Translate this page into:
Exploring the efficacy of endophytic Diaporthe caatingaensis as a biocontrol agent targeting Fusarium strains afflicting coffee plants in Saudi Arabia
⁎Corresponding author. myassin2.c@ksu.edu.sa (Mohamed Taha Yassin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
The coffee plant is a strategic crop in Saudi Arabia that makes a substantial contribution to the country’s economy. Therefore, it is crucial to continuously monitor and control the fungal phytopathogens that affect coffee plants in order to minimize crop losses and ensure sustainable cultivation. This is the first surveillance report of Fusarium phytopathogens associated with coffee plants in Saudi Arabia. Moreover, the antagonistic efficiency of the endophytic fungus, Diaporthe caatingaensis, was evaluated against the isolated Fusarium phytopathogens.
Methods
The isolated strains were preliminary identified using cultural and microscopic methods and the identification was confirmed using internal transcribed spacer (ITS) sequencing technique. The detached leaf assay was conducted to assess the disease severity of Fusarium phytopathogens against detached coffee leaves. Moreover, dual culture assay was utilized to assess the antagonistic activity of D. caatingaensis.
Results and conclusion
Fusarium oxysporum was found to be the most frequent isolated strain followed by F. solani, F. proliferatum and F. verticillioides strains. Fusarium proliferatum strain was found to be the most severe strain whereas F. solani strain showed the lowest disease severity. On the other hand, propiconazole fungicide was tested for its efficiency against Fusarium pathogens using food poisoning technique, showing that F. oxysporum strain 1 of accession number OP955665 was the most sensitive strain. However, F. proliferatum, F. oxysporum st. 2 (OP959851) and F. solani strains showed no significant response when the propiconazole concentration increase from 150 to 200 ppm. Scanning electron microscope proved the potent antagonistic activity of D. caatingaensis against F. proliferatum and F. oxysporum OP959874 strains through mycoparasitic modes of action as coiling, appressorium formation resulting in complete lysis of fungal mycelium. Accordingly, the current investigation provides the first surveillance data about Fusarium strains associated with coffee plants and also the utilization of D. caatingaensis as a potential biocontrol agent for effective management of Fusarium phytopathogens, avoiding the incidence of fungal resistance to fungicides and the harmful effects of commercial fungicides for sustainable cultivation of coffee in Saudi Arabia.
Keywords
Fusarium
Phylogenetic analysis
Diaporthe caatingaensis
Biological control
Mycoparasitism
Dual culture assay
SEM
1 Introduction
The Coffea genus belongs to the Rubiaceae family and is native to the African continent, particularly Ethiopia (Melese and Kolech, 2021). Within this taxonomic classification, there are two subgenera, Baracoffea and Coffea, which together consist of around 103 distinct species (Davis and Rakotonasolo, 2021). The two most prevalent and commercially cultivated commercial species globally are C. arabica L. (Arabica) and C. canephora (Robusta) (Martins et al., 2022). Robusta coffee is native to the humid lowland forests of tropical regions in Africa. It has a large natural geographic distribution, ranging from Guinea to Uganda and Angola. It thrives in many forms and ecotypes. The area has been surveyed and prospected by ORSTOM and FAO missions (Akpertey et al., 2022). Historically, the origin of coffee may be traced back to the Kaffa area of Ethiopia. It was then brought to other regions of the globe by merchants from Yemen around the 15th century (Krishnan, 2022). Saudi Arabia is situated near Ethiopia, where the cultivation and dissemination of coffee began many centuries ago, particularly in the southwest of the Arabian Peninsula (Yemen and the southwest of Saudi Arabia). Coffee cultivation is mostly concentrated in the Al Baha, Asir, Jazan, and Najran areas of Saudi Arabia. According to the data from the Jazan Mountains Development Authority (MJDA), a government organization, Saudi Arabia has over 78,000 coffee trees (Alasmari et al., 2020). The majority, around 84 %, are found in the Addayer district of the Jazan area. The projected yearly coffee bean yield from these plants in Saudi Arabia is around 500 tons (Alhudaib and Ismail, 2024).
The severity of Fusarium diseases in crops has been increasing during the previous century, presenting a significant menace to global food security (Petronaitis et al., 2021). It is now clear that Fusarium diseases are the biggest threat to many of the world's main crops (Corbu et al., 2023). They devastate both the vital calorie crops, such as rice, maize, wheat, and soybean, as well as the commercial crops, such as coffee, bananas, and barley (Ekwomadu and Mwanza, 2023). In this context, Fusarium is a very significant group of fungi in terms of its impact on the economy, since it causes a decrease in crop productivity via its pathogenic effects on plants. The presence of mycotoxins in food and feed products not only makes them unsuitable for sale, but also contributes to significant financial losses in the agricultural sector. Additionally, the ingestion of mycotoxins presents a serious risk to the health of both humans and animals (Rampersad, 2020). Managing Fusarium diseases in plants is challenging due to their presence in both seeds and soil (Nikitin et al., 2023). The pathogen Fusarium xylarioides is responsible for causing wilt in Coffea excelsa (C. liberica) according to Steyaert's research in 1948 (Peck and Boa, n.d.). The discovery of this illness dates back to 1927 in Oubangui-Chari, which is today known as the Central African Republic. Initially, it was believed to be caused by a root rot (Flood, 2010). A previous report indicated that F. oxysporum isolates from coffee corky roots were able to inhabit the xylem of coffee seedling roots (López-Lima et al., n.d.). According to the average coffee yields, the anticipated losses caused by coffee wilt disease in Uganda were 350 kg per hectare per year, whereas in Ethiopia they were 276 kg per hectare. The yearly losses per hectare were $232 and $275, respectively, which were significant amounts for small-scale farmers with tiny plots of land (Peck and Boa, n.d.).
Managing the harmful fungal strains that affect coffee plants is crucial to minimize potential economic losses caused by these pathogens. However, the use of chemical fungicides to control these fungal strains poses health risks and has negative effects on both land and water ecosystems (Yassin et al., 2021). Multiple detrimental impacts of fungicides on human health have been documented, including neurological, dermatological, gastrointestinal, and carcinogenic consequences (Punia et al., 2023). Endophytic fungi and/or bacteria were identified residing within the tissues of nearly every vascular plant species. These microorganisms are present in various organs of the host plants, with even their seeds serving as habitats for endophytes (Segaran and Sathiavelu, 2019). Endophytic fungi function as biocontrol agents, safeguarding the host plant against diseases throughout the whole of the endophytes' life cycle. These fungi exhibit a high degree of adaptability to unfavorable environmental circumstances and play a crucial role in defending the host plant (Wu et al., 2018). The ongoing surveillance of Fusarium pathogens associated with coffee plants is necessary to gather comprehensive data on pathogenic fusarial strains and identify the most effective methods for controlling coffee wilt disease. This will ultimately help minimize the economic losses caused by this disease. Hence, the current study was conducted for the isolation of the Fusarium pathogens associated with coffee plants in Saudi Arabia and identifying them using morphological and molecular methods. Moreover, in the current study, we evaluate the potentiality of an endophytic fungus, namely, Diaporthe caatingaensis strain isolated from coffee plant leaves, against the isolated Fusarium pathogens associated with coffee plants in Saudi Arabia.
2 Materials and methods
2.1 Isolation of Fusarium phytopathogens from coffee plants
Eighty samples were collected from different parts of coffee plants (roots, fruits and leaves) and these plants located in different farms which located in southwest region of Saudi Arabia (Table 1). Fig. 1 showed a geographical map of sample collection sites. The plant parts were collected in the period from May to December 2021, September to December 2022, and January to June 2023. Moreover, the collected leaves were symptomized by the presence of black and necrotic spots. The plant parts were disinfected by washing with ethanol (70 %) and then sterilized using sodium hypochlorite (1 %). After that, they were washed with sterilized distilled water and let for dryness over sterile filter papers. Afterwards, small pieces were cut using a sterilized cutter from different plant parts and then plated over freshly prepared potato dextrose agar (PDA) medium supplemented with chloramphenicol 0.5 g/L to prevent bacterial contamination. The plates were incubated for 25 ± 2 ◦C for 5 days and then were observed for fungal growth. The fungal strains were then purified onto fresh PDA plates using single spore or hyphal tip separating techniques, and subsequently, the pure cultures were preserved over sterile PDA slants at 4 °C for subsequent identification.
District
Farm no.
Latitude (N)
Longitude (E)
Al Baha
1
N 19.72379
E 41.37959
2
N 19.84973
E 41.30132
3
N 19.84438
E 41.31080
Jazan
4
N 17–29201
E 43.09986
5
N 17–25647
E 43.10591
6
N 17–25712
E43.10561
7
N 17–25726
E43.10515
8
N 17–25571
E43.10597
9
N 17.28707
E43.08406
10
N17.28680
E43.08498
11
N17.29330
E43.08656
12
N17.29200
E43.09990
13
N17.2403
E43.1014
14
N17.2104
E 43.1216
15
N17.2100
E43.0638
Asir
16
N18.0829
E42.1853
17
N18.1512
E42.1733
18
N17.5328
E42.1913
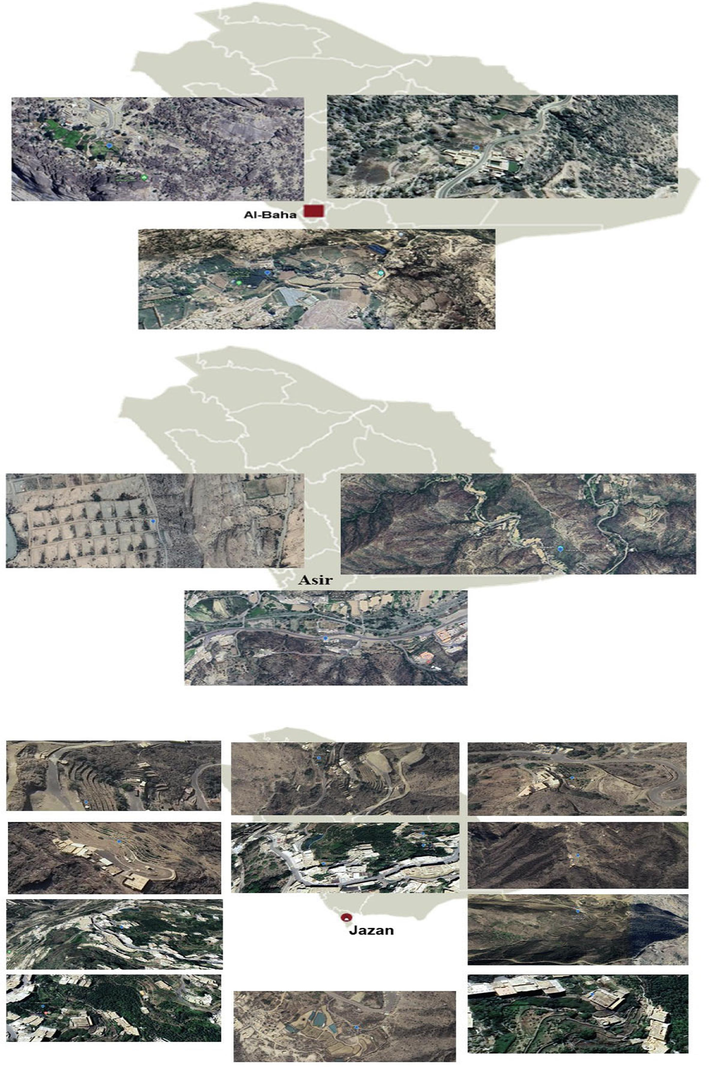
Geographical map of sample collection sites.
2.2 Morphological and molecular identification of the isolated Fusarium strains
The fungal strains were purified and identified by the examination of their visible and microscopic characteristics, using accepted criteria for the identification of fungi. The Fusarium species were precisely identified using the methodology outlined by Leslie and Summerell (2006) (Leslie and Summerell, 2008). The morphological identification was confirmed by the use of molecular tools, namely the internal transcribed spacer (ITS) sequencing approach. In this context, the genomic DNA of fusarial strains was obtained by using the Plant Genomic DNA Mini Kit (GP100) Geneaid technique, following the guidelines provided by the manufacturer. Subsequently, the procedure of DNA electrophoresis was carried out in accordance with Sambrook et al. (1989) (Sambrook et al., 1989). The internal transcribed spacer (ITS) region was amplified using the universal primer combination ITS4 (F) (5′-TCC TCC GCT TAT TGA TAT GC-3′) and ITS5 (R) (5′-GGA AGG AGA AGT CGT AAC AAG G-3′). The amplification of genomic DNA was performed in a 25 μl reaction mixture including 5 μl of Master Mix (Bioneer, Korea), 1.5 μl of each primer, 5 μl of genomic DNA as a template, and 12 μl of deionized sterile distilled water. The PCR reactions were conducted in a Thermo-cycler (MyGenie96 Thermal Block, Bioneer, Korea). The amplification programme began with a denaturation step at 95 °C for 5 min to activate the tag-polymerase. This was followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 45 s. The final extension step was carried out at 72 °C for 5 min. For the last step, a 1.5 % agarose gel electrophoresis was mixed with 1 × Tris-borate-EDTA (TBE) buffer. It was then stained with ethidium bromide and seen under a UV transilluminator. Finally, it was shot using the GeneSnap picture imaging system (SynGene).
2.3 DNA sequencing and phylogenetic analysis
The ITS1-5.8S rDNA sequencing and purification of the amplified PCR products from fungal isolates were performed at MACROGEN Company in South Korea, which could be found at the website https://dna.macrogen.com. The acquired sequences were compared to those included in GeneBank and NCBI https://www.ncbi.nlm.nih.gov using BLAST. The acquired sequences were cleaned and assembled using Bioedit software. The ITS nucleotide sequences were analyzed using MEGA 11. The ITS sequences were compared to the most similar sequences using the Basic Local Alignment Search Tool (BLAST) service available on GenBank. We analyzed a total of 86 ITS sequences of Fusarium strains that were linked to our sequences. The ClustalW programme with default settings was used to analyze the sequences, including an opening penalty of 15 and an extension penalty of 6.66 (MEGA). A phylogenetic tree was constructed using a minimum Bayesian Information Criterion, which selected the most appropriate model for nucleotide replacement. Genetic distances were determined using the Kimura 3-parameter model (Supplementary Table S1). The phylogenetic analysis abridged the names of all isolates using the formula: Species/Country/Isolate/Year/Type. The isolated fusarial strains were highlighted in red color.
2.4 Pathogenicity assay using detached leaf assay technique
A total of eight isolates were selected as typical samples from the described species. The inoculation was performed on healthy detached coffee leaves. The healthy leaves underwent surface sterilization by immersing them in 70 % ethanol for 1 min, followed by three rinses with distilled water. The leaves were then dehydrated using sterilized filter paper and arranged on a tray for the purpose of drying. The inocula were made by using a 7-day-old culture that was cultivated on PDA. A pathogenicity test was conducted utilizing the conidial suspension inoculation technique. To prepare the conidial suspension, the conidia from a pure culture were collected by adding a little quantity of sterile distilled H2O and gently swirling. The conidial suspension was transferred to a beaker and the concentration was adjusted to 1x106 conidia/mL, as determined using a haemocytometer. Incisions of about 1 cm were created on the surface of the leaves using a sterilized scalpel. Afterwards, a micropipette was used to apply 20 µL of the conidial suspensions to the wounded regions. In order to prepare the control, a sterile solution of distilled water was used to substitute the conidial suspension.
Three duplicates were done for each isolate of the same species, and they were inoculated onto the identical leaves. Following inoculation, the leaf petioles were enveloped with moist cotton and the trays were shielded with a plastic wrapping (Cling wrap) to maintain a relative humidity of about 90 % and prevent the leaves from becoming dry. Leaf spot symptom development was examined, and the size of the lesion was assessed daily for a period of 10 days. The fungal isolates were reisolated using direct isolation and then identified based on their morphology. The pathogenicity tests were run twice to assess the reliability of the findings.
2.5 Antifungal efficiency of standard fungicide against the tested Fusarium stains
The fungicidal efficacy of Propiconazole against the investigated Fusarium pathogens was determined using a food poisoning assay. The efficacy of the standard Propiconazole fungicide was assessed by adding various concentrations of the fungicide (0.025, 0.05, 0.10, 0.15, and 0.20 ppm) to the newly prepared PDA medium. In this context, 8 mm mycelial discs were removed from a fungal culture after 7 days using a sterilized cork borer. The discs were then inserted into the middle of PDA plates treated with varying concentrations of fungicide, while a control group of newly made PDA plates without fungicide was also inoculated with mycelial discs. Finally, the inoculated plates were incubated for 7 days at 25 ± 2 °C then the mycelial growth diameter was measured using Vernier calipers for both control and treated plates. The formula for calculating mycelial inhibition percentage is as follow:
The mycelial inhibition %=(A–B)/A×100, where A represents the growth diameter in the control plates and B represents the growth diameter in the treatment plates.
2.6 Investigation of antagonistic activity of Diaporthe caatingaensis against the tested Fusarium pathogens
Diaporthe caatingaensis strain was isolated from coffee plant leaves and identified using ITS sequencing as previously described. The strain was registered in GenBank with accession number of OP957006 which showed 99.12 % similarity with D. caatingaensis OL964065 and 99.10 % with D. caatingaensis KY085925 registered previously in the Genbank. The antagonistic activity of the biocontrol agent, D. caatingaensis, was examined utilizing a dual culture experiment. A mycelial disc measuring 8 mm from the biocontrol fungus was removed from a fungal culture that had been growing for 7 days. The mycelial disc was then placed 1.5 cm away from the border of the prepared PDA plates. On the opposite side of the plates, 8 mm discs of Fusarium pathogens were inoculated. Alternatively, another set of PDA plates was inoculated only with mycelial discs of Fusarium strains in the middle of plates, serving as the control group. Finally, the plates were incubated for 7 days at 25 ± 2 °C then the mycelial growth diameter was measured using Vernier calipers for both control and treated plates. The inhibition percentages were calculated according to the following equation: The inhibition percentage % =(A–B)/A×100 %, where A represents the colony diameter of Fusarium pathogens in the control plate, B represents the Fusarium colonies diameter in the dual culture plates.
2.7 Investigation of the interactions between the biocontrol agent and the tested Fusarium pathogens
The area of contact between the biocontrol agent and the fusarial pathogens was removed using a sterilized cutter. The interaction zone between the antagonistic fungus and the Fusarium strains was examined using scanning electron microscopy (SEM) analysis. The agar sections were then submerged in a solution containing 3 % (v/v) glutaraldehyde, which was buffered with 0.1 M sodium phosphate at a pH of 7.2. This immersion lasted for 60 min at a temperature of 25 °C. After that, the agar pieces were subjected to four rinses in a buffer solution and then treated with a 1 % (w/v) osmium tetroxide (OsO4) solution for 1 h to complete the fixation process. The samples were subjected to alcoholic dehydration by immersing them in ethanol solutions with concentrations ranging from 30 % to 100 % for a duration of 15 min. Once the specimens were fully dried, they were attached to stubs using double-sided carbon tape. The surface was coated with a thin layer of gold using a Polaron SC 502 sputter coater, and the changes in shape and structure were examined using a scanning electron microscope (JEOL JSM-6380 LA).
3 Results and discussion
3.1 Cultural and microscopic characteristics of Fusarium isolates
The identified Fusarium pathogens include three strains of F. oxysporum, which is the most frequent isolated strain, as well as one strain each of F. solani, F. proliferatum, and F. verticillioides. The strains were designated with the following abbreviations: F. solani (F.s), F. proliferatum (F.p), F. verticillioides (F.v), F. oxysporum strain 1 (F.o.S1), F. oxysporum strain 2 (F.o.S2), and F. oxysporum strain 3 (F.o.S3). The cultural characteristics were recorded for the isolated fusarial pathogens after 7 days of incubation. In this context, F. oxysporum, which is the most frequently isolated fusarial pathogen, was found to produce a white to beige colour in young colonies on PDA that darkened to violet after 7 days of incubation (Hussein et al., 2024). Conversely, F. moniliforme, also known as F. verticillioides, forms colonies that range in colour from white to violet on PDA media (Munkvold, 2017). Moreover, F.p and F.s strain colonies cultivated on PDA at a temperature of 25 °C for a duration of 7 days exhibited a dense-cottony white mycelium (Fig. 2) (Masratul Hawa et al., 2013; Zhu et al., 2019). The graduation behavior of the development of colonial colour in Fusarium species seemed to be a common characteristic, however it could not be relied upon for their precise identification. The morphological traits and rapid colonial colour changes of Fusarium spp. posed challenges in identifying them at the species level (Santos et al., 2019). Hence, the cultural identification was further confirmed by using internal transcribed spacer (ITS) sequencing technique. The microscopic features of F.v strain revealed the presence of aseptate microconidia that are oval to clavate in shape and the presence of false heads that arise from the polyphialides and monophialides in the aerial mycelium, whereas the macroconidia are produced in sporodochia and have a slender shape, thin walls, and are generally straight. They typically have 3–5 septa, and no chlamydospores are produced (Leslie and Summerell, 2008). Nevertheless, the microscopic characteristics of F.v were indistinguishable from those of the F.p strain, providing additional evidence that cultural and microscopic characteristics are insufficient for distinguishing between distinct Fusarium species. On the other hand, F. oxysporum's microscopic features include the presence of chlamydospores, typically produced singly or in pairs, and non-septated, oval to kidney-shaped microconidia. Moreover, we found that the macroconidia of F. oxysporum are short to medium in length, straight to slightly curved, thin-walled, and typically have three septa (Munkvold, 2017). Furthermore, the macroconidia of F.s are typically straight to slightly curved, with generally three to four septa, while the microconidia are abundant and have an oval to reniform shape, with zero to two septa (Aoki et al., 2014). Collectively, the isolated Fusarium species exhibit certain cultural and microscopic characteristics that provide challenges in reliably differentiating between various species. This highlights the need for precise identification using molecular ITS sequencing.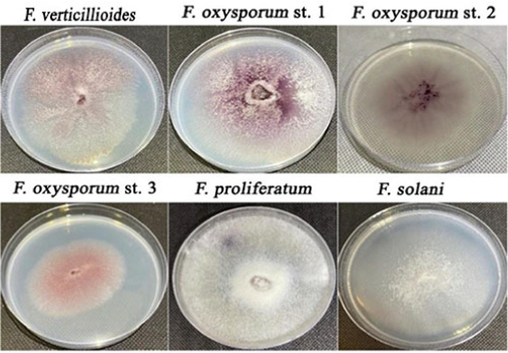
Culture characteristics of the isolated Fusarium strains.
3.2 Molecular identification of the isolated Fusarium pathogens
The F. oxysporum species complex (FOSC) includes cosmopolitan, asexual, soil borne species that are pathogenic (on plants, animals, humans) and nonpathogenic, revealing a complex phylogenetic structure. From a practical point of view, F. oxysporum strains ranked among the top 10 most economically and scientifically important plant pathogenic genera on a wide range of agronomic, ornamental, and horticultural crops (Edel-Hermann and Lecomte, 2019).
Fusarium oxysporum was the most frequent isolated fusarial strain followed by F. solani, F. proliferatum and F. verticillioides. F. oxysporum isolates were registered in Genbank with accession number of OP955665, OP959851, OP959874 and OQ680015 (Fig. 3). The isolated F. oxysporum strains were represented in the phylogenetic tree as sample 9, 45, 46 and 54 whereas F.s strains were highlighted in the phylogenetic tree as sample 36, 44, and 47. F.s isolates were registered in the Genbank with accession numbers of OP959797, OP959849 and OP987000. The isolated F. oxysporum strains from sample no 45 and 54 showed the closest relationship with the Chinese isolates number MT530242, MT530203, MT529672, MT476745, MK640600, MT180464, MT672442, OR789481, OR789476, OP363852, and OQ195132. Moreover, F. oxysporum isolates no 45 and 54 exhibited close relationship with Uzbekistan strains registered with numbers of OR816038 and OR819467. Furthermore, F. oxysporum (sample 46) strain revealed close genetic similarities with the Tiwanian and Indian isolates of MK336584 and MH100701, respectively. The isolated F.v strain (sample 3) revealed close relationships with the Chinese isolate of accession number MT549849 whereas F.s (sample 44) exhibited close relationship with the Chinese isolate number OP363852. Moreover, F.s isolates (sample 36 and 47) revealed close genetic relationships with MK409320 from USA and the Brazilian isolate number KT211516. However, the isolated F.p strain (sample 20) displayed genetic similarities with Chinese and Japanese isolates of Fusarium sp. registered with accession numbers of MK640559 and LC503276, respectively.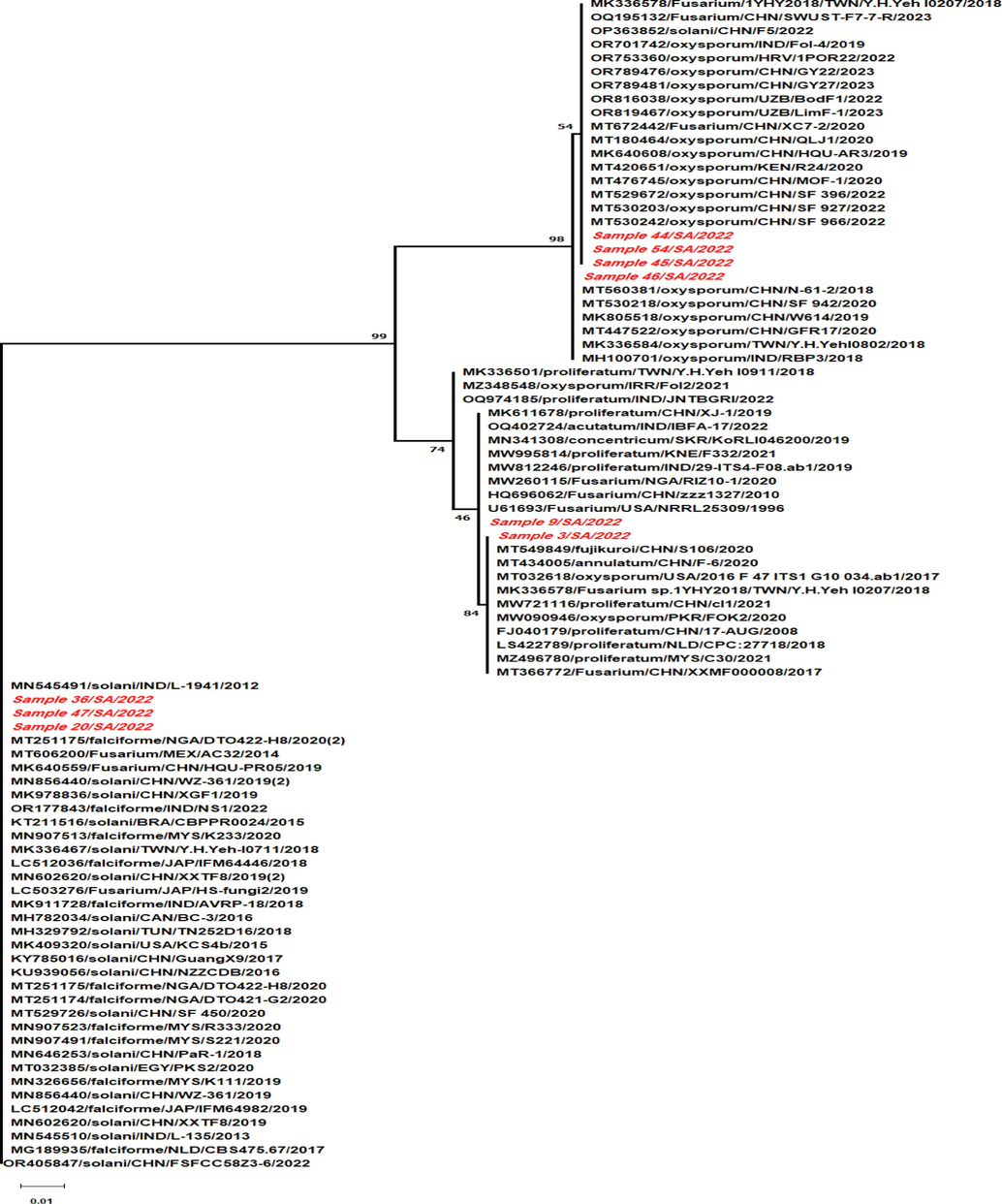
Phylogenetic analysis of the isolated Fusarium strains isolated from coffee plants in Saudi Arabia.
Recent progress in the fields of molecular biology and morphological surveys has resulted in the identification of many hidden species within F. oxysporum. As a result, the classification of the genus has undergone significant revisions (Lombard et al., 2019). Hence, the phylogenetic analysis of Fusarium strains is recommended to be conducted using multilocus sequence analysis (MLSA) of partial gene sequences for the translation elongation factor 1-α (tef1), the DNA-directed RNA polymerase II largest subunit (rpb2), β-tubulin (tub2), and the internal transcribed spacer (ITS) with the primer pairs Tef1F/Tef1R, RPB2-5F/RPB2-7cR, Tub2F/Tub2R, and ITS1/ITS4, respectively as described in different reports (O’Donnell et al., 2022). Another study determined that beta-tubulin sequences are not universally informative in Fusarium however, TEF, RPB1, and RPB2 sequences are informative. Therefore, the study suggested using beta-tubulin only in species complexes where it has been demonstrated to accurately reflect the species phylogeny inferred from orthologous marker loci (O’Donnell et al., 2022).
3.3 Pathogenicity assay
The pathogenicity assay of Fusarium strains was conducted on detached leaves of coffee plants. In this context, the disease severity was calculated according to the following equation:
Disease severity (S) = (area of diseased tissue /total tissue area) x 100. Pathogenicity assays demonstrated that all isolates caused the formation of black and necrotic lesions on detached coffee leaves (Fig. 4). The isolated F.p strain of accession number OP355441 showed the highest disease severity index of about 76.20 % followed by F.o.S3 of accession number OP959874. In this context, F.p showed a large dark necrotic lesion on detected coffee leaves of a diameter measuring 37.53 mm which signifies that the strain was highly virulent. The disease severity percentage of the isolated F.p strain was found to be significantly higher than that of a previous study (Wang et al., 2018). However, the F.s strain exhibited the lowest level of disease severity compared to the other isolated Fusarium strains, with a recorded relative disease severity of 15.65 % (Table 2). The detached lesion diameter of F.s strain in a prior report was found to range from 7.1 to 7.8 mm in diameter on detached wheat leaves and this result was in accordance with that of our current investigation (Sakr, 2020). However, the F.o.S3 strain with the accession number OP959874 exhibited the most severe disease symptoms on detached coffee leaves when compared to the other F.o.S1 and F.o.S2 with the accession numbers OP955665 and OP959851, respectively. Furthermore, F.v strain expressed a higher disease incidence of 66.44 % and a lesion diameter ranged of 26.95 mm. Taken together, the isolated Fusarium strains showed the formation of dark lesions which range from mild to severe on the detached coffee leaves. Different superscript letters indicated that values were significantly different at p ≤ 0.05.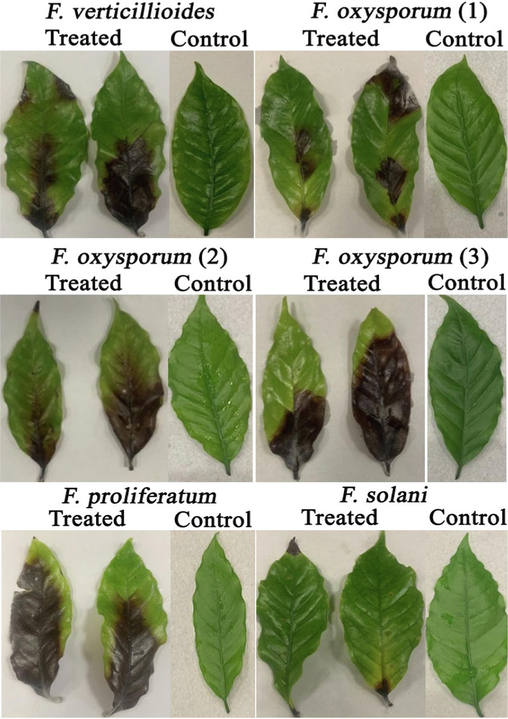
Pathogenicity assay of different Fusarium isolates on detached leaves of coffee plants.
Fusarium strains
Accession number
Lesion diameter (mm)
Leaf area (mm)
Disease severity
F.v
OP962009
26.95 ± 1.46 mm
40.56
66.44 a
F.o.S1
OP955665
16.73 ± 0.92 mm
42.78
39.11b
F.o.S2
OP959851
24.18 ± 0.81 mm
45.61
53.01c
F.o.S3
OP959874
31.23 ± 1.58 mm
45.73
68.29d
F.p
OP355441
37.53 ± 1.21 mm
49.25
76.20e
F. s
OP959797
6.5 ± 0.78 mm
41.54
15.65f
3.4 Antifungal efficiency of propiconazole fungicide against Fusarium stains
The severity of the isolated Fusarium strains necessitated an investigation of their susceptibility to fungicides to provide insights into their control and management. Hence, the current study investigated the efficiency of different concentrations of propiconazole fungicide in inhibiting the mycelial growth of the isolated Fusarium strains. In this context, F.o.S1 showed the highest susceptibility to the tested propiconazole antifungal agent at all of the tested concentrations, and this coincided with the pathogenicity assay results, as F.o.S1 showed the least disease severity compared to F.o.S2 and F.o.S3 (Table 3). However, F.o.S2 showed the highest resistance to propiconazole at the high concentration of 200 ppm recording relative mycelial growth diameter of 30.14 ± 0.25 mm. The suppression of mycelial growth was shown to be dependent on the concentration. However, it was noted that at higher doses of propiconazole (150 and 200 ppm) against F.p, F.o.S2, and F. s, there was no significant reduction the diameter of mycelial growth. In addition, the percentage of inhibition of mycelial growth was measured and compared to a control. It was observed that the F.p strain showed the lowest percentage of mycelial inhibition at the highest concentration of propiconazole (200 ppm), with a value of 53.92 % (Fig. 5). This finding supports the previous description of the severity of this strain in the detached leaf assay. Fusarium oxysporum strain 3 showed 75 % mycelial inhibition at 200 ppm of propiconazole fungicide whereas a previous study indicating 74 % inhibition of mycelial growth of Fusarium incarnatum-equiseti at 150 ppm of propiconazole (Prabhas et al., 2023). Taken together, propiconazole fungicide showed the highest fungicidal efficiency against F.o.S1 whereas F.o.S2 expressed the lowest susceptibility. Moreover, moderate antifungal efficiency of propiconazole fungicide was detected against F.p, F.v, F.o.S3, and F.s strains. A statistical analysis was conducted to determine the mean of different treatments of propiconazole for each strain and the other strains. The analysis aimed to identify any significant differences between the different strains by comparing each strain to the others. In this context, significant different was detected between F.v. and F.o.S1 strains of p value < 0.0001. No significant differences were detected between F.v and other strains of F.o.S2, F.p and F.s strains with relative p values of 0.2972, 0.9999 and 0.1673, respectively. In contrast, significant difference was detected between F.v and F.o.S3 (p value = 0.0094). Moreover, significant different between F. s and F.o.S1 was detected (p < 0.0001) however, no significant differences were detected between F.s and other strains. Furthermore, significant difference was detected between F. p and both of F.o.S1 and F.o.S3 with p-values of < 0.0001 and 0.0075, respectively. Significant differences were detected between F.o.S3 and both of F.v, F.p and F.o.S1 with relative p values of 0.0094, 0.0075 and 0.0007, respectively however significant difference was detected between F.o.S2 and F.o.S1 (p < 0.0001). Significant difference was detected between F.o.S1 and other strains and this could be explained due to that the F.o.S1 expressed the highest susceptibility to propiconazole fungicide at all tested concentrations. The use of fungicides to control fungal infections may pose health risks owing to the harmful effects these fungicides have on land and water ecosystems. Multiple deleterious impacts of fungicides on human health have been documented, including gastrointestinal, neurological, dermatological, and carcinogenic consequences. In addition, the frequent application of fungicides leads to the development of fungal resistance, even at higher concentrations. This has been observed in the current study, where propiconazole at 150 and 200 ppm did not significantly reduce the growth of F.p, F.o.S2, and F.s (Yassin et al., 2022). Therefore, the use of environmentally friendly techniques to control Fusarium pathogens is crucial for preserving a clean and sustainable environment. The present work aimed to examine the biocontrol efficacy of the endophytic fungus Diaporthe caatingaensis, which was isolated from coffee plant leaves, against the isolated Fusarium phytopathogens. Different superscript letters indicated that values were significantly different at p ≤ 0.05.
Propiconazole
Mycelial growth diameter (mm)
Concn. (ppm)
Control
25
50
100
150
200
F.p (Sample 20)
59.34 ± 0.53a
49.58 ± 0.28a
32.55 ± 0.67a
31.87 ± 0.38a
28.33 ± 0.41a
27.34 ± 0.27a
F.v (sample 3)
68.35 ± 0.51b
48.13 ± 0.35a
37.51 ± 0.63b
36.93 ± 0.56b
35.57 ± 0.42b
24.78 ± 0.58b
F.o.S1 (sample 9)
67.23 ± 0.57b
0.00 ± 0.00b
0.00 ± 0.00c
0.00 ± 0.00c
0.00 ± 0.00c
0.00 ± 0.00c
F.o.S2 (sample 45)
85.00 ± 0.00c
51.18 ± 0.62c
42.17 ± 0.54d
35.86 ± 0.71b
30.88 ± 0.34d
30.14 ± 0.25d
F.o.S3 (sample 46)
85.00 ± 0.00c
35.54 ± 0.54d
29.85 ± 0.37e
29.87 ± 0.29d
25.39 ± 0.25e
21.25 ± 0.62e
F.s(sample 36)
69.67 ± 0.38b
43.16 ± 0.49e
34.02 ± 0.53f
29.94 ± 0.58d
27.31 ± 0.44f
26.87 ± 0.31f
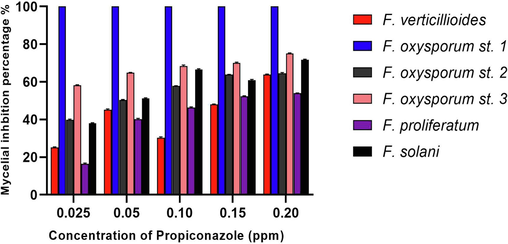
Mycelial inhibition percentages of the isolated Fusarium strains treated with different concentrations of Propiconazole fungicide.
3.5 Investigation of the antagonistic activity of D. caatingaensis strain utilizing dual culture assay
The antagonistic activity of D. caatingaensis was evaluated against six Fusarium strains using dual culture assay. Furthermore, the endophytic fungus D. caatingaensis presumably produces compounds that restrict the development of phytopathogenic fungi. This is shown by the collapse of pathogen hyphae in the contact zones, as seen in the dual culture experiment on plates containing F.p, F. s, and F. v (Fig. 6). The antibiosis process is characterized by the formation of a transparent interaction zone, which occurs when fungal growth ceases as a result of the biocontrol agent's secondary metabolites (Yassin et al., 2022). In this context, antibiosis was clearly visible against F.p, F. v, and F. s strains, however the region of antibiosis was determined to be small against F. oxysporum strains. However, the potent biological effects of D. caatingaensis strain may also be attributed to their ability to colonize at high rates, which leads to the suppression of competing microorganisms (Bizos et al., 2020). Fig. 7 showed that the isolated D. caatingaensis expressed the highest antagonistic activity against F.o.S2 whereas the least inhibition of mycelial growth was detected against F.s strain. The study used SEM analysis to explore the antagonistic behavior of the strain D. caatingaensis against the tested Fusarium strains, focusing on the area of antibiosis. The fungal endophytes produce bioactive compounds that function as powerful agents to regulate plant pathogens in the surrounding habitats. The examination of the types of endophytic species, the number of species, and the densities of colonization showed that the bioactive substances produced by endophytic fungus play a role in the mutually beneficial relationship between plants and fungi, helping to safeguard the host in different situations (Bullington and Larkin, 2015). The metabolites of the endophytic Diaporthe sp. were reported to possess antimicrobial activities and these compounds were 2(5H)-furanone, pyrrolidine, 9- octadecenoic acid (Z)-, methyl ester, hexadecanoic acid, methyl ester, pyrrolidine, 1-[2-(1,3- cyclopentadien-1-yl)ethyl], pyrrolidine, N-(4-methyl-4- pentenyl)-, pyrrolidine, N-(4-methyl-3- pentenyl), pyrrolidin-1-propionic acid, cyclopentanone, 2-(1-methylpropyl)-, 2-propyl cyclopentanone and pyrrolidine-5-one, 2-[3- hydroxypropyl]- as detected by gas chromatography mass spectrometry analysis (Saravanakumar et al., 2021). Furthermore, another research shown that the D. caatingaensis MT192326 strain produces bioactive compounds such as camptothecin (CPT) and its derivatives, which have been found to have antibacterial properties against bacterial pathogens (Dhakshinamoorthy et al., 2021).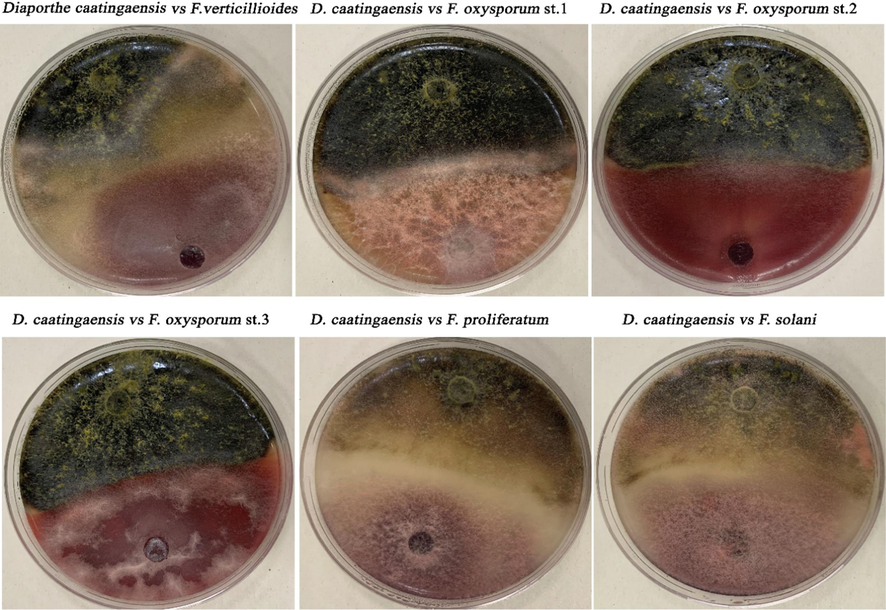
Antagonistic activity of the isolated Diaporthe caatingaensis strain against the isolated Fusarium strains.
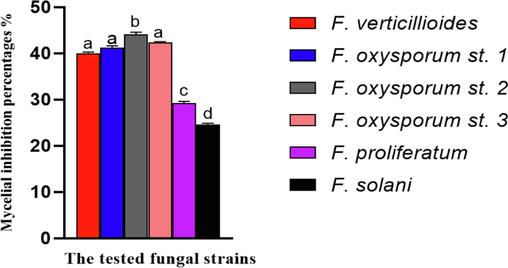
Antagonistic activity of Diaporthe caatingaensis strain against the tested fungal pathogens. Different letters indicated that values were significantly different at p ≤ 0.05.
3.6 Scanning electron microscope (SEM) analysis of the antibiosis zone
A scanning electron microscopy (SEM) examination was performed to examine the antibiosis area of the biocontrol agent against F.p and F.o.S3. The purpose was to evaluate the antagonistic mechanism of action of D. caatingaensis against these plant phytopathogens. The selection of these strains was based on their high disease severity, as determined by the results of pathogenicity tests. These strains were selected to investigate the parasitic mode of action of the biocontrol agent against them. SEM analysis revealed the lysis of the mycelium of F.p strain and this could be assigned to the action of fungal active metabolites as seen in Fig. 8a. Furthermore, the antibiosis in the dual culture plate of F. oxysporum was investigated, and other forms of antagonism were observed, such as the coiling of D. caatingaensis around the mycelium of F.o.S3, and the formation of appressorium, which ultimately resulted in the breakdown of the fungal mycelium, as depicted in Fig. 8b. Previous studies have shown that Trichoderma harzianum acts against fungal phytopathogens through a process called mycoparasitism. This involves the bioagent mycelium adhering to the mycelium of the fungal phytopathogens, coiling around it, forming an appressorium, and releasing active fungal metabolites and enzymes. These substances ultimately cause the fungal mycelium to break down and initiate cell death (López-Mondéjar et al., 2011; Ojha and Chatterjee, 2011). The SEM analysis clearly verified that the D. caatingaensis strain is highly effective in controlling Fusarium phytopathogens of coffee plants. This is due to the biocontrol agent's ability to inhibit the growth of fungal phytopathogens through various mycoparasitic mechanisms.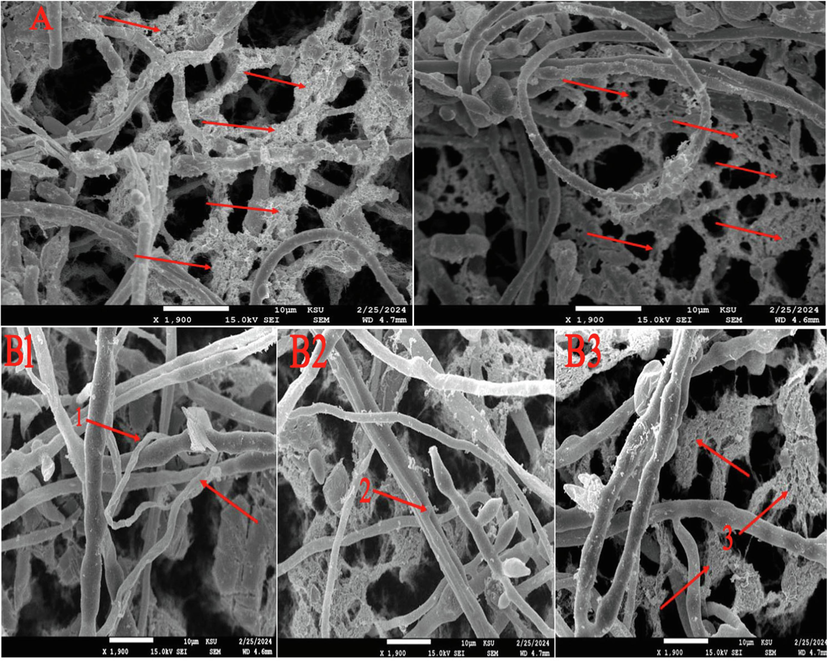
A- Lysis of F.p mycelium owing to the action of biocontrol agent metabolites, B- mycoparasitic behavior of D. caatingaensis against F.o.S3 (B1: coiling of D. caatingaensis around fungal phytopathogens, B2: formation of appressorium, B3: lysis of mycelium).
4 Conclusion
This is the first screening report of Fusarium phytopathogens linked with coffee plants in Saudi Arabia, as well as the first to describe the antagonistic effect of D. caatingaensis strain OP957006, isolated from coffee plant leaves, as a biocontrol agent against the identified fusarial pathogens. However, future studies need to be done on the phylogenetic analysis of Fusarium pathogens using multilocus sequence analysis of partial gene sequences for the translation elongation factor 1-α (tef1), the DNA-directed RNA polymerase II largest subunit (rpb2), and β-tubulin (tub2).
The endophytic fungus, D. caatingaensis, could be used to formulate safe and effective fungicides to control the fusarial pathogens and manage crop losses caused by fungal phytopathogens, for sustainable cultivation of coffee plants in Saudi Arabia. Furthermore, the biocontrol agent might be utilized to replace conventional fungicides, avoiding their negative environmental impacts.
Funding
This research project was supported by a grant from the Researchers Supporting Project number (RSP2024R114), King Saud University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Maha Alsubaie: Conceptualization, Investigation, and Data curation. Abdulaziz A. Al-Askar: Supervision and Dara curation. Fatimah Olyan Al-Otibi: Writing, reviewing and editing, Data curation and Funding acquisition. Khalid Maniah: Formal analysis and Data curation. Abdulrahman Alkathiri: Data curation. Mohamed Taha Yassin: Writing – review & editing, Writing – original draft, Validation, Formal analysis, Conceptualization.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R114), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Genetic-base broadening of Robusta coffee: Assessment of genetic variability for vigor and yield of introduced germplasm. Ecol. Genet. Genomics. 2022;25:100151
- [CrossRef] [Google Scholar]
- Alasmari, K., Abu Zeid, I., al-attar, A., 2020. Coffee Arabica in Saudi Arabia: An Overview ISSN (Online). Int. J. Pharm. Phytopharm. Res. 10, 71–78.
- First Occurrence of Coffee Leaf Rust Caused by Hemileia vastatrix on Coffee in Saudi Arabia. Microbiol. Res.. 2024;15:164-173.
- [CrossRef] [Google Scholar]
- Systematics of key phytopathogenic Fusarium species: current status and future challenges. J. Gen. Plant Pathol.. 2014;80:189-201.
- [CrossRef] [Google Scholar]
- The Role of Microbial Inoculants on Plant Protection, Growth Stimulation, and Crop Productivity of the Olive Tree (Olea europea L.) Plants. 2020;9:743.
- [CrossRef] [Google Scholar]
- Using direct amplification and next-generation sequencing technology to explore foliar endophyte communities in experimentally inoculated western white pines. Fungal Ecol.. 2015;17:170-178.
- [CrossRef] [Google Scholar]
- Current insights in fungal importance—a comprehensive review. Microorganisms. 2023;11:1384.
- [CrossRef] [Google Scholar]
- Santos, A.C. da S., Trindade, J.V.C., Lima, C.S., Barbosa, R. do N., da Costa, A.F., Tiago, P.V., de Oliveira, N.T., 2019. Morphology, phylogeny, and sexual stage of Fusarium caatingaense and Fusarium pernambucanum, new species of the Fusarium incarnatum-equiseti species complex associated with insects in Brazil. Mycologia 111, 244–259. DOI: 10.1080/00275514.2019.1573047.
- Six new species of coffee (Coffea) from northern Madagascar. Kew Bull.. 2021;76:497-511.
- [CrossRef] [Google Scholar]
- Endophytic fungus Diaporthe caatingaensis MT192326 from Buchanania axillaris: An indicator to produce biocontrol agents in plant protection. Environ. Res.. 2021;197:111147
- [CrossRef] [Google Scholar]
- Martins, D. dos S., Fornazier, M.J., Ventura, J.A., Pirovani, V.D., Uramoto, K., Guarçoni, R.C., Culik, M.P., Fiuza Ferreira, P.S., Zanuncio, J.C., 2022. Coffea arabica and C. canephora as host plants for fruit flies (Tephritidae) and implications for commercial fruit crop pest management. Crop Prot. 156, 105946. DOI: 10.1016/j.cropro.2022.105946.
- Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology®. 2019;109:512-530.
- [CrossRef] [Google Scholar]
- Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture. 2023;13:1810.
- [CrossRef] [Google Scholar]
- The importance of plant health to food security. Food Secur.. 2010;2:215-231.
- [CrossRef] [Google Scholar]
- Cumulative mortalities in white leg shrimp, Litopenaeus vannamei Boone 1931, cultured in biofloc system in Egypt reflected new record of Fusarium verticillioides infection. Aquac. Int. 2024
- [CrossRef] [Google Scholar]
- Krishnan, S., 2022. Coffee: Genetic Diversity, Erosion, Conservation, and Utilization, in: Priyadarshan, P.M., Jain, S.M. (Eds.), Cash Crops: Genetic Diversity, Erosion, Conservation and Utilization. Springer International Publishing, Cham, pp. 55–80. DOI: 10.1007/978-3-030-74926-2_3.
- The Fusarium Laboratory Manual. John Wiley & Sons; 2008.
- Epitypification of Fusarium oxysporum – clearing the taxonomic chaos. Persoonia Mol. Phylogeny Evol. Fungi. 2019;43:1-47.
- [CrossRef] [Google Scholar]
- López-Lima, D., Carrión, G., Sánchez-Nava, P., Desgarennes, D., Villain, L., n.d. Fungal diversity and Fusarium oxysporum pathogenicity associeted with coffee corky-root disease in Mexico.
- Mycoparasitism-related genes expression of Trichoderma harzianum isolates to evaluate their efficacy as biological control agent. Biol. Control. 2011;56:59-66.
- [Google Scholar]
- Characterization and pathogenicity of Fusarium proliferatum causing stem rot of Hylocereus polyrhizus in Malaysia. Ann. Appl. Biol.. 2013;163:269-280.
- [CrossRef] [Google Scholar]
- Coffee (Coffea arabica L.): Methods, Objectives, and Future Strategies of Breeding in Ethiopia—Review. Sustainability. 2021;13:10814.
- [CrossRef] [Google Scholar]
- Munkvold, G.P., 2017. Fusarium Species and Their Associated Mycotoxins, in: Moretti, A., Susca, A. (Eds.), Mycotoxigenic Fungi: Methods and Protocols. Springer, New York, NY, pp. 51–106. DOI: 10.1007/978-1-4939-6707-0_4.
- Diversity, Ecological Characteristics and Identification of Some Problematic Phytopathogenic Fusarium in Soil: A Review. Diversity. 2023;15:49.
- [CrossRef] [Google Scholar]
- DNA Sequence-Based Identification of Fusarium: A Work in Progress. Plant Dis.. 2022;106:1597-1609.
- [CrossRef] [Google Scholar]
- Mycoparasitism of Trichoderma spp. in biocontrol of fusarial wilt of tomato. Arch. Phytopathol. Plant Prot.. 2011;44:771-782.
- [CrossRef] [Google Scholar]
- Peck, L.D., Boa, E., n.d. Coffee wilt disease: The forgotten threat to coffee. Plant Pathol. n/a. DOI: 10.1111/ppa.13833.
- Importance of Fusarium spp. in Wheat to Food Security: A Global Perspective. In: Scott P., Strange R., Korsten L., Gullino M.L., eds. Plant Diseases and Food Security in the 21st Century, Plant Pathology in the 21st Century. Cham: Springer International Publishing; 2021. p. :127-159.
- [CrossRef] [Google Scholar]
- Prabhas, S., Singh, Gupta, P., Dahariya, N., Kaur, M., Singh, K., Gupta, N., Dahariya, M., Kaur, K., Kushwaha, K.P., 2023. Screening of chemical fungicides against Fusarium incarnatum-equiseti inciting pod rot of mungbean [Vigna radiata (L.) Wilczek] under in vitro condition.
- Evidence of the Toxic Potentials of Agrochemicals on Human Health and Biodiversity. In: Ogwu M.C., Chibueze Izah S., eds. One Health Implications of Agrochemicals and Their Sustainable Alternatives, Sustainable Development and Biodiversity. Singapore: Springer Nature; 2023. p. :105-135.
- [CrossRef] [Google Scholar]
- Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens. 2020;9:340.
- [CrossRef] [Google Scholar]
- Aggressiveness of Fusarium species causing head blight on wheat plants determined in detached leaf and seedling in vitro assays. Indian Phytopathol.. 2020;73:483-491.
- [CrossRef] [Google Scholar]
- Molecular cloning: a laboratory manual. Cold spring harbor laboratory press; 1989.
- Molecular identification, volatile metabolites profiling, and bioactivities of an indigenous endophytic fungus (Diaporthe sp.) Process Biochem.. 2021;102:72-81.
- [CrossRef] [Google Scholar]
- Fungal endophytes: A potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol.. 2019;21:101284
- [CrossRef] [Google Scholar]
- Identification of Fusarium proliferatum causing leaf spots on Cymbidium orchids in Taiwan. J. Phytopathol.. 2018;166:675-685.
- [CrossRef] [Google Scholar]
- An endophytic Fungi of Ginkgo biloba L. produces antimicrobial metabolites as potential inhibitors of FtsZ of Staphylococcus aureus. Fitoterapia. 2018;128:265-271.
- [CrossRef] [Google Scholar]
- Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro. J. King Saud Univ. - Sci.. 2021;33:101363
- [CrossRef] [Google Scholar]
- In vitro antagonistic activity of Trichoderma spp. against fungal pathogens causing black point disease of wheat. J. Taibah Univ. Sci.. 2022;16:57-65.
- [CrossRef] [Google Scholar]
- First Report of Cotton (Gossypium) Wilt Caused by Fusarium proliferatum in New Mexico, U.S.A. Plant Dis.. 2019;103:2679.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103396.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







