitory concentration (MIC) of the selected bacterial strains was assessed, revealing that strains Zn-P-1 and Zn-P-4 exhibited the highest levels of growth and were submitted to NCBI GenBank. Halozone of solubilization ranged from 7 mm to 16 mm. The highest SI and SE (%) value was found to be in the 10 mM concentration of zinc sulphate heptahydrate. The lowest SI and SE (%) value was found to be in 1 mM as well as 15 mM concentration of ZnSO4·7H2O by Zn-P-1 and Zn-P-4 isolates respectively. (Fig. 4). MIC value up to 11.5 mmol/L and 24 mg/ml was also reported by Sen and Joshi (2017) and is in consonance with our observations. Halozone diameter ranged from 7.0 mm to 11 mm, 8 mm to 14 mm, and 8 mm to 16 mm at 6th day, 10th day, and 24th day respectively. High zinc tolerance ability in 10 mM concentration suggests their potential for detoxifying mechanism as reported by Mazalan et al. (2020).Fig. 5a.Fig. 5b..
Media
Control(mg/l)
Zn-P-1
(mg/l)Zn-P-2
(mg/l)Zn-P-3
(mg/l)Zn-P-4
(mg/l)
ZnCO3
14.06
39.42
18.26
6.64
148.57
ZnEDTA
30.95
55.61
147.32
130.31
149.4
ZnO
25.31
134.87
121.59
117.86
123.67
KH2PO4
14.44
26.97
54.14
21.99
48.14
CaH2PO4
19.42
71.79
68.47
69.30
72.32
ZnS broth
14.10
43.25
39.87
41.75
44.25
SEm(±)
0.44
0.52
0.53
0.41
0.57
CD at 5 %
1.39
1.68
1.72
1.33
1.83
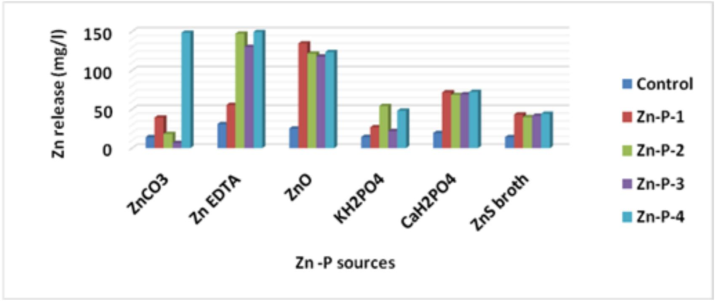
Zinc release (mg/l) using Zn and P sources at 13th day.
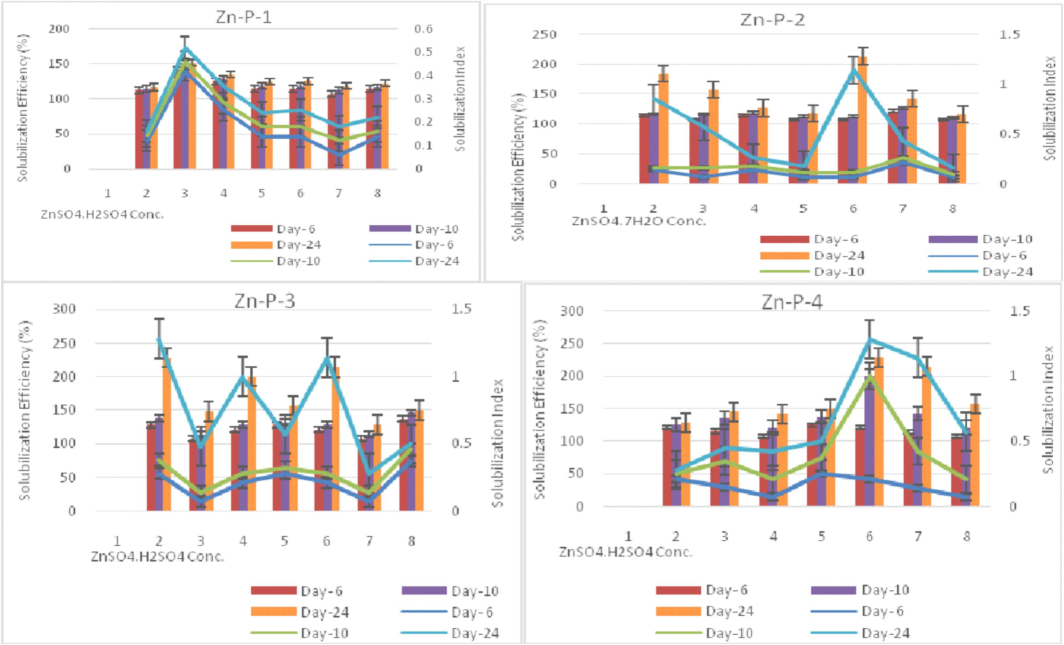
Zinc Tolerance ability*Horizontal axis entries = 2 = 2 mM, 3 = 2.5 mM, 4 = 5 mM, 5 = 7.5 mM, 6 = 10 mM, 7 = 12.5 mM and 8 = 15 mM.
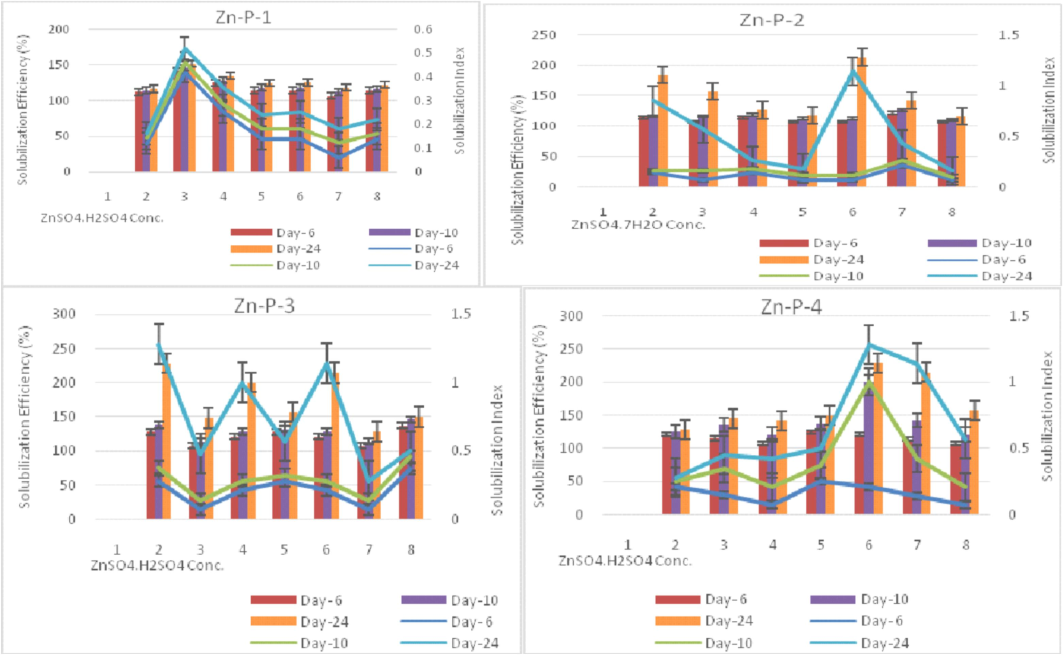
Zinc Tolerance ability*Horizontal axis entries = 2 = 2 mM, 3 = 2.5 mM, 4 = 5 mM, 5 = 7.5 mM, 6 = 10 mM, 7 = 12.5 mM and 8 = 15 mM.
PCR amplified 16s rRNA,.Lane Description: L- Ladder(500 bp);1-M1.
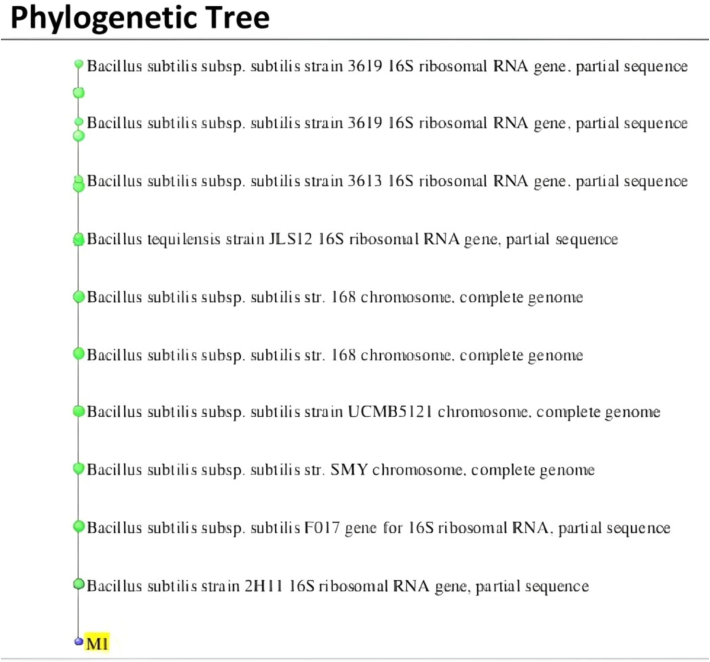
Phylogenetic tree.
3.5 Cultural and molecular characterization of potent isolate(Zn-P-1)
The most potent Zn-solubilizing rhizobacteria isolated from the rhizosphere wheat cultivating soil from different locations were subjected to biochemical identification viz, Citrate utilization, Urease, Nitrate reduction and Catalase. All biochemical tests exhibited positive results except gram staining which exhibited negative results and was creamish yellow in colour. The most potent Zn Solubilizing bacteria was Bacillus strain (Zn-P-1) identified as (Bacillus subtilis subsp. subtilis strain- 3619) following molecular identification which was further submitted to NCBI genbank and coded as ON024399.1. The highest % similarity with other accessions was OK135798-100 % and MT538491- 99.84 %.
3.6 Effect of P & Zn sources on solubilization and PGPR activities in wheat
All the isolated strains effectively solubilized the insoluble phosphorus (P) and zinc (Zn) sources. Among them, Zn-P-1 (Bacillus) isolate showed maximum potential of P and Zn solubilization (Table 4), resulting in an elevation in soil-available phosphorus, phosphatase activity, soil microbial biomass carbon, and available zinc, as outlined in Table 4. along with a parallel decline in soil pH and phytic acid in the grain of wheat crop as compared to control. Similar results were also reported by Raghuveer et al. (2017). This might be due to the release of organic substances from the roots to the rhizosphere soil, thereby elevating the microbial biomass in the rhizosphere, as well as the supply of mineralization of carbon (C) and nitrogen (N), resulting in the enhancement of indigenous microflora (Kumar et al., 2021). The increase in Zn availability and decline in pH might be attributed to the production of organic acids, which serve as key mechanisms to solubilize the complex Zn into a soluble form by lowering the pH of the microbial surrounding (Kushwaha et al., 2021), thus increasing Zn availability and assimilation in plants as reported by Kusale et al., 2021.
Treatments
P Solubilization
Zn Solubilization
Soil av. P(mg kg−1)
Soil pH
Phosphatase activity
(µg- PNP g-1soil hr -1).SMBC
(µg-1soil)Soil avail. Zn
(mg kg−1)Phytic acid(%)
Quantitative at 10th Day
(µg ml−1)Quantitative at 13h Day
(µg ml−1)
TCP
KH2PO4
CaH2PO4
ZnCO3
ZnO
Zn EDTA
Control
–
–
–
–
–
–
11.34
7.29
37.90
87.53
1.11
1.29
P1-TCP
–
–
–
–
–
–
18.25
7.26
48.62
93.33
1.29
1.13
P2-ZnCO3
–
–
–
–
–
–
16.92
7.11
46.89
93.17
1.67
0.72
Isolate
B0 (Control)
2.18
3.21
5.25
14.06
25.31
30.95
12.17
7.31
40.10
89.63
1.19
1.24
B1
18.73
11.24
10.12
39.42
134.87
55.61
18.06
7.14
48.02
92.81
1.49
1.11
B4
7.25
5.24
6.69
148.57
123.67
149.4
14.43
7.24
42.27
90.91
1.32
0.87
SEm(±)
0.30
0.39
0.55
0.93
0.41
0.36
0.48
0.08
0.21
0.11
0.41
0.03
P*B(CD at 5 %)
1.00
1.13
1.60
2.40
1.63
1.18
1.28
0.21
0.46
0.36
NS
0.09
3.7 Interaction effect of P (TCP) &Zn (ZnCO3) sources along with isolates at harvest stage
The data from (Table 5) revealed significant differences in various types of phosphorus (P) and zinc (Zn) efficiencies, including agronomic efficiency (AE), physiological efficiency, recovery efficiency, P and Zn use efficiency, as well as P and Zn harvest index, P and Zn uptake, and yield. The highest values were recorded in treatment combinations involving TCP along with Bacillus isolate (P1B1), as well as ZnCO3 along with Bacillus isolate (P2B1) as compared to control(B0). This increase in efficiency might be attributed to the enhanced P release and increased P solubilization capacity of the Bacillus isolate, resulting in an upsurge in nutrient uptake by the plant, which aligns with findings by Kshetri et al. (2018). The combined inoculation of ZnCO3 and the Bacillus isolate demonstrated a synergistic relationship, leading to increased Zn solubilization and Zn uptake, ultimately enhancing plant growth parameters. This finding is consistent with studies by Akhtar et al. (2013). In Capsicum annum also it has been observed that Zn solubilizing Bacillus sp. promoted different plant growth parameters (Khan et al. 2021). PUE- Phosphorus Use Efficiency (%), PHI- Phosphorus Harvest Index, PE- Physiological Efficiency, RE −Recovery Efficiency, AE- Agronomic Efficiency, ZUE- Zinc Use Efficiency, ZHI- Zinc Harvest Index.
Treatments
P1-TCPPUE(%)
PHI (%)
PE
(g/g)RE
(g/g)AE
(g/g)P-Uptake(Grain + Straw)(g/pot)
Zn- Uptake(Grain + Straw)(g/pot)
Grainyield(g/pot)
Strawyield(g/pot)
P1B0
3.32
0.519
0.90
332
18.03
7.22
86.32
17.05
21.32
P1B1
5.93
0.512
0.83
593
20.01
11.44
93.31
20.24
25.31
P1B2
P2-ZnCO3
4.83ZUE
(%)0.514ZHI
(%)0.89
483
19.34
9.66
92.10
19.17
23.92
P2B0
1.07
0.289
0.15
107
5.96
6.36
87.06
12.89
16.19
P2B
2.54
0.298
0.35
334
6.99
9.92
96.61
17.21
21.51
P2B2
2.57
0.305
0.31
316
6.78
8.86
95.84
16.33
20.42
4 Conclusions
Our study highlights the significant potential of Zn-P-1 (Bacillus isolate) in solubilizing phosphorus (P) and zinc (Zn), enhancing soil availability of P and Zn, promoting phosphatase activity and soil microbial biomass carbon, and increasing available Zn in the soil. Additionally, it underscores the concurrent decline in soil pH and phytic acid levels in wheat crop grains. Furthermore, our findings suggest that treatment combinations involving TCP with Bacillus isolate (P1B1) and ZnCO3 with Bacillus isolate (P2B1) demonstrated the highest levels of various phosphorus (P) and zinc (Zn) efficiencies, encompassing agronomic efficiency, physiological efficiency, recovery efficiency, as well as P and Zn use efficiency. Additionally, they exhibited superior P and Zn harvest index, uptake, and yield. The combined inoculation of ZnCO3 and the Bacillus isolate demonstrated a synergistic relationship, leading to increased P and Zn solubilization, uptake, and ultimately, improvements in plant growth parameters. The identified isolate, Zn-P-1, holds promise for further utilization as a component in consortium mixtures and biofertilizers aimed at enhancing sustainable soil and plant health. This suggests a practical application for agricultural improvement strategies using bacillus species and exploring more substrates type.
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Renu Gupta: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Data curation, Conceptualization. Ravi Kumar: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Wahidah H. Al-Qahtani: Writing – review & editing, Funding acquisition, Formal analysis. Mostafa A. Abdel-Maksoud: Writing – review & editing, Funding acquisition, Formal analysis, Conceptualization.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R293) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Some characteristics of phosphate solubilizing rhizobacteria as an ecological strategy for sustainable agriculture. Mater. Today Proc.. 2019;13:1224-1228.
- [Google Scholar]
- Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiol.. 2021;66
- [Google Scholar]
- Co-inoculation with Rhizobium and Bacillus sp. to improve the phosphorus availability and yield of wheat (Triticum aestivum L. JAPS. J. Animal Plant Sci.. 2013;23
- [Google Scholar]
- Indigenous African agriculture and plant associated microbes: Current practice and future transgenic prospects. Scientific Research and Essays 7; 2012.
- Evaluation of zinc solubilization potential by different strains of fluorescent pseudomonads. Manage: J. Appl. Sci. Environ; 2012. p. :16.
- Borah, M., Das, S., Boruah, H., Boro, R., Barooah, M., 2018. Diversity of culturable endophytic bacteria from wild and cultivated rice showed potential plant growth promoting activities.
- Cavite, H., Mactal, A., Cruz, J., Khermkhan, J., 2018. Phosphate-solubilizing bacteria from upland rice (Oryza sativa L.) rhizosphere and their tricalcium phosphate solubilizing abilities.
- Chen, Y.P., Rekha, P.D., Arun, A.B., Shen, F.T., Lai, W.A., Young, C.C., 2006. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied soil ecology 34.
- Isolation and screening of phosphate solubilizing bacteria from paddy rhizosphere soil. Int. J. Curr. Microbiol. App. Sci.. 2020;9:477-485.
- [Google Scholar]
- Fageria, N.K., Baligar, V.C., 2003. Fertility management of tropical acid soils for sustainable crop production. In: Handbook of soil acidity.
- Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. Eur. J. Soil Biol.. 2016;76:1-8.
- [Google Scholar]
- Sensitive method for the rapid determination of phytate in cereals and cereal products. In: Journal of the Science of Food and Agriculture 34. 1983.
- [Google Scholar]
- Soil chemical analysis, prentice hall of India Pvt, Ltd. Int. J. Curr. Microbiol. App. Sci.. 1973;8
- [Google Scholar]
- Insights into the interactions among roots, RHIZOSPHERE, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells. 2021;10:1551.
- [Google Scholar]
- Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J. Agri. Bio. Sci.. 2009;1:48-58.
- [Google Scholar]
- Role of phosphate-solubilizing microorganisms in sustainable agriculture. Agron. Sustain. Dev.. 2006;26:29-43.
- [Google Scholar]
- Zinc solubilizing Bacillus strains that modulate growth, yield and zinc bio fortification of soybean and wheat. Rhizosphere. 2017;4:126-138.
- [Google Scholar]
- Rhizosphere mediated nutrient management in allium Hookerithwaites by using phosphate solubilizing rhizobacteria and tricalcium phosphate amended soil. J. Plant Interact.. 2018;13:256-269.
- [Google Scholar]
- Forest soil nutrient stocks along altitudinal range of Uttarakhand Himalayas: An aid to nature based climate solutions. Catena. 2021;207:105667
- [Google Scholar]
- Inoculation of klebsiella variicola alleviated salt stress and improved growth and nutrients in wheat and maize. Agronomy. 2021;11:927.
- [Google Scholar]
- Enhancement in plant growth and zinc biofortification of chickpea (Cicer arietinum L.) by Bacillus altitudinis. J. Soil Sci. Plant Nutr.. 2021;21:922-935.
- [Google Scholar]
- Ladha, J.K., Pathak, H., Krupnik, T.J., Six, J., Kessel, C., 2005. Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects.
- Lavakush, Y., J, V., P.j, 2012. Isolation and characterization of effective plant growth promoting rhizobacteria from rice rhizosphere of Indian soil. Asian Journal of Biological Sciences 5, 294–303.
- Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J.. 1978;42:421-428.
- [Google Scholar]
- Hexavalent chromium-resistant bacteria isolated from river sediments. In: Applied and Environmental Microbiology 46. 1983.
- [Google Scholar]
- Mazalan, N.Z.S., Oyeleye, A., Rahman, R.N.Z.R.A., Aris, A.Z., Salleh, A.B., Normi, Y.M., 2020. Isolation and characterization of an acid and metal tolerant Enterobacter cloacae NZS strain from former mining lake in Selangor, Malaysia. Beni-Suef University Journal of Basic and Applied Sciences 9.
- Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng.. 2014;73:526-535.
- [Google Scholar]
- Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17
- [Google Scholar]
- Piper, C.S., 1966. Soil and Plant Analysis, Indian ed.Hans Publ.
- Draft genome sequence of Bacillus altitudinis Lc5, a biocontrol and plant growth-promoting endophyte strain isolated from indigenous black rice of Manipur. Genome Announc.. 2018;6:00601-00618.
- [Google Scholar]
- Impact of phosphorus levels and PSB strains on soil microbial and enzymatic activities under acidic soils of North EastIndia. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:2061-2067.
- [Google Scholar]
- Assessment of zinc solubilization potential of zinc-resistant Pseudomonas oleovorans strain ZSB13 isolated from contaminated soil. Braz. J. Biol.. 2021;83
- [Google Scholar]
- Characterization of phosphate solubilizing bacteria isolated from mine tailings of zawar mines, Udaipur, India. Int. J. Curr. Microbiol. App. Sci.. 2017;6:588-596.
- [Google Scholar]
- Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2013;2:587.
- [Google Scholar]
- Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem.. 1969;1:301-307.
- [Google Scholar]
- An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem.. 1987;19:703-707.
- [Google Scholar]
- Dihydropteroate synthase of mycobacterium leprae and dapsone resistance. Antimicrob. Agents Chemother.. 2000;44:1530-1537.
- [Google Scholar]
- Wilson, K., 2001. Preparation of genomic DNA from bacteria.. Current Protocols in Molecular Biology (1997) 2.4.1-2.4.5.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103509.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







