Translate this page into:
Exploring metabolic pathway alterations in obese fermented feces mediated by individual fruit extracts of Triphala components using untargeted metabolomics
⁎Corresponding author at: Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University, 50, Ngamwongwan Rd., Ladyao, Chatuchak, Bangkok 10900, Thailand. paiboon.tu@ku.ac.th (Paiboon Tunsagool)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective
This work aimed to explore the modified pathways impacted by changes in fecal metabolites among female obese adults during the human gut model period, both in the presence and absence of individual Triphala constituent fruit extract.
Methods and results
The human gut model employed individual fruit extracts from Phyllanthus emblica, Terminalia bellerica, and T. chebula, comparing them to a control group. Fermentation used fecal samples from female obese adults over 24 h. Metabolite extraction, untargeted metabolomics, and pathway analysis identified metabolic changes. Treatments with P. emblica extract, T. bellerica extract, and T. chebula extract revealed the statistical detection of 128, 734, and 757 up-regulated metabolites, respectively, while 31, 90, and 92 down-regulated metabolites were identified. Pathway analysis revealed that P. emblica extract primarily influenced vitamin B6 metabolism, whereas the treatments with T. bellerica extract and T. chebula extract predominantly engaged glycine, serine, and threonine metabolism for metabolic regulation within the human gut model.
Conclusion
By examining obese fecal metabolite changes and their association with metabolic pathway modulation through individual fruit extracts of Triphala constituents in a human gut model, this study provides a comprehensive understanding of Triphala components’ potential for managing obesity and its applications in the food industry.
Keywords
Feces
Metabolomics
Phyllanthus emblica
Terminalia bellerica
Terminalia chebula
1 Introduction
Obesity management options include weight loss drugs, and changing the gastrointestinal tract through surgical techniques which require the necessary considerations for safe application in the obese patient (May et al., 2020) including gut microbiota management. Appetite control and weight management, including several biological processes, were also monitored by gut microbiota-derived metabolites (Ejtahed et al., 2020). At the same time, holistic treatment has been introduced for obesity management. A separate study highlighted the remarkable properties of Triphala, incorporates the fruits of three plant species such as Phyllanthus emblica L., Terminalia chebula Retz, and T. bellerica Roxb, (Ahmed et al., 2021) in enhancing lipid profile, regulating blood glucose levels, managing body weight, body mass index, and waist circumference, (Phimarn et al., 2021) thus contributing to effective obesity management.
P. emblica fruits contain hydrolyzable tannins as key bioactive components, (Yang and Liu, 2014) which relate to the suppression of lipid buildup in 3T3-L1 cells (Nobushi et al., 2022). In addition, P. emblica has been documented to possess potential against obesity in male Wistar rats that were subjected to a diet rich in fats, resulting in considerable reductions in the rate of body weight gain (Nazish and Ansari, 2017). The main active constituents of T. bellerica are ellagitannins, classified as hydrolyzable tannins, including corilagin, chebulagic acid, galloylpunicalagin, and digalloyl-hexahydroxydiphenoyl-hexoside (Sobeh et al., 2019). The extract of T. bellerica demonstrated notable potential in combating obesity in spontaneously obese type 2 diabetic TSOD mice. This positive outcome was attributed to its ability to decrease the absorption of dietary lipids and strengthen the suppression of pancreatic lipase activity, primarily facilitated by gallic acid (Makihara et al., 2012). T. chebula is a source of tannins that contain pyrogallol (hydrolyzable) type as a major active ingredients (Bag et al., 2013). The extract of T. chebula presented the anti-obesity properties in mice that were induced to become obese through a high-fat diet. These effects were observed through the regulation of lipogenesis, stimulation of fatty acid oxidation, and activation of anti-inflammatory responses (Subramanian et al., 2021). Furthermore, P. emblica extract, T. bellerica extract, and T. chebula extract have been associated with gut microbiota regulation (Kim et al., 2006, Kong et al., 2022, Li et al., 2022), resulting in changes to metabolites in the gastrointestinal tract (Zhang and Dang, 2022). Although in vivo experimental models, such as mice, have been employed to investigate the impact of individual Triphala components in relation to gut microbiota and its derived metabolites, focusing on their potential anti-obesity effects, it should be noted that in vivo models might not be a suitable representation of human physiology.
The human gut model employs an approach of batch fermentation conducted in a laboratory setting to mimic the composition and behavior of the human colonic microbiota, utilizing fecal samples (Takagi et al., 2016). To examine the interactions between a model of simulated human gut microbiota and various molecules, such as antibiotics, DNA-based techniques combined with UHPLC-MS/MS analyses were employed during in vitro batch fermentation (El Houari et al., 2022). Additionally, in vitro batch fermentation or batch fermentation conducted in a laboratory setting was employed to investigate the generation of gut microbiota-dependent trimethylamine from dietary precursors, and LC-MS was used for quantifying the resulting metabolites (Day-Walsh et al., 2021). In vitro batch fermentation coupled with untargeted metabolomics might be a tool to reveal the metabolic mechanism for obesity management.
Because Triphala is a three-fruit mixture, determination of individual Triphala constituents on metabolic pathway alterations related to anti-obesity effect is required. The current work describes the action of P. emblica fruit extract, T. bellerica fruit extract, and T. chebula fruit extract on gut microbiota-derived metabolite alterations of female obese adults during fecal batch fermentation as a human gut model. We employ untargeted metabolomics to discern how each Triphala constituent individually regulates metabolism in the context of obesity management and its potential applications within the food sector.
2 Materials and methods
2.1 Ethics approvals
The present study protocol was reviewed and approved by The Ethics Committee of Kasetsart University, Bangkok, Thailand (License number COA64/068) and it was registered with the Thai Clinical Trials Registry (TCTR20220204007). Written informed consent was obtained from all participants before their inclusion in the study.
2.2 Individual fruit extracts of P. emblica, T. bellerica, and T. chebula preparation and composition analysis
Dried fruits of P. emblica, T. bellerica, and T. chebula were ground to a fine powder. To prepare the fruit extract, each sample was boiled, filtrated, and evaporated according to the methods in another work (Kwandee et al., 2023). The extracted samples were sent to Mae Fah Luang University, Chiang Rai, Thailand for determination of total phenolic content, total hydrolyzable tannin level, and total accumulation of condensed tannins by the Center of Excellence in Natural Products Innovation. Additionally, the content of total flavonoids in each sample was determined following another study (Kwandee et al., 2023).
2.3 Collection of fecal samples
The samples (fresh feces) were provided by three Thai female volunteers aged 23–33 years with BMI values in the range 35.0–40.0 kg/m2. The volunteers had no record of antibiotic usage, no previous instances of diarrhea or gastrointestinal tract disorders, and had not incorporated pro- or pre-biotics into their daily routine for three months. Prior to the collection of fecal samples, all volunteers provided written informed consent. The completed questionnaire indicated that each volunteer had a consistent eating history with identical proportions of carbohydrates, proteins, fats, and fruit and vegetable consumption. The sample preparation was carried out following the published work (Kwandee et al., 2023). Subsequently, the fecal solution from each sample was introduced into the batch fermentation conducted in a laboratory setting, serving as the human gut model.
2.4 Preparation of human gut model
The construction of human gut model was accomplished by adopting in vitro batch fermentation methods described in the previous study (Kwandee et al., 2023). The fermented batch consisting of the individual extract of P. emblica, T. bellerica, and T. chebula (1 mg/mL) with the fecal solution and basal medium was used as a treatment. A control group was established using the fermented batch, which solely consisted of the solution of fecal material and the fundamental nutrient solution (basal medium). Following a 24-hour fermentation period, samples were collected from the human gut model, including all its contents. The samples of each treatment were directly kept at −20 °C for metabolite extraction.
2.5 Extraction of metabolites and analysis of untargeted metabolomics
Metabolite extraction was carried out following the method of another study (Kwandee et al., 2023). Prior to analyzing the untargeted metabolomics samples, blank sample (water) preparation and quality control (QC) sample preparation were conducted following procedures described in a previously conducted study (Nazish and Ansari, 2017). For metabolite profiling, the untargeted metabolomics were executed utilizing a system of Vanquish Flex ultra-high-performance liquid chromatography (UHPLC) (Thermo Scientific, Germany) connected to a mass spectrometer using the system of the Orbitrap Exploris 120 (Thermo Scientific, Germany). A Hypersil Gold C-18 column (Thermo Fisher Scientific, USA) with dimensions of 2.1 × 150 mm and an internal diameter of 1.9 μm was utilized as a UHPLC column. The UHPLC and injection conditions were set as in another report (Kwandee et al., 2023). The mass-to-charge ratio (m/z) range, full MS resolution, data-dependent MS2, MS2 spectrometry data acquisition, and collision energy levels were configured following the previously conducted work (Kwandee et al., 2023). Metabolite identification was conducted using Compound Discover software version 3.3 from Thermo Fisher Scientific in the USA. The MyCompoundID MS was utilized in combination with the Human Metabolome Database (HMDB) library and the Evidence-based Metabolome Library (EML) database to aid in the identification of putatively identified metabolites (Li et al., 2013).
2.6 Statistical analysis
Statistical analysis of the LC-MS data, including Partial least squares discriminant analysis (PLS-DA), differential analysis (volcano plot and heatmap), and pathway analysis, was performed using MetaboAnalyst 5.0 (Pang et al., 2022) with a significance threshold of p-value < 0.05.
3 Results
3.1 The individual fruit extract composition of P. emblica, T. bellerica, and T. chebula
Supplementary Table S1 revealed that the concentration of total hydrolyzable tannins was the most prominent among all extracts. P. emblica extract, T. bellerica extract, and T. chebula extract contained total hydrolyzable tannin content at 222.30 ± 12.97, 115.86 ± 1.04, and 25.28 ± 0.07 mg gallotannin equivalent/mL, respectively. Other constituents of P. emblica extract, T. bellerica extract, and T. chebula extract were total phenolic compounds (126.97 ± 3.81, 62.34 ± 1.22, and 13.06 ± 0.15 mg gallic acid equivalent/mL, respectively), total flavonoids (0.96 ± 0.03, 0.23 ± 0.02, and 0.60 ± 0.03 mg catechin equivalent/mL, respectively), and total condensed tannins (0.37 ± 0.03, 0.35 ± 0.02, and 0.26 ± 0.01 mg catechin equivalent/mL, respectively).
3.2 Profiling of sub-metabolome and identification of metabolites
The analysis of fecal samples from all treatments, using the data obtained from electrospray ionization in both modes of positive and negative ions, led to the identification of 1,649 metabolites in duplicate. Metabolites that aligned with the database through m/z and retention time were categorized as metabolites with positive identification. A total of 31 metabolites were effectively aligned with both the mzCloud and ChemSpider compound databases, using m/z and retention time as criteria (Supplementary Table S2). Additionally, the mzCloud database yielded 93 matched metabolites, and the ChemSpider compound database resulted in 1,006 matched metabolites through the use of m/z and retention time (Supplementary Tables S3–S4). Metabolites that aligned with the HMDB library and the EML library database based on mass matching were regarded as metabolites with putative identification. A total of 28,719 metabolites were matched to the HMDB library, while the EML library yielded 53,950 matched metabolites through mass matching (Supplementary Tables S5-S6).
3.3 Analysis of fecal metabolome profiles and their comparative evaluation
Distinct separations in all extract treatments including the control group were captured in PLS-DA plot (Fig. 1). The metabolome data was categorized into four groups, each represented by distinct colors, along with a separate QC group (Supplementary Fig. S1). In the PLS-DA plot, red, blue, and yellow, and green dots represent the metabolome data of P. emblica extract, T. bellerica extract, T. chebula extract, and the control group, respectively. The metabolome data for P. emblica extract and the group of control show a close clustering pattern, whereas the metabolome data for T. bellerica extract and T. chebula extract exhibit noticeable separation, signifying distinct differentiation.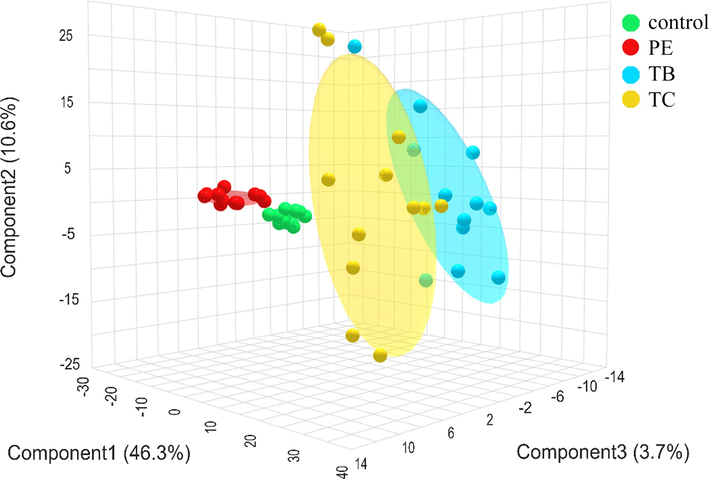
Partial least squares discriminant analysis (PLS-DA) of metabolomic data obtained from Phyllanthus emblica extract (PE)-treated group, Terminalia bellerica extract (TB)-treated group, and Terminalia chebula extract (TC)-treated group, and control group.
In Fig. 2, the volcano plots depict the binary comparison, highlighting the modified fecal metabolome within the human gut model when comparing the individual extract treatment to the control group. By conducting a binary comparison of the sub-metabolome in each extract treatment against the group of control, the impact of control was removed. Significance was attributed to any metabolite concentration change exceeding a 2-fold difference and possessing a p-value below 0.05. It was shown that 128, 734, and 757 up-regulated metabolites (shown as red dots) and 31, 90, and 92 down-regulated metabolites (shown as blue dots) were detected in the treatments of P. emblica extract (Fig. 2A), T. bellerica extract (Fig. 2B), and T. chebula extract (Fig. 2C) versus the control group, respectively. In the volcano plot, the grey dots indicate the absence of any significant difference.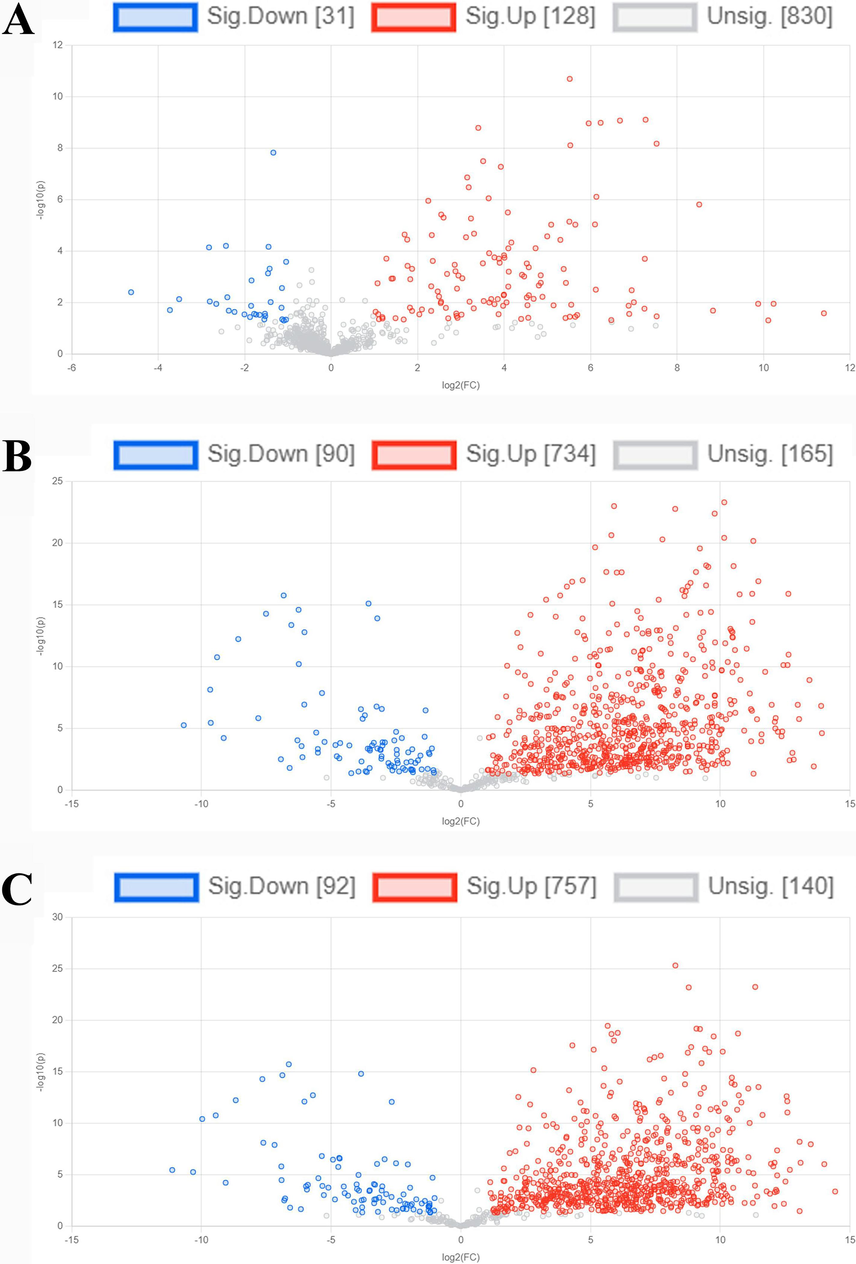
Comparing metabolites between treatment and control groups using a volcano plot. (A) Phyllanthus emblica extract versus control. (B) Terminalia bellerica extract versus control. (C) Terminalia chebula extract versus control. Metabolites showing significance are represented in red and blue, meeting the criteria of a fold change greater than 2 and a p-value below 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4 Modulation of fecal metabolites within the human gut model
Based on the number of recorded hits and the significance of the detected metabolites, the metabolites with positive identification from each extract were found to participate in sixty pathways. These pathways were characterized by their impact and p-value. Following the pathway analysis depicted in Fig. 3, each extract exerted an influence on numerous metabolites, particularly amino acids, which are associated with diverse metabolic pathways within the human gut model. There were eighteen amino acids which were detected in all treatments as shown in heatmap (Supplementary Fig. S2). In addition, P. emblica extract predominantly stimulated vitamin B6 metabolism as a key pathway (Fig. 4A), while the treatments of T. bellerica extract and T. chebula extract primarily influenced glycine, serine, and threonine metabolism (Fig. 4B). These pathways exhibited the highest impact and statistical significance among the sixty pathways analyzed within the human gut model.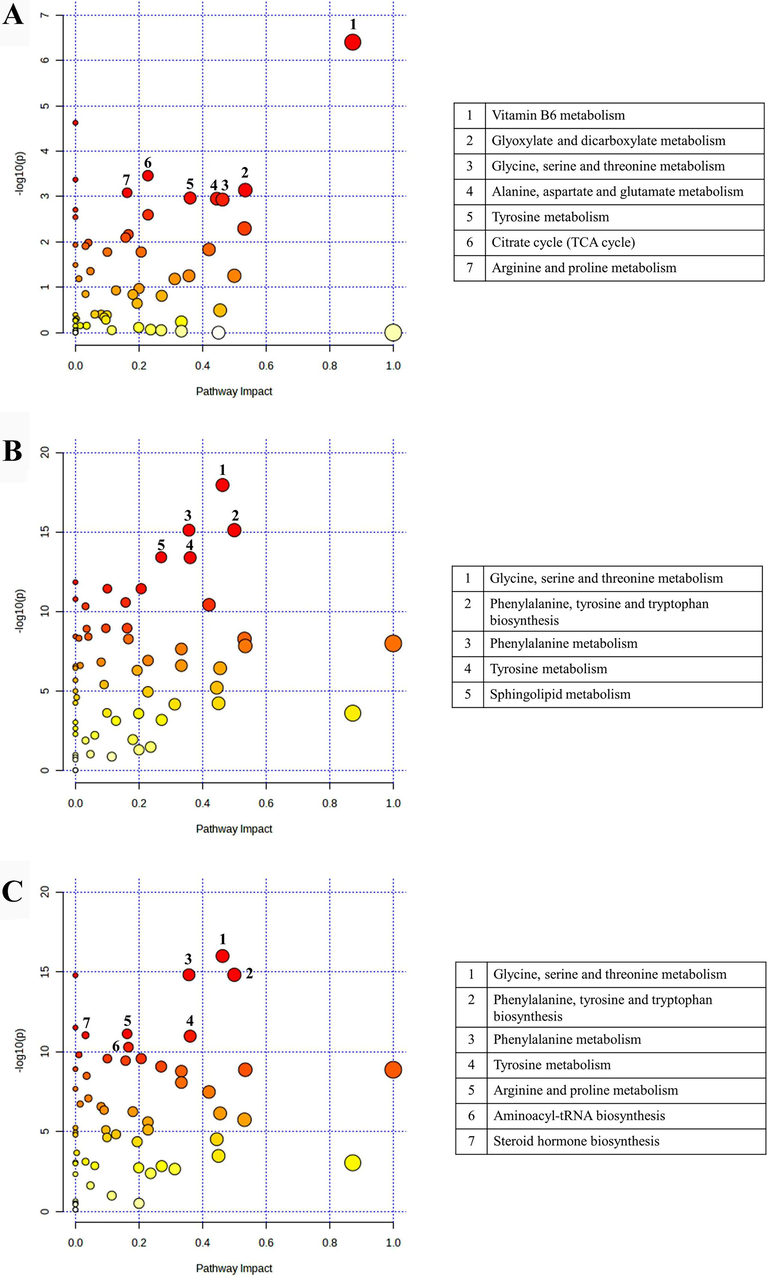
Overview of metabolic pathway analysis. (A) Phyllanthus emblica extract versus control. (B) Terminalia bellerica extract versus control. (C) Terminalia chebula extract versus control.
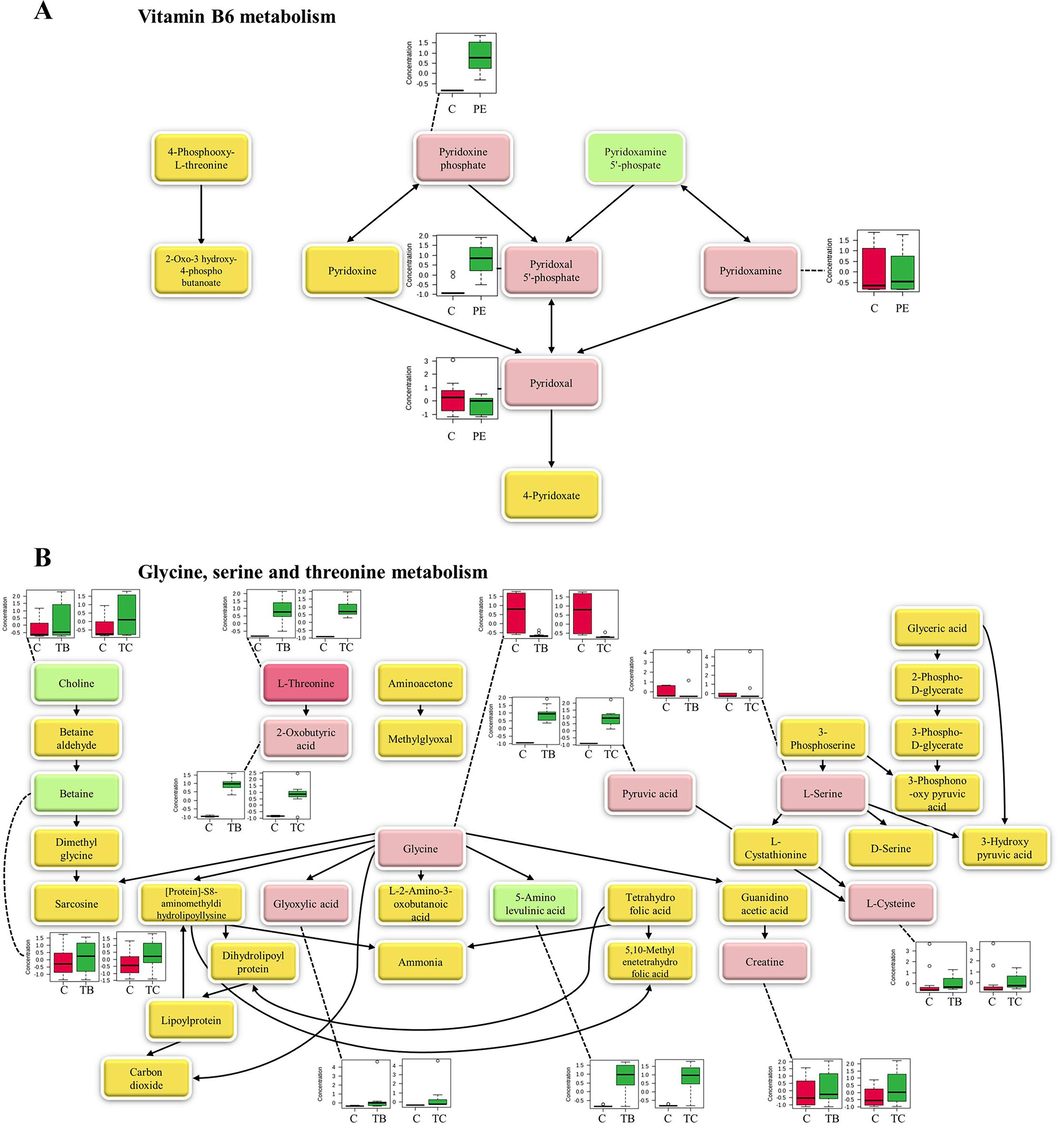
Metabolic pathway, the metabolites that corresponded to entries in both the mzCloud and ChemSpider compound databases were classified as positive identification, which were labeled in the dark pink boxes. The light pink boxes within the figures indicate the metabolites with positive identification that corresponded to entries in either the mzCloud or ChemSpider compound databases. The green box denotes the metabolites with putative identification, while the yellow boxes represent the metabolites that have not yet been identified (not found in the current work). Box plots showing the identified metabolites are presented alongside their respective metabolites. (A) Metabolic pathway of vitamin B6 metabolism. PE, Phyllanthus emblica extract group; C, the group of control. (B) Metabolic pathway of glycine, serine and threonine metabolism. TB, Terminalia bellerica extract group; TC, Terminalia chebula extract group; C, control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4A illustrates the presence of metabolites with positive identification, including pyridoxine phosphate, pyridoxal 5′-phosphate, pyridoxamine, and pyridoxal, in vitamin B6 metabolism. Additionally, pyridoxamine 5′-phosphate was found to be a putative metabolite within this pathway. Fig. 4B displays the presence of several metabolites with positive identification, including L-threonine, 2-oxobutyric acid, glyoxylic acid, glycine, pyruvic acid, L-serine, creatine, and L-cysteine, within the glycine, serine, and threonine metabolism pathway. Furthermore, choline, betaine, and 5-amino levulinic acid were identified as putative metabolites in this pathway.
4 Discussion
The main content of each fruit extract was hydrolyzable tannins, that was similar to other studies that reported hydrolyzable tannins as major bioactive components of each fruit (Bag et al., 2013, Yang and Liu, 2014, Sobeh et al., 2019). Hydrolyzable tannins had a therapeutical property by decreasing nutrient intake and anti-nutritional activity in rats, resulting in the management of body weight (Manzoor et al., 2021). Thus, hydrolyzable tannins might be a key compound of each fruit extract of Triphala components that play an important role in metabolic changes, resulting in obesity management.
Moreover, phenolic compounds were found as a minor content in each herb extract (in this study, gallic acid was employed as the standard for assessing the total phenolic accumulation). Gallic acid, which is a phenolic compound, has been reported to provide a reduction property of redundant lipid storage, including inhibition of lipogenesis in adipose tissue (Dludla et al., 2018). In addition, gallic acid contributes to the modulation of peroxisome proliferator-activated receptor gamma expression, which aids in enhancing insulin sensitivity and improving glucose metabolism (Behera et al., 2023). In the individual fruit extract, there was small amounts of flavonoids and condensed tannins compared to other compounds. Flavonoids are concerned anti-obesity agents with properties of reducing fat absorption, increasing energy disbursement, regulating lipid metabolism, and modulating gut microbial profile, resulting in obesity management (Rufino et al., 2021). Another study demonstrated that the combination of condensed tannins and bile salts affected lipid digestion, resulting in fat absorption reduction (Li et al., 2019). It could be suggested that phenolic compounds, flavonoids, and condensed tannins might work together with hydrolyzable tannins for the actions of each fruit extract on obesity management.
In PLS-DA plot, it could be observed two distinct patterns of metabolome data, that was the data pattern of the P. emblica extract plus the control and the data pattern of T. bellerica extract plus T. chebula extract. It could be suggested that the action of P. emblica extract on metabolite changes was different from the effect of T. bellerica extract and T. chebula extract, indicating different metabolic regulation. Even the metabolome data in the extract of P. emblica was clustered closely with the group of control in PLS-DA plot, but the volcano plot of P. emblica extract versus the control group reveals the differential metabolites between both groups. In addition, each herbal extract exhibited a more pronounced impact on the alteration of fecal metabolites within the human gut model compared to the group of control.
For pathway analysis, it was discovered that the P. emblica extract, T. bellerica extract, and T. chebula extract induced the accumulations of metabolites, such as amino acids, linked to sixty pathways. In this study, eighteen amino acids were altered mediated by each fruit extract. Another study reported that changes of essential amino acids involved in maintaining energy homeostasis through various mechanisms and reducing fat mass body weight, (Xiao and Guo, 2022) resulting in obesity management. Furthermore, P. emblica extract prominently influenced vitamin B6 metabolism as a primary pathway, whereas both T. bellerica extract and T. chebula extract triggered the major pathway of glycine, serine, and threonine metabolism in the human gut model. In addition, a result from another study was reported that T. bellerica extract impacted the metabolism of glycine, serine, and threonine in the intestinal bacterial community of mice, (Zhang et al., 2023) which aligns with the findings of this study.
Pathway analysis results revealed that the top three pathways activated by P. emblica extract were the metabolism of vitamin B6, glyoxylate and dicarboxylate metabolism, and glycine, serine, and threonine metabolism. In contrast, T. bellerica and T. chebula extracts induced the top three pathways, which included glycine, serine, and threonine metabolism, phenylalanine, tyrosine, and tryptophan biosynthesis, and phenylalanine metabolism. In a previous study, Triphala extracts were employed in fecal batch fermentation to model the human gut and evaluate their effects on metabolites originating from the gut microbiota of obese adult women (Kwandee et al., 2023). The findings indicated that Triphala notably influenced metabolites related to phenylalanine, tyrosine, and tryptophan biosynthesis, vitamin B6 metabolism, and phenylalanine metabolism. Based on these pathways, the study suggests that P. emblica extract may activate vitamin B6 metabolism, while T. bellerica and T. chebula extracts may stimulate phenylalanine, tyrosine, and tryptophan biosynthesis, as well as phenylalanine metabolism during the Triphala actions in the fecal batch fermentation period.
In the vitamin B6 metabolism pathway, this study identified the following metabolites with positive identification: pyridoxine phosphate, pyridoxal 5′-phosphate, pyridoxamine, and pyridoxal, which are forms or vitamers of vitamin B6 (Mascolo and Vernì 2020). A published study reported that vitamin B6 supplementation contributed to BMI reduction and improved body composition by decreasing weight, fat mass, waist circumference, total cholesterol, triglycerides, low-density lipoprotein, insulin resistance, and fasting insulin in obese women who received vitamin B6 supplementation for a duration of eight weeks (Haidari et al., 2021). According to another study, there was a statistically significant correlation between elevated vitamin B6 levels and an increase in the percentage of fat-free mass in obese women (Rodríguez-Rodríguez et al., 2008). Activation of vitamin B6 metabolism might be one of the strategies for obesity improvement by the action of P. emblica extract.
In glycine, serine and threonine metabolism, there were 4 altered amino acids that were metabolites with positive identification (L-threonine, glycine, L-serine, and L-cysteine). The administration of threonine supplements was reported to have the ability to reduce body weight, as well as the weights of perirenal and epididymal fat pads, in obese mice. Additionally, it led to decreased serum concentrations of glucose, total cholesterol, triacylglycerols, and LDL-cholesterol in the same group of mice (Ma et al., 2020). Glycine supplementation had potential to reduce insulin resistance in the liver, resulting in management of obesity-related metabolic diseases (Alves and Morio, 2023). Supplementation of L-serine was involved in reduction of weight regain in mice (López-Gonzales et al., 2022). Cystine (two cysteines join together via a disulfide bond) was reported to effectively hinder the increase in body weight and the accumulation of visceral fat (Kobayashi et al., 2009). The alterations of amino acids affected lipid metabolism might be one of the mechanisms for obesity treatment mediated by the action of T. bellerica extract and T. chebula extract. However, the determination of synergistical stimulation effect of each fruit extract on fecal metabolite changes influencing signal transduction, as well as, the relationship between the metabolites and the chemicals of hydrolyzable tannins, phenolic compounds, flavonoids, and condensed tannins, is challenging and still requires future study.
5 Conclusions
The findings from the present study offer a thorough understanding of the impact of P. emblica extract, T. bellerica extract, and T. chebula extract on alterations in gut-derived metabolites, particularly concerning the regulation of metabolic pathways, in comparison to the group of control within the human gut model. In summary, the distinct fruit extracts of Triphala components demonstrated notable effects on metabolic regulation, thus highlighting Triphala as a promising natural option for obesity management and its applications in the food industry.
CRediT authorship contribution statement
Pincha Kwandee: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing - original draft. Surasawadee Somnuk: Conceptualization, Methodology, Resources, Writing - review & editing. Massalin Nakphaichit: Conceptualization, Methodology, Resources, Validation, Writing - review & editing. Bandhita Wanikorn: Conceptualization, Data curation, Methodology, Resources, Writing - review & editing. Sittiruk Roytrakul: Conceptualization, Methodology, Resources, Validation, Writing - review & editing. Paiboon Tunsagool: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Acknowledgments
This work was funded by Kasetsart University through the Graduate School Fellowship Program and supported by Kasetsart University Research and Development Institute (KURDI) (FF (KU)13.65).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exploring scientific validation of Triphala Rasayana in ayurveda as a source of rejuvenation for contemporary healthcare: an update. J. Ethnopharmacol.. 2021;273:113829
- [CrossRef] [Google Scholar]
- Alterations in glycine metabolism in obesity and chronic metabolic diseases – an update on new advances. Curr. Opin. Clin. Nutr. Metab. Care. 2023;26(1):50-54.
- [CrossRef] [Google Scholar]
- The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop Biomed.. 2013;3, 3:244-252.
- [CrossRef] [Google Scholar]
- Therapeutic potential of gallic acid in obesity: considerable shift! Obes Med.. 2023;37:100473
- [CrossRef] [Google Scholar]
- The use of an in-vitro batch fermentation (human colon) model for investigating mechanisms of TMA production from choline, L-carnitine and related precursors by the human gut microbiota. Eur. J. Nutr.. 2021;60(7):3987-3999.
- [CrossRef] [Google Scholar]
- Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients. 2018;11:1.
- [CrossRef] [Google Scholar]
- Gut microbiota-derived metabolites in obesity: a systematic review. Biosci. Microbiota Food Health. 2020;39, 3:65-76.
- [CrossRef] [Google Scholar]
- Development of an in vitro model of human gut microbiota for screening the reciprocal interactions with antibiotics, drugs, and xenobiotics. Front. Microbiol.. 2022;13:828359
- [CrossRef] [Google Scholar]
- The effect of pyridoxine hydrochloride supplementation on leptin, adiponectin, glycemic indices, and anthropometric indices in obese and overweight women. Clin Nutr Res.. 2021;10(3):230-242.
- [CrossRef] [Google Scholar]
- Growth-inhibiting activity of active component isolated from Terminalia chebula fruits against intestinal bacteria. J. Food Prot.. 2006;69(9):2205-2209.
- [CrossRef] [Google Scholar]
- Kobayashi, H., Hirabayashi, Y., Murakami, H., et al., 2009. Anti-obesity effects of amino acid in high-fat diet induced obese mice. FASEB J. 23, S1, 227.225-227.225. 10.1096/fasebj.23.1_supplement.227.5.
- Preventive effect of Terminalia bellirica (Gaertn.) Roxb. extract on mice infected with Salmonella typhimurium. Front. Cell. Infect. Microbiol.. 2022;12:1054205.
- [CrossRef] [Google Scholar]
- Efficacy of Triphala extracts on the changes of obese fecal microbiome and metabolome in the human gut model. J. Tradit. Complement. Med.. 2023;13(2):207-217.
- [CrossRef] [Google Scholar]
- Characterizing the interactions of dietary condensed tannins with bile salts. Agric Food Chem.. 2019;67(34):9543-9550.
- [CrossRef] [Google Scholar]
- MyCompoundID: using an evidence-based metabolome library for metabolite identification. Anal. Chem.. 2013;85(6):3401-3408.
- [CrossRef] [Google Scholar]
- Aqueous extract of Phyllanthus emblica L. alleviates functional dyspepsia through regulating gastrointestinal hormones and gut microbiome in vivo. Foods.. 2022;11:10,1419.
- [CrossRef] [Google Scholar]
- L-serine supplementation blunts fasting-induced weight regain by increasing brown fat thermogenesis. Nutrients. 2022;14:9.
- [CrossRef] [Google Scholar]
- Threonine, but not lysine and methionine, reduces fat accumulation by regulating lipid metabolism in obese mice. J. Agric. Food Chem.. 2020;68(17):4876-4883.
- [CrossRef] [Google Scholar]
- Preventive effect of Terminalia bellirica on obesity and metabolic disorders in spontaneously obese type 2 diabetic model mice. J. Nat. Med.. 2012;66(3):459-467.
- [CrossRef] [Google Scholar]
- Effect of hydrolysable tannin on nutrient intake obesity and other associated metabolic risk factors in polycystic rats. Transl. Med. Commun.. 2021;6(1):10.
- [CrossRef] [Google Scholar]
- Vitamin B6 and diabetes: relationship and molecular mechanisms. Int. J. Mol. Sci.. 2020;21:10.
- [CrossRef] [Google Scholar]
- Modern pharmacological treatment of obese patients. Ther. Adv. Endocrinol. Metab.. 2020;11 2042018819897527
- [CrossRef] [Google Scholar]
- Emblica officinalis - Anti-obesity activity. J. Complement Integr Med.. 2017;15:2.
- [CrossRef] [Google Scholar]
- Inhibitory effects of hydrolysable tannins on lipid accumulation in 3T3-L1 cells. Biol. Pharm. Bull.. 2022;45(10):1458-1465.
- [CrossRef] [Google Scholar]
- Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc.. 2022;17, 8:1735-1761.
- [CrossRef] [Google Scholar]
- Effects of Triphala on lipid and glucose profiles and anthropometric parameters: a systematic review. J. Evid. Based Integr. Med.. 2021;26 2515690x211011038
- [CrossRef] [Google Scholar]
- Vitamin B6 status improves in overweight/obese women following a hypocaloric diet rich in breakfast cereals, and may help in maintaining fat-free mass. Int. J. Obes.. 2008;32(10):1552-1558.
- [CrossRef] [Google Scholar]
- Flavonoids as antiobesity agents: a review. Med. Res. Rev.. 2021;41(1):556-585.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant and hepatoprotective activities of methanol extracts from leaves of Terminalia bellirica and Terminalia sericea (Combretaceae) PeerJ. 2019;7:e6322.
- [Google Scholar]
- Anti-obesity effect of T. chebula fruit extract on high fat diet induced obese mice: a possible alternative therapy. Mol. Nutr. Food Res.. 2021;65, 10:e2001224.
- [Google Scholar]
- A single-batch fermentation system to simulate human colonic microbiota for high-throughput evaluation of prebiotics. PLoS One. 2016;11(8):e0160533.
- [Google Scholar]
- Impacts of essential amino acids on energy balance. Mol Metab.. 2022;57:101393
- [CrossRef] [Google Scholar]
- Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem.. 2014;62(3):529-541.
- [CrossRef] [Google Scholar]
- Roles of gut microbiota and metabolites in overweight and obesity of children. Front. Endocrinol.. 2022;13:994930
- [CrossRef] [Google Scholar]
- Terminalia bellirica ethanol extract ameliorates nonalcoholic fatty liver disease in mice by amending the intestinal microbiota and faecal metabolites. J. Ethnopharmacol.. 2023;305:116082
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103115.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







