Translate this page into:
Exploitation of selected plant extracts as bio-control against fungal contaminants in animal feed
⁎Corresponding authors. isfahan@hu.edu.pk (Isfahan Touseef), shakira_akmal@parc.gov.pk (Shakira Ghazanfar) shakira_akmal@yahoo.com (Shakira Ghazanfar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Pakistan is among the top agricultural countries around the globe, but mycotoxin contamination causes a major commercial loss every year. The present study aimed to isolate the fungi and their mycotoxins present in contaminated feed of animals in the local market of Peshawar.
Methodology
The antifungal activity of certain plant extracts was to be tested against pathogenic fungi. TLC method was used for mycotoxin analysis and it was found that Aflatoxin G1, G2, B1 and B2 were present in different amounts both qualitatively and quantitatively based on samples.
Result
This study found the presence of contamination in all the tested feed samples. All ten samples were found positive for Aspergillus flavus. B1 toxin was found in high quantity in all ten samples, while G2 was found in a lower quantity as compared to other toxins such as G1, B1 and B2. The quantity of aflatoxin was from 48.6 to 284.7 ppb in 25 g of samples. In the case of antifungal potency, all plant extracts showed important antifungal potency against tested fungi. The MIC and MFC values noted ranged from 110 to 300 mg/ml and 100–300 mg/ml respectively. Citrus aurantium and Myrtus communis were absorbed to have antifungal potency against all test fungi. Citrus aurantium extracts were also found to inhibit the growth of Aspergillus flavus.
Conclusion
According to the results of the present research, a variety of fungal strains and aflatoxins were present in animal feed in numerous parts of Peshawar, Pakistan and different plant extracts can be used in animal feed to reduce this type of contamination.
Keywords
Citrus aurantium
Mycotoxins
Peshawar
Pakistan
Aflatoxin
- FAO
-
Food and Agriculture Organization
- PCSIR
-
Pakistan Council of Scientific and Industrial Research
- PDA
-
Potato Dextrose Agar
- PDB
-
Potato Dextrose Broth
- MIC
-
Minimum Inhibitory Concentration
- MFC
-
Minimum Fungicidal Concentration
- TLC
-
Thin Layer Chromatography
- UV
-
Ultra violet
Abbreviations
1 Introduction
Pakistan is mainly dependent on its agriculture sector. About half of Pakistan’s population is dependent upon agriculture. Different researchers recommended that agricultural development is directly related to the national economy of the country (Ali and Iqbal, 2004). In addition to other agriculture products, livestock is also thought to be an important contributor to Pakistan’s GDP because it adds almost 37% value-addition to the national GDP. According to the Food and Agriculture Organization (FAO) reports, 25% of the cereals in the world are destroyed by mycotoxins (Klopfenstein,2000).
Mycotoxins are diverse types of molecules that are injurious to living things. They are generally present in cereals and grains during the pre or post-harvesting stage and due to their varied toxicity, they are considered injurious for the users (Yiannikouris and Jouany,2002). Many species of fungi account for the production of mycotoxins in animal feedstuff (Yiannikouris and Jouany,2002; Salari et al., 2012). The threat of mycotoxin contamination especially aflatoxin in cereals, grains and other field crops is a serious safety concern for the world (Reddy et al.,2009).
In olden times, many techniques were used to inhibit the progression of mycotoxins i.e. microorganisms, absorbents, chemicals and ionizing radiations (Sinha, 1998). Nevertheless, natural plants and plant extracts have expanded the attention of researchers due to their less toxic effects. Natural compounds from higher plants are cheap and ecofriendly and are considered as antimicrobial agents (Reddy et al., 2009; Gonçalez et al., 2003).
Plants possessing powerful antimicrobial properties can be used to protect food from different types of toxins. Natural compounds from the plant can also be served as antifungal agents (Abid et al., 2022). Additionally, citrus plants are reported to have medicinal importance. Crude extracts from these plant's families can be used as natural remedies to cure many diseases i.e. diarrhea, constipation and vomiting, etc (Gonçalez et al., 2003). Citrus fruits have an ample amount of carotenoids, minerals, limonoids, essential oils, flavonoids, acridone alkaloids, and vitamin B and C complex. Limonoids, glycosides, flavones and polymethoxyflavones are the flavenoids naturally produced by citrus plants. (Gonçalez et al., 2003; Sinha, 1998). They have many health regulating activities i.e. anti-viral, anti-cancerous, anti-fungal, anti-bacterial, anti-oxidant, and anti-allergic potencies. Lemon juice is employed as a diuretic agent, antiscorbutic and also used against the common cold (Salari et al., 2012). Moreover, the antifungal and anti-aflatoxigenic efficacy of Aloe vera, Zizyphusspina, Cassia italica, Lavandula vera, Olea europaea, Ricinus communis, Lawsonia inermis, Prosopisjuliflora, Datura stramonium and Eucalyptus globulus is also documented to be used as inhibitors of aflatoxin production (Reddy et al.,2009). The current study aimed to isolate the fungal species and also analyze their mycotoxins in the samples of animal feeds available in the local markets of Peshawar. Also, it was aimed to test the antifungal activity of different plant extracts against those fungal species. The synergistic effects of toxin inhibition and mycoflora promotion were also aimed in the present study.
2 Methods and materials
2.1 Plant samples
Six different medicinal plant leaves i-e; Aloe vera (Aloe), Myrtus communis (Myrtle), Citrus sinensis (Sweet orange), Emblica officinalis (Amla), Citrus limon (Lemon) and Citrus aurantium (Sour orange) were collected from Pakistan Council of Scientific and Industrial Research (PCSIR) Peshawar, Pakistan (Table 1). The plant samples (N = 6) were shade dried and crushed to obtain a powder.
Plant name
Family
Urdu/local name
English name
Aloe vera
Liliaceae
Aloe vera
Aloe
Citrus aurantium
Rutaceae
Narangi
Bitter orange
Citrus sinensis
Rutaceae
Malta
Sweet orange
Emblica officinalis
Phyllanthaceae
Amla
Indian gooseberry
Citrus limon
Rutaceae
Leemu
Lemon
Myrtus communis
Myrtaceae
Mehndi/hina
Myrtle
2.2 Preparation of aqueous extracts
Concisely, 50 g of dried powder of selected plants was soaked in about 500 ml sterile purified water. Then the mixture was filtered with a muslin cloth. After filtration, the mixture was centrifuged at 4000 rpm for 30 mins. The supernatant was further processed for filtration through Whatman filter paper No.1. The extract was then stored at 4 °C.
2.3 Sample collection areas
Different areas of Peshawar were visited and ten animal feed samples were purchased. The samples were processed within 48 h of being collected. Table 2 shows the animal feed samples.
S No
Location
1
Board bazzar
2
Bacha khan chouk
3
Bashir-abad
4
Firdos-stop
5
Fakkir-kely
6
Hashtnaagri
7
Palosi-bazar
8
Terahi-bala
9
Tehkaal
10
Warsaak-road
2.4 Determination of total count of fungal colonies
The pour plate technique was used to determine fungal colonies. In 450 ml butterfield solution, 50 g of animal feed was added and mixed for 2 min after that, serial dilutions were made up to 10-6 folds. Potato Dextrose Agar (PDA) was used for the culturing of fungal isolates. A total of 20 ml media was transferred to each petri plate. After solidification of the media, the petri plates were incubated for 25 °C. Then 1 ml sample from each serially diluted tube was inoculated in sterilized petri plates. The plates were again placed in an incubator and after 3 to 5 days fungal colonies were appeared and calculated per 1 g of sample (Bhatti et al.,1990).
2.5 Isolated fungal colonies characterization
The isolated colonies were identified through microscopic analysis. The glass was prepared by adding 2 drops of lactophenol cotton blue dye on the glass. A small amount of the fungal sample to be tested was taken from the media and placed on the glass slide portion where the dye was added. A cover slide was placed over the sample and pressed a little until a small bubble was created in order to release the in-between air. To improve the observation, the hyphal growth was broken. The slides were observed under various magnifications. Characteristics like conidial head, conidial organization and hyphae were observed and noted (Habib et al., 2015).
2.6 Maintenance of fungal culture
The cultures were placed in PDA media and were served for further analysis (Satish et al., 2007).
2.7 Inoculum preparation
The strains were grown on PDA media at 27 °C and 7 days after their, the spores of these fungal species were grown on agar plates. The cells from the stored cultures were transferred and diluted with fresh potato Dextrose Broth (PDB). The spores suspension was kept at the level of 2x105cfu/ml spores (de Lima et al., 2013; Abril et al., 2008).
2.8 Antifungal activity assay
The leaf extract of six selected plants was analyzed for antifungal activities through the disc diffusion method (Yaseen et al.,2022). The process was performed in tight septic conditions and the tests were performed in triplicate to avoid errors. The zone of inhibition was measured in millimeters. The antifungal activity test was done under extreme aseptic conditions.
2.9 Determination of minimum inhibitory concentration (MIC) of prepared extracts
The MIC value was determined using the standard methodology documented by (Awang et al., 2011).
2.10 Determination of minimum fungicidal concentration (MFC) of prepared extracts
MFC value was determined using the standard method documented by (Dahham et al., 2010). The minimum concentration required to inhibit the growth of fungi was recorded.
2.11 Percent mycelial inhibition of A. flavus using poison food technique
To check the antifungal activity of selected plants against A. flavus, aqueous extracts of these plants were made. The food poison technique previously used by (Mohana and Raveesha, 2007) was used for this process. Autoclaved distilled water was taken as a control sample. Four replicates were maintained for each concentration of aqueous extract. The efficacy of these extracts was observed in terms of percent inhibition (%) of the fungal mycelial growth using the following formula: I% = (dc-dt) × 100/dc (Thippeswamy et al., 2014).
dc = ‘average diameter of mycelial growth in the control’.
dt = ‘average diameter of mycelial growth in the treatment’.
2.12 Analysis and quantification of mycotoxins/aflatoxin by TLC technique
For the analysis of mycotoxins in the feed samples, the thin layer chromatography (TLC) technique was used (Bhatti et al., 1990). For visualization of aflatoxins on TLC plates, the plates were observed under Ultra-Violet light at 366 nm and 254 nm in a closed system and their spot intensities were compared.
2.12.1 Calculation
Effective Weight:
Comparison of Standard:
2.13 Statistical analysis
Experiments were performed in triplicates and the values of these experiments were presented as mean values ± standard errors. The analysis was done with ANOVA using SPSS 20 software. The difference of 0.05 between values was considered statistically significant.
3 Results
3.1 Quantification of fungus in animal feed
All the samples were found to have a variable number of fungal colonies that were presented as cfu/g in Fig. 1. The highest TPC value of 4x1021 was shown by sample C while A was found to have the lowest TPC value of 5x1014. All other samples showed moderate TPC values.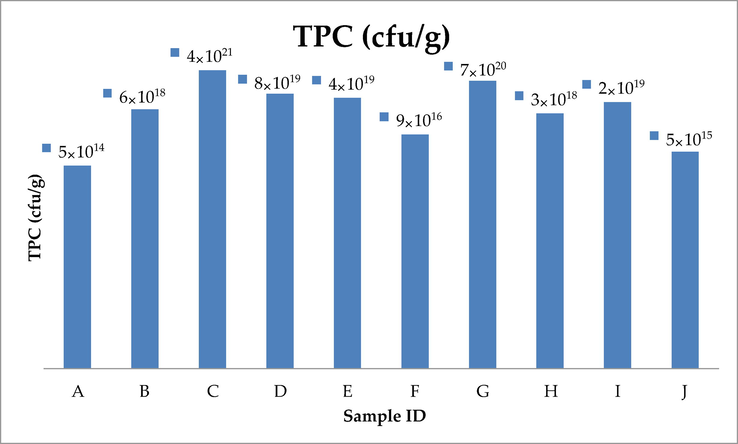
All samples showing different TPC values.
3.2 Fungal flora of animal feed samples
The plating technique was used for the investigation of feed samples. A representative figure of a couple of isolates is shown in Fig. 2 and Fig. 3. Table 3 shows the list of isolated fungi samples. Seven species of Aspergillus i-e; A. parasiticus, A. flavus, A. niger, A. ochraceus, A. clavatus, A. fumigatus, A. carbonarius and three species of Penicillium i-e; P. citrinum, P. verrucosum and P. notatum were obtained from feed samples. All ten samples were found positive for Aspergillus flavus while only one sample was positive for Aspergillus notaum i-e; sample G.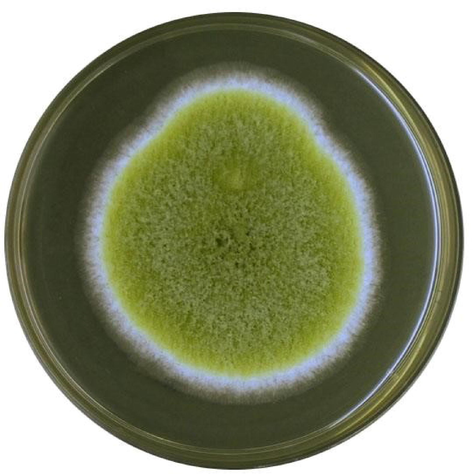
Growth of Aspergillus flavus on PDA medium.
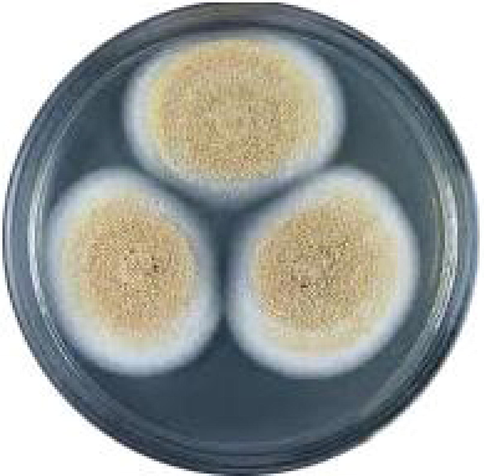
Growth of Aspergillus ochracheus on PDA medium.
S. No
Sample ID
Identified Fungus
01
A
Aspergillus niger
Aspergillus flavus
Aspergillus parasiticus
Aspergillus carbonarius
02
B
Aspergillus niger
Aspergillus flavus
Aspergillus ochraceus
03
C
Aspergillus niger
Aspergillus flavus
Aspergillus parasiticus
Aspergillus carbonarius
04
D
Aspergillus flavus
Aspergillus parasiticus
05
E
Penicillium citrinum
Aspergillus parasiticus
Aspergillus carbonarius
Aspergillus flavus
06
F
Aspergillus niger
Aspergillus flavus
Aspergillus parasiticus
Aspergillus carbonarius
07
G
Aspergillus notatum
Aspergillus flavus
Aspergillus parasiticus
Aspergillus carbonarius
08
H
Aspergillus flavus
Aspergillus parasiticus
Penicillium citrinum
09
I
Aspergillus niger
Aspergillus flavus
Aspergillus parasiticus
Aspergillus carbonarius
Aspergillus ochraceus
Penicillium citrinum
10
J
Aspergillus niger
Aspergillus flavus
Aspergillus parasiticus
Aspergillus carbonarius
3.3 Antifungal activity of plant’s leaves aqueous extracts
Table 4 shows the antifungal activity of plant extracts. Citrus sinensis showed the highest activity 13 ± 0 mm) against Aspergillus niger while Aloe vera was reported to have the least inhibition (07 ± 1 mm). Aspergillus niger and Citrus lemon showed no antifungal activity. In the case of Aspergillus flavus, the widest zone of inhibition (15 ± 1) was shown by Citrus aurantium., while the least activity (09 ± 1 mm) was shown by Citrus limon. Emblica officinalis showed no activity. The activity of Citrus sinensis was highest (15 ± 0 mm) against Aspergillus parasiticus, and lowest was that of Citrus lemon (07 ± 1 mm) against Aspergillus parasiticus. The antifungal activity of all the water extracts of selected plants against tested fungi is summarized in Table 4. NZ: No zone of inhibition.
Name of Fungus
Zone of inhibition in milimeter (mm)
Aloe vera
Citrus limon
Citrus aurantium
Citrus sinensis
Emblica officinalis
Myrtus communis
A. parasiticus
13 ± 1.0
7 ± 1.0
11 ± 1.0
15 ± 00
6 ± 1.0
13 ± 00
A.. flavus
11 ± 0.6
9 ± 1.0
15 ± 1.0
11 ± 00
NZ
14 ± 1.0
A. ochracheus
09 ± 1.0
10 ± 00
17 ± 1.0
11 ± 1.0
9 ± 2.0
11 ± 1.0
A. fumigatus
14 ± 0.0
13 ± 1.0
10 ± 2.0
14 ± 2.0
11 ± 1.0
13 ± 00
A. niger
07 ± 1
NZ
9 ± 2.0
13 ± 00
10 ± 1
10 ± 1.0
A. carbonarius
10 ± 2.0
8 ± 2.0
12 ± 1.0
9 ± 2.0
6 ± 1.0
9 ± 00
A. clavatus
08 ± 0.0
11 ± 1.0
12 ± 00
8 ± 1.0
7 ± 2.0
17 ± 1.0
P. citrinum
NZ
9 ± 00
11 ± 1.0
11 ± 1.0
7 ± 00
8 ± 1.0
P. notatum
15 ± 00
7 ± 1.0
9 ± 1.0
10 ± 00
10 ± 1.0
14 ± 1.0
P. verrucosum
06 ± 00
8 ± 2.0
10 ± 0.5
7 ± 0.5
9 ± 1.0
11 ± 1.0
3.4 MIC of plant’s leaves aqueous extracts
The Minimum Inhibitory Concentration (MIC) of the selected plant's leaves aqueous extracts was carried out by microdilution method which is listed as MIC value mg/ml in Table 5. The MIC value Citrus sinensis 160 mg/ml against Aspergillus niger, MIC value of Citrus aurantium was 240 mg/ml against Aspergillus niger. In the case of Aspergillus flavus, Citrus aurantium exhibited MIC at 130 mg/ml, whereas Citrus limon had the highest MIC (210 mg/ml). Against Aspergillus parasiticus, the lowest MIC was observed in Citrus Sinensis (140 mg/ml) whereas the highest MIC was observed in Emblica officinalis (210 mg/ml). Against Aspergillus ochraceus the lowest MIC value was found in Citrus aurantium (110 mg/ml) whereas the highest MIC value was found in Emblica officinalis (210 mg/ml) and Aloe vera (210 mg/ml). A summary of the MIC value of all the test plants against the isolated fungi is described in Table 5. NA: not applied.
Name of Fungus
MIC Value mg/ml
Myrtus communis
Emblica officinalis
Aloe vera
Citrus sinensis
Citrus aurantium
Citrus limon
A. niger
180
180
230
160
240
NA
A. flavus
160
NA
170
170
130
210
A. parasiticus
170
290
150
140
170
230
A. ochracheus
170
210
210
180
110
190
A. carbonarius
210
300
190
230
170
230
A. fumigatus
160
180
170
150
200
160
A. clavatus
100
240
230
240
160
170
P. notatum
150
200
150
190
210
160
P. citrinum
210
250
NA
180
170
150
P. verrucosum
170
240
280
250
180
180
3.5 MFC of plant’s leaves aqueous extracts
The MFC value of the aqueous extracts of selected plants was performed by the microdilution method. In the case of Aspergillus niger MFC value was 180 mg/ml in Citrus sinensis, while the highest MFC value was noted in Aloe vera i-e; 240 mg/ml. In the case of Aspergillus flavus, Citrus aurantium showed the lowest MFC value i.e 150 mg/ml, however, Citrus limon showed the highest MFC value i.e. 230 mg/ml. In the case of Aspergillus parasiticus the least MFC value was shown by Citrus sinensis (160 mg/ml), while the highest MFC was observed in Emblica officinalis (290 mg/ml). A summary of the MIC value of all the test plants against the isolated fungi is described in Table 6. NA: not applied.
Name of Fungus
MFC Value mg/ml
Myrtus communis
Emblica officinalis
Aloe vera
Citrus sinensis
Citrus aurantium
Citrus limon
A. niger
200
200
240
180
240
NA
A. flavus
170
NA
200
190
150
230
A. parasiticus
180
290
180
160
190
240
A. ochracheus
190
230
230
190
130
210
A. carbonarius
230
300
210
230
200
230
A. fumigatus
170
200
180
170
210
180
A. clavatus
110
260
250
260
180
190
P. notatum
160
210
170
210
230
170
P. citrinum
230
260
NA
190
190
180
P. verrucosum
190
220
300
250
200
200
3.6 Toxicological analysis of animal feed
In the current study, the amount of aflatoxin was calculated by the TLC method in 25 g of each feed sample. Feed samples were screened for the presence of aflatoxins B1, B2, G1 and G2. The number of aflatoxins found in ppb in each 25 g is summarized in Table 7. Results showed that aflatoxin B1 was found in the highest quantity in sample C and also present in all the samples in variable amounts. Whereas aflatoxin G2 was found to rarely occur with the lowest quantity and also present a small quantity in sample I (12.7 ppb). Furthermore, the highest quantity of total aflatoxin was detected in sample C i.e. 248.7 ppb, whereas the lowest quantity of aflatoxin was detected in sample E i-e; 48.6 ppb. ND = Not detected.
Sample ID
B1 (ppb)
B2 (ppb)
G1 (ppb)
G2 (ppb)
Total Aflatoxin (ppb)
A
48.6
26.7
ND
ND
75.3
B
53.8
30.6
ND
ND
84.4
C
136.5
63.9
67.7
16.6
284.7
D
57.6
23.1
17.6
ND
98.3
E
36.8
ND
11.8
ND
48.6
F
88.5
24.2
13.5
ND
126.2
G
67.3
19.6
9.7
ND
96.6
H
34.3
17.8
ND
ND
52.1
I
112.8
51.9
28.2
12.7
205.6
J
79.2
34.4
7.5
ND
121.1
3.7 Percent mycelial inhibition of A. flavus
The antifungal activity of the aqueous extract of each plant was determined against A. flavus by poison food technique for the determination of percent mycelial inhibition. The result is presented in Fig. 4. Citrus aurantium showed the highest (62.3 ± 0.33) mycelial inhibition of A. flavus, while Citrus limon showed the lowest mycelial inhibition (46.5 ± 0.46).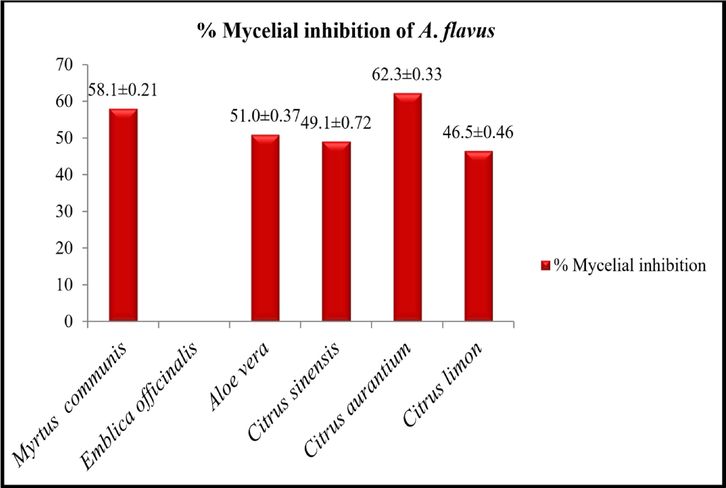
% Mycelial inhibition of A. flavus by selected plant extract. Data given are the mean of four replicates ± standard error.
4 Discussion
The present study was carried out to check the antifungal effect of the plant leaves aqueous extract of specific plants to inhibit or lower the fungal growth and their mycotoxins in animal feed samples. In the current study, six medicinal plants (listed in Table 1) were used to investigate their antifungal and antimycotoxinigenic capacity. The highest activity among six plants was shown by Myrtus communis against aspergillus clavatus. It has been previously reported that Myrtus communis has significant antifungal ability against dermatophytic fungi. The research has confirmed that methanolic extract of M.communis leaves inhibits Mycosporum canis (Mehrabani et al., 2013). In another study, the growth of R. solani was inhabited by 60% through essential oils of Myrtus communis at a concentration of 1600 ppm (Curini et al., 2003). The difference between the results of our study and Curini et al., (2003) may be due to the techniques used to calculate antifungal activity and selection of test fungi.
Numerous studies exposed the noteworthy biological activity of Aloe vera against bacteria, viruses, and fungi. Ethanol and aqueous extract of Aloe vera had shown exciting results against a number of bacteria and fungi. (Nidiry et al., 2011). In the current study aqueous, extracts of aloe vera leaves were tested that inhibited the growth of different species of Aspergillus and Penicillium.
Shireen et al., (2015) documented that Aloe vera extract showed strong antifungal activity against Candida albicans (Shireen et al., 2015). In the present study, an aqueous extract of Aloe vera leaves was also subjected to the percentage (%) mycelial inhibition of Aspergillus flavus and results showed that about 51 ± 0.37% growth was inhibited. Coopoosamy and Magwa et al., (2007) documented the antifungal activity of Aloe vera and reported that Aloe vera restricts the growth of Aspergillus niger to 24.29%, 9.26% of Aspergillus flavus growth and 6.24% of Penicillium digitatum growth (Coopoosamy and Magwa et al., 2007). Moreover, Kawai and his group (1998) showed that crude extracts of Aloe vera controlled the production of aflatoxin (Kawai et al., 1998).
Sharma and Tripathi, 2009 reported that citrus essential oils comprise a complex mixture of volatile compounds having a potential antifungal activity that reduces the growth of fungi in a dose–response manner or inhibits fungal growth. In the present research, the Citrus sinensis leaves were found to be an effective inhibitor against Penicillium and Aspergillus. The highest antifungal activity of Citrus sinensis was recorded against Aspergillus parasiticus whereas MIC and MFC were recorded as 140 mg/ml and 160 mg/ml respectively and their results showed MIC 4.09.0 µl/ml against the Trichoderma harzianum and Verticillium fungicola.
In the present research, the C. sinensis leaves extract was also subjected to the percentage (%) mycelial inhibition of Aspergillus flavus. (Chutia et al., 2009) studied C. sinensis essential oil for the percentage (%) growth inhibition of diverse fungal species, they found that C. sinensis inhibited Fusarium oxysporum Alternaria alternate (84%) (42%), Curvularia lunata (93.25%) and Helminthosporium oryzae (54%). The variation of our results in comparison with earlier studies is possible because of the selection of different fungal species and methods.
In our study, Citrus aurantium presented high antifungal efficiency. The highest activity was found in the case of Aspergillus ochraceus, the zone of inhibition was calculated as 17 ± 1 mm, where MIC and MFC were noted to be 110 mg/ml and 130 mg/ml respectively. (Hsouna et al., 2013) found that the antifungal potency of Citrus aurantium against the growth of, Fusarium, Alternaria alternata and Aspergillus. According to this research, Citrus aurantium essential oil inhibited the growth of Fusarium graminearum and Aspergillus flavus with a zone of inhibition of 22 mm and MIC value of 78 µg/ml respectively. Also, Citrus aurantium showed high antifungal potential against Aspergillus niger. Fusarium oxyporum and Aspergillus flavus. The zone of inhibition ranged 14–34 mm and MIC was 0.078–1.25 mg/ml.
In the current investigation, Citrus limon showed modest antifungal activity. The most important activity was recorded in the case of Aspergillus clavatus and its zone of inhibition was 11 mm. In the present research, the percentage (%) of mycelial inhibition of A. flavus was also done. Citrus limon inhibited the growth of A. flavus up to 46.5 ± 0.46%. Similarly, the percentage (%) of mycelial inhibition of A. flavus was also done by (Chutia et al., 2009). Furthermore, Citrus limon essential oil also restricted the growth of A. flavus up to 44.0 ± 0.07%. Fisher and Phillips, (2006) found that the spread of foodborne microbes can be controlled by using essential oils of different citrus plants such as C. sinensis, C. limon, C. bergamia and their constituents. Also, (Hosni et al., 2010) reported that three monoterpenes are effective antifungal components that are 1,8-cineole, thymol, and (S)-limonene. (Hosni et al., 2010) found that essential oils from C. limon showed antifungal activity against Glomerella cingulata and Fusarium oxysporum.
5 Conclusions
Despite the existence of plentiful agricultural-based food resources for livestock in Pakistan, some pathogenic fungal species are accountable for polluting livestock feed by making mycotoxins. Moreover, mycotoxin contamination, mainly aflatoxins, is the main distress for the safety of grains and other field crops around the world. Natural plants and plant extracts, on the other hand, have captured the attention of the scientific community due to their less toxic effects. Natural products derived from higher plants are environmental friendly, biodegradable, substantial, and have antifungal and antimycotoxigenic properties. Results of the present study showed that all the aflatoxins i.e., G1, G2, B1 and B2 were present in varying amounts depending on the plants. The highest quantity was present of B1 in all ten samples while G2 was in a lower amount as compared to other samples. Relating to the antifungal properties of the samples Citrus aurantium and Myrtus communis were absorbed to have antifungal potency against all test fungi. Citrus aurantium extracts were found to inhibit the growth of Aspergillus flavus. Future research is needed to investigate the proper genomic understanding of these mycotoxins as an effective treatment therapy. Furthermore, good surveillance and proper hygienic measures should be implemented to reduce the likelihood of pathogenic contaminants in animal feed.
CRediT authorship contribution statement
Raza Ullah: Conceptualization, Methodology. Isfahan Touseef: Conceptualization, Methodology, Supervision. Rameesha Abid: Data curation, Writing – review & editing, Visualization, Validation. Arshad Farid: Writing – review & editing, Visualization, Formal analysis, Supervision. Sohail Ahmad: Writing – review & editing, Formal analysis. Hesham Ali El Enshasy: Writing – review & editing, Resources, Validation, Formal analysis. Adil Aksoy: Resources, Investigation, Supervision. Nada H. Aljarba: Funding acquisition. Tahani Mohamed Al–Hazani: Funding acquisition. Muhammad Muzammal: Resources, Investigation, Supervision. Shakira Ghazanfar: Validation, Formal analysis, Writing – original draft, Writing – review & editing.
Acknowledgment
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R62), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Authors also would like to thank the financial support from RMC, UTM through industrial projects no. R.J.130000.7609.4C465 and R.J.130000.7609.4C359. The authors acknowledge the services and facilities provided by the Department of Microbiology, Hazara University Mansehra, Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pharmacological properties of 4′, 5, 7-Trihydroxyflavone (Apigenin) and Its Impact on cell signaling pathways. Molecules. 2022;27(13):4304.
- [Google Scholar]
- Improved microassays used to test natural product-based and conventional fungicides on plant pathogenic fungi. Plant Dis.. 2008;92:106-112.
- [Google Scholar]
- Total factor productivity growth in pakistan's agriculture: 1960–1996. Pak. Dev. Rev. 2004:493-513.
- [Google Scholar]
- Preparation, characterization and antimicrobial assay of 1, 10-Phenanthroline and 2, 2’-Bipyridyl adducts of Cadmium II N-Sec-Butyl-N-Propyldithiocarbamate: crystal structure of Cd [S CN i-CH CH ] 2, 2’-Bipyridyl. World Appl. Sci. J.. 2011;12:1568-1574.
- [Google Scholar]
- Association of aflatoxin producing strains of Aspergillus with some stored cereals. Pakistan J. Phytopathol.. 1990;2:74-82.
- [Google Scholar]
- Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT-Food Sci. Technol.. 2009;42:777-780.
- [Google Scholar]
- Coopoosamy, R. M., & Magwa, M. L., 200. Traditional use, antibacterial activity and antifungal activity of crude extract of Aloe excelsa. African J. Biotechnol, 6.
- Composition and in vitro antifungal activity of essential oils of Erigeron canadensis and Myrtus communis from France. Chem. Nat. Compd.. 2003;39:191-194.
- [Google Scholar]
- Studies on antibacterial and antifungal activity of pomegranate Punica granatum L. Am. Eurasian J. Agric. Environ. Sci.. 2010;9:273-281.
- [Google Scholar]
- Isolation, identification, and activity in vitro of killer yeasts against Colletotrichum gloeosporioides isolated from tropical fruits. J. Basic Microbiol.. 2013;53:590-599.
- [Google Scholar]
- The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol.. 2006;101:1232-1240.
- [Google Scholar]
- Inhibition of aflatoxin production by Polymnia sonchifolia and its in vitro cytotoxicity. Arq. Inst. Biol.. 2003;70:159-163.
- [Google Scholar]
- Isolation and identification of Aspergillus species from poultry feeds in Kaduna State. Nigeria. Microbiol. Res. Int.. 2015;3:27-32.
- [Google Scholar]
- Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem.. 2010;123:1098-1104.
- [Google Scholar]
- Characterization of essential oil from Citrus aurantium L. flowers: antimicrobial and antioxidant activities. J. Oleo Sci.. 2013;62:763-772.
- [Google Scholar]
- In vivo effects of Aloe arborescens Miller var. natalensis Berger Kidachi aloe on experimental tinea pedis in guinea-pig feet. Phytother. Res.: Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv.. 1998;12:178-182.
- [Google Scholar]
- Nutritional quality of cereal-based foods. In: Handbook of cereal science and technology. CRC Press; 2000. p. :705-723.
- [Google Scholar]
- Evaluation of antifungal activities of Myrtus communis L. by bioautography method. Jundishapur J. Microbiol.. 2013;6:1T.
- [Google Scholar]
- Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. J. Agric. Technol.. 2007;4:119-137.
- [Google Scholar]
- Nidiry, E. S. J., Ganeshan, G., & Lokesha, A. N., 2011. Antifungal activity of some extractives and constituents of Aloe vera.
- Reddy, K. R. N., Reddy, C. S., & Muralidharan, K., 2009. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol, 26 1 27-31..
- Assessment of the microbiological quality and mycotoxin contamination of Iranian red pepper spice. J. Agric. Sci. Technol.. 2012;14:1511-1521.
- [Google Scholar]
- Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J. Agric. Technol.. 2007;3:109-119.
- [Google Scholar]
- Anti-fungal activity of Aloe vera: In vitro study. SRM J. Res. Dent. Sci.. 2015;6:92.
- [Google Scholar]
- Detoxification of mycotoxins and food safety. Mycotoxins Agric. food Saf.. 1998;1:381-406.
- [Google Scholar]
- Inhibitory activity of plant extracts on aflatoxin B1 biosynthesis by Aspergillus flavus. J. Agric. Sci. Technol.. 2014;16:1123-1132.
- [Google Scholar]
- Antibacterial, Hemagglutination, and Insecticidal Activity Studies on the Solvent Extracts of the Roots of Olea ferruginea. Makara J. Sci.. 2022;26:8.
- [Google Scholar]
- Mycotoxins in feeds and their fate in animals: a review. Anim. Res.. 2002;51:81-99.
- [Google Scholar]







