Translate this page into:
Evaluation of molecular mechanisms responsible for in vivo anti-Alzheimer’s property of Euphorbia cotinifolia methanol extract
⁎Corresponding authors. rphrashad@gmail.com (Muhammad Rashad), muhammad.rashad@unich.it (Muhammad Rashad), abid.hussain.sayyid@ki.se (Abid Hussain Sayyid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Alzheimer's is considered as the most prevalent neurological disease; it is a degenerative and progressive disease that affects brain’s neurons causing memory, language and thinking loss, as well as behavioral abnormalities. This study was performed to assess the anti-Alzheimer features of Euphorbia cotinifolia methanol extract (ECM). Antioxidant activity was performed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and high-performance liquid chromatography (HPLC) was performed to identify some constituents present in ECM. Aluminum chloride-induced Alzheimer's Wistar rats were used to access the therapeutic potential of ECM. Several behavioral experiments were conducted to assess the impact of ECM treatment on motor dysfunction and cognitive abilities. Quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA) studies were conducted to explore the effect of ECM treatment of different biomarkers. Half-maximal inhibitory concentration (IC50) of ECM and standard (ascorbic acid) in DPPH assay was found to be 18.61 and 11.37 µg/mL, respectively. The HPLC confirms the presence of chlorogenic acid, gallic acid, caffeic acid, sinapic acid, quercetin, and benzoic acid in ECM. Data from in vivo behavioral studies showed a significant improvement in motor dysfunctions and cognitive abilities of animals treated with ECM. ECM treatment also elevated the acetylcholine, superoxide dismutase, catalases, glutathione peroxidase, and glutathione levels. Histopathological examination showed a reduction in neurofibrillary tangles and neuronal loss. qPCR studies showed reduced expression of interleukins (IL-1-α, IL-1-β), tumor necrosis factors (TNF-α and TNF-β) secretase in ECM-treated groups. Findings of the current study highlight the anti-Alzheimer’s potential of E. cotinifolia.
Keywords
Molecular mechanisms
Anti-Alzheimer
Euphorbia cotinifolia
Behavioral studies
TNF-α, qPCR
1 Introduction
In some countries, Alzheimer's disease (AD) is very familiar neurological disorder; it is a degenerative and progressive disease that affects brain’s neurons and manifests as memory, thinking and language loss, along with abnormal behavior. Neurons that slow down this disease use the chemical neurotransmitter acetylcholine to contact the remaining neurons. In AD, changes in enzymes such as acetylcholinesterase (AChE) as well as butyrylcholinesterase (BuChE) can be detected, although acetylcholine deficiency is significant and most significant sign (Rachakonda et al., 2004). Senile external plaques (containing β-amyloid peptides) and the intracellular neurofibrillary tangles (NFT, which is composed of bovine head protein) are two abnormalities that block the brain of AD patients. Aβ is formed through the enzymatic breakdown of the original amyloid precursor protein (APP). The proteins used to degrade APP are secretases α, β, and γ (Rachakonda et al., 2004). NFT is responsible for degeneration of synaptic or nerve cells atrophy caused by damage to synchronously connected axons. Paired helical filament (PHF) NFT is majorly formed by hyperphosphorylated tau protein (EI-Agnaf and Irvine 2002). Oxidative stress plays a significant function in the synthesis of β-amyloid plaques and intracellular neurofibrillary in AD (Bhatt et al., 2021). Moreover, various pro-inflammatory cytokines, comprising IL–1 as well as TNF–α, are upregulated in ADcontributing to the cascade synthesis of β-amyloids plaque and the intracellular neurofibrillary tangle (Wang et al., 2015).
Medicinal plants contain a large number of chemical substances that have a significant contribution to drug development, their particular chemical structure giving them properties (biological, biochemical, chemical, physical, physiological, etc.) absolutely specific to each of them (Glevitzky et al., 2019). The genus Euphorbia is used as folklore medicine in various parts of the world, most commonly in traditional Chinese-medicine. Various parts of different species of this family are used in traditional medicine to treat gastric, migraine, respiratory disorders, gonorrhea, skin, inflammation, intestinal parasites, and warts (Salehi et al., 2019). E. cotinifolia, commonly known as the Caribbean copper plant, has been scientifically proved to have molluscicidal, antiviral and antibacterial properties. The in-vitro antioxidant potential of E. cotinifolia has been discovered in several researches. The phytochemical assays confirm the occurrence of terpenoids, steroids, tannin, glycosides, and flavonoid in ethyl-acetate and methanolic extracts. Steroids, glycosides, carbohydrates were also found in chloroform and petroleum-ether extracts. The HPLC equipped photodiode-array (HPLC-DAD) detection assay confirmed the occurrence of significant concentrations of caffeic acid and phenolic compounds (Jayalakshmi et al., 2021).
Oxidative stress is considered as the main culprit of AD. As this disease is directly linked with age, one of the theories predicts that oxidative stress leads to aging at later stages. Following the age, accumulation of β-amyloid peptides in the mitochondria occurs which can itself generate ROS catalyzed by Cu2+ and Fe2+ ions. At advanced age, the balance between ROS production and antioxidants (SOD, CAT and glutathione peroxidase) enzymes disturbed which leads to oxidative damage and ultimately AD progression. In mouse models and autopsy analysis of AD patients, the elevated levels of ROS cause mitochondrial dysfunction and vice versa which in turn trigger accumulation of β-amyloid peptides hence leads to AD.
However, until now, according to our knowledge, no scientific study is available that reports the anti-Alzheimer activity of E. cotinifolia. Therefore, our study performed to evaluate the in vivo anti-Alzheimer properties of methanol extract of E. cotinifolia.
2 Material and methods
2.1 Plant collection
E. cotinifolia collection was made from two different sources: half from main lawn of the Government College University of Faisalabad, and the other half from the model town park Lahore and both were mixed to obtain better results. The plants were identified by Professor Mansoor Ahmed, chairman of the Department of Botany, Agricultural University Faisalabad. After verification voucher specimen number EC-527 was allotted and plant sample was added to the botany department's herbarium, University of Agriculture, Faisalabad.
2.2 Preparation of extract
The plant was washed to remove dirt and excess materials and dried under shade for 20 days to obtain an effective extract. Dried whole plant was subjected to grind into fine powder and weighed. The extraction was carried out using a cold immersion process. A predetermined amount (500 g) of grounded plant was put into a glass bottle, and a predetermined volume (1000 mL) of methanol (Sigma-Aldrich, USA) also added thereto. The jar was placed in an ultrasonic bath to ensure optimal extraction. After 24 h, it was filtered via Whatman filter paper (#1). The process is repeated thrice. The rotary evaporator is used to concentrate the extract at 37 °C under reduced pressure. The extract was assigned the code ECM. The semisolid extract was brownish green in color with an aromatic odor and yield obtained was 4.47% (22.35 g).
2.3 Determination of antioxidant activity by DPPH
This experiment was carried out in line with the procedure reported by Queiroz et al. (2009) considering minor alterations. The ability of ECM to scavenge the DPPH (2,2-diphenyl-1-picrylhydrazyl) stabilized free radicals demonstrates its antioxidant potential. Established concentrations of ECM (200, 400, 600, 800, 1000 µg/mL), and 100 µL from each concentration was mixed with 500 µL of 0.1 mM of DPPH (Sigma-Aldrich, USA) in the ethanol solution. Then absorbance was measured at 517 nm wavelength after 30 min of incubation at ideal room temperature. Different concentrations of ascorbic-acid were utilized as control and an equation for linear regression with a dose–response curve (R2) were plotted. The experiment was carried out thrice to obtain the average inhibition percentage and IC50 value was measured by graphical method by drawing curves of sample and standard. A low absorbance means a high free radical scavenging effect, which can be calculated by the following formula.
2.4 High-performance liquid chromatography (HPLC)
Shimadzu HPLC with an autosampler was used, that was attached with a pump (LC10AT) and an ultraviolet visible detector (SPD10AV). A column with diameter 25 cm × 4.6 mm having particle size 5 μm of shim-pack CLCODS (C-18) and 40 μL injection utilized. Mobile-phase was prepared by mixing two solvents A and B; Acetic acid and water 6:94 (solvent-A), the other 100% acetonitrile (solvent-B). Mobile phase's flow rate was fixed at 1 mL/min. The wavelength used to test the absorbance was 280 nm. Sonicator was used to degas the sample stock solution and mobile phase, filtering through a membrane with a 0.45 μm pore size (Millipore). By comparing the sample's absorption spectra and peak retention durations to a chemical library, substances were identified.
2.5 Institutional review board/Animal ethics committee (AEC) approval
Animal research was granted by the AEC of the GC University Faisalabad with an authorization code GCUF/ERC/2266, Research No. 19,866 and the IRB (Institution Review Board) No. 866.
2.6 Exploration of anti-Alzheimer activity
For the neuroprotective studies, healthy male Wistar’s rat weighing about 200–250 g recruited which were at least nine weeks old. Aluminum chloride 300 mg/kg for three weeks p.o. caused AD associated behavioral, biochemical, and molecular inadequacies in Wistar rats to produce chronic aluminum deposit and disposal in the brain’s tissues.
2.6.1 Study design
In this study, healthy Wistar’s rat (male) were included and segregated into six group. Apiece group comprised six rats as mentioned in Table 1.
Group
Treatment/Dosage
Group-I
Kept as control group; administered carboxymethylcellulose (CMC, vehicle) 1 mL/kg p.o.
Group-II
Diseased-control group; administered AlCl3 300 mg/kg p.o.
Group-III
Standard group; administered AlCl3 300 mg/kg and drug rivastigmine 3 mg/kg p.o.
Group-IV
AlCl3 300 mg/kg, and ECM 100 mg/kg administered p.o.
Group-V
AlCl3 300 mg/kg, and ECM 300 mg/kg administered p.o.
Group-VI
AlCl3 300 mg/kg, and ECM 800 mg/kg administered p.o.
The dose of ECM was chosen randomly to determine the pharmacological and toxic effect if it has any. Each group of animals was treated for 21 days, and the behavior and body weight at the beginning and end of study periods were recorded. Twenty-four hours apart from the last dose, animals were slaughtered through cervical dislocation using a mild anesthesia to remove the brains, washed them with phosphate buffered saline, stored at −20 °C for biochemical and histological evaluations. For biological evaluation, each test was performed in triplicate to minimize human error.
2.6.2 Behavioral studies
2.6.2.1 Morris water maze test
The purpose of the Morris test is to observe memory, spatial learning, and task strategies. Morris's water is carried out according to the reported method. In this test, a round plastic bucket with a diameter of 1 m and 1.5 feet depth. It is preserved at 26 °C and was filled with tap water. In the middle of the pool, a platform was positioned one inch below the water surface and exposed during the training test. Opaque water was added to the dry milk powder so that the rats could not see the platform during the experiment. The group is evenly divided into the East, West, North, and South quadrants. The rats were trained on the platform for five days in a row (4 trials per day), after which they were positioned on the edge of the pool and submerged for 90 s. Four tests were performed from different directions every day to un-reveal the central platform. Animals guided towards platform by the very first training’s day and stayed on the platform for 20 s. Time consumed to cross the underwater platform and emerge from water was noted. On the 6th day, The mouse couldn't see the platform, and a duration of up to 120 s was recorded to cross the submerged platform from four alternate directions.
2.6.2.2 Open field test
This is designed to simultaneously examine animal exploration, movement, and anxiety. It is made of plywood with dimensions of 72 × 72 cm and walls of 36 cm and is coated with white resin to make it look like a hollow square room. To observe the movement of rats in the equipment, a plexiglass wall was built. Using black lines, the compartment floor was segmented into 16 squares (18 × 18 cm). To distinguish the center point from other locations around it, an 18 × 18 cm square with a red line was created. After each animal experiment, clean the chamber with 70% isopropyl alcohol. Gently handled the rat's tail and place it in a corner of the device, and measure complete distance of movement and numbers of box crossing its four legs for 10 mins. We observed the time spent in centering, grooming, stretching, paying attention to posture, lifting, freezing, and defecation.
2.6.2.3 Passive avoidance test
The equipment is made of plexiglass with a grid on the bottom and measures 27 × 27 × 27 cm (3 mm S.S rods, 8 mm apart). The battery was placed on the floor of the grid to provide a 20 V electric current. A wood platform (dimension: 10 × 7 × 1.7 cm) was placed in the center. Starting from their tails, these animals were carefully handled and placed on a platform. On the first attempt, it took 15–20 s for the mouse to cross the platform to the grid floor. Two hours after the first test, the second test starts. During the testing phase, a mouse kept on a wooden platform, and measured its reduced latency.
2.6.2.4 Y-maze test
In behavioral neuroscience, this activity is conducted to evaluate short-term and spatial memory, and cognitive deficits in rats. The equipment used for this task consists of three wooden arms connected at 120°, and the other wooden arm is Y-shaped. With a triangular central area, these arms are 25 cm height, 35 cm length and 10 cm width. Record the animal's exploratory behavior in Y-maze apparatus for 8 mins. The rat was held on one side of one arm of the device, and the number of entrances in every arm and the frequency of triples (entered into 3 arms in continuous pattern) were decoded to calculate spontaneous changes. Only when the hind legs were completely crossed, the entry of one arm was counted. The formula for calculating spontaneous alteration is as follows:
Equation was used to determine the laterality index and examine the animal's preferred side.
2.6.2.5 Hole board test
The rat’s curiosity and anxiety behavior were studied by immersing the head in the hole-board equipment. The device is made of plexiglass with dimensions of 25 cm × 25 cm containing height 30 cm. Floor is evenly divided into 16 holes at a height of 1.5 m from the floor. The rat was gently placed on the floor of the device and observed for 8 min. Record the number of heads immersed in the hole, and the distance moved around the periphery and center of the device. The head immersion score is only performed after the animal’s two eyes have entered the hole.
2.6.2.6 Wire hanging test
This experiment's goal is to assess the neuromuscular strength of the animals. The equipment used for a given test consisted of a horizontal metal grid mounted on a wooden wall 50 cm high and 3 in. wide. Gently manipulate the animals by their tails and place them on the rack and gain support until they grasp the rack with their front and hind legs and hang them from the rack in an upright position. The animal is required to stay on the cable for up to 30 sec. The recorded suspension time ranges from 30 sec to 1 min.
2.6.2.7 Elevated plus-maze test
In the external perception behavior paradigm, this test is conducted to evaluate memory and exploration. The device is made of a wooden base with a diameter of 10 cm × 10 cm, 2 open arms with a diameter 50x10 cm, and 2 closed arms with a diameter of 50 × 10 cm and 50 × 40 × 10 cm. On first day (20th day of the investigation), the rat was carefully placed on the open arm with its back to the platform, and the latency shift (time required by animal to shift with an open arm toward closed arm was measured, since this was their inherent behavior to hide themselves from exploring. The same process was performed after 24 h (on the 21st day of the research) and transferring latency was collected to assess learning, cognition, memory deficits.
2.6.3 Estimation of biochemical parameters
Each rat was sacrificed using isoflurane as a mild anesthetic and the brain was removed by cervical dislocation. To remove blood from previously separated brain, it was washed by using ice-cold normal saline and then preserved at −20 °C. 0.1 M phosphate-buffer, pH 7.4, composed of 1 mmol EDTA (sigma-Aldrich, USA), 1 mM phenylmethylsulfonyl fluoride, 25 M sucrose and 10 mM potassium chloride utilized to prepare the brain’s homogenate in a tissue homogenizer. To obtain the supernatant, it was centrifuged at 800 rpm and 4 °C for at least 30 min. For measuring the biological activities, the Perkinelmer’s EnSight® Multimode Plate Reader (USA) was used. All analytical grade chemicals were bought from Sigma-Aldrich, USA, having high purity.
2.6.3.1 Estimation of malondialdehyde (MDA) level
MDA level is a measure of the peroxidation of lipids. In a test tube, brain’s homogenate (200 µL) was muddled with 8.1% of sodium dodecyl sulphate (200 µL), 1.5 mL of both 20% acetic acid and 0.8% TBA (thiobarbituric acid), and 4 mL of distilled water. In a water bath, heat for an hour at 90 °C, then cool under running water. The mixture was then combined with 5 mL n-butanol and 1 mL distilled water, agitated violently, centrifuged for 10 min at 4000 rpm. The absorbance measurement of the overlying butanol layer was determined at 532 nm. To plot calibration curve, different concentrations of TBA were produced, and MDA level was computed using the equation.
2.6.3.2 Estimation of catalase (CAT) activity
To evaluate the CAT activity 50 μL brain-homogenate, 50 mM phosphates buffer, pH 7.0 (1.95 mL), and 30 mM H2O2 (1 mL) were mixed. At 240 nm, the absorbance was measured. Equation used for determination of CAT activity is given below. where, E = 0.071 mmol cm−1 (hydrogen peroxide extinction coefficient), δOD = per minute absorbance shift.
2.6.3.3 Estimation of superoxide dismutase (SOD) activity
For the estimation of SOD, 1.2 mL of sodium-phosphate buffer (0.052 M, pH 8.3), 100 μL of tissue homogenate, phenazine-methosulphate (186 μm) 100 μL, nitrobluetetrazolium (300 μm) 300 μL, and 200 μL triton-X were used to make the reaction mixture. At 30 °C, it was incubated for 95 s. To stop reaction added glacial acetic acid. After adding 4 mL n-butanol, it was rapidly agitated for 10 min before centrifuging to separate n-butanol layer. The absorption of the chromogen was measured at wavelength 560 nm and compared to a known standard curve of SOD, given in unit/mL, using n-butanol as the blank.
2.6.3.4 Estimation of glutathione peroxidase (GPx) level
Test mixture contained 0.1 mL of brain’s homogenate, 0.2 mL of sodium azide, 0.2 mL ethylenediaminetetraacetic acid (EDTA), and 0.2 mL distilled H2O. Trichloroacetic-acid (TCA) was used to inhibit the chemical reaction. The supernatant was centrifuged for 10 min at 2000 rpm. Disodium hydrogen phosphate (4 mL) and 0.5 mL 5,5′-dithiobis nitro-benzoic acid (DTNB) also added to the supernatant. Absorbance calculated at 420 nm. The GPx activity was measured in μmoles of glutathione oxidized/min/mg of protein.
2.6.3.5 Estimation of reduced glutathione (GSH) level
For the estimation of GSH 1 mL of each tissue homogenate and potassium chloride was dissolved in 4 mL distillated water. In addition, 1 mL of trichloroacetic acid was mixed to the above-obtained mixture, then centrifuged at 3000 rpm for 30 min. Mixed 2 mL of the obtained supernatant with 4 mL of Tris-buffer (0.4 M) and 0.1 mL DTNB (0.001 M). Measure absorbance at 412 nm, i.e. blank and sample (except brain-homogenate). Used the given formula to determine the concentration of GSH. where A denotes absorbance, D denotes dilution, and E denotes molar extinction coefficient (C = 13,000 M−1cm−1).
2.6.3.6 Estimation of acetylcholinesterase activity
2.6 mL of 0.1 M Phosphate buffer (pH 8.0) treated with a small amount of tissue homogenate (0.4 mL) in 100 mL 2,4-dithiodinitrobenzoic acid and 20 mL acetylthiocholine iodide. In this test, 2,4-dithiobisnitrobenzoic acid reacts with thiocholine to produce a yellow color with an absorption wavelength of 412 nm. The given formula is used to determine the activity of acetylcholinesterase: where CO is the initial tissue concentration (mg/mL); while A represents change in absorb./min, and R is substrate hydrolyzed rate (in moles/minute/gram of tissue).
2.6.3.7 Observation of brain’s histopathological changes
The isolated brain was fixed in 4% paraformaldehyde at room temperature for 24 h. A microtome was used to cut the circumferential sections of the paraffin-fixed brain tissue (5 µm thick), stained by hematoxylin and eosin dye, and assessed the pathological features underneath an optical microscope.
2.6.4 Estimation of mRNA expression of AD linked genes transcripts
After 21 days of treatment, the animals were sacrificed, and the brain tissues were removed, and washed with phosphate buffer. For total mRNA extraction by the triazole method, 100 mg of tissue in 1 mL of triazole reagent (Molecular Research Center Corporation, USA) were homogenized. Total mRNA was quantified by Nanodrop-2000 (ThermoFisher Scientific, Waltham, USA) at a wavelength of 260/280 nm. The Revert Aid First Strand cDNA Synthesis Kit is used to create single-stranded cDNA (Thermo Scientific, Waltham, USA). The following settings are used for quantitative PCR thermal cycling: denaturation for 15 sec at 95 °C, hybridization for 20 sec at 60 °C, extension for 20 sec at 72 °C, then 40 cycles (denature at 95 °C for 15 sec, anneal at 60 °C for 20 sec and prolong at 72 °C for 20 sec (Biorad-T100 96-well thermal cycler, California, USA). The sequences and sizes of the reference gene primers and expected PCR products of the targeted genes are given in Table S1. (GADPH) Glyceraldehyde-3-phosphate dehydrogenase is used as an internal reference also known as housekeeping genes. Relative quantification was calculated by Realplex software using the CT method. The average CT of the three values, the measurement target, and the reference gene were used to calculate ΔCT. The difference between the sample and the control ΔCT is measured to calculate the ΔΔCT. The change in mRNA expression was measured by 2ΔΔCT.
2.6.5 Statistical analysis
The post-hoc test in Graphpad prism 9th edition was used for statistical analysis. Bonferroni post-hoc test was applied to analyze the results by one-way and two-way ANOVA (analysis of variance). Data reported as mean ± SEM and p-value < 0.05 was kept statistically significant.
3 Results
3.1 In vitro antioxidant activity of ECM
The antioxidant potential of ECM was measured by DPPH method. The percent inhibition of ECM was 82% at 1000 µg/mL, indicating an IC50 value of 18.61 ± 0.23 µg/mL when compared to the reference (ascorbic acid), which exhibited an inhibition activity of 90% at 1000 µg/mL and display the IC50 value of 11.37 ± 0.35 µg/mL (Table S2). Percent inhibition of ECM (y = 0.0339x + 49.369) and ascorbic acid (y = 0.0444x + 49.495) are depicted in Fig. 1a.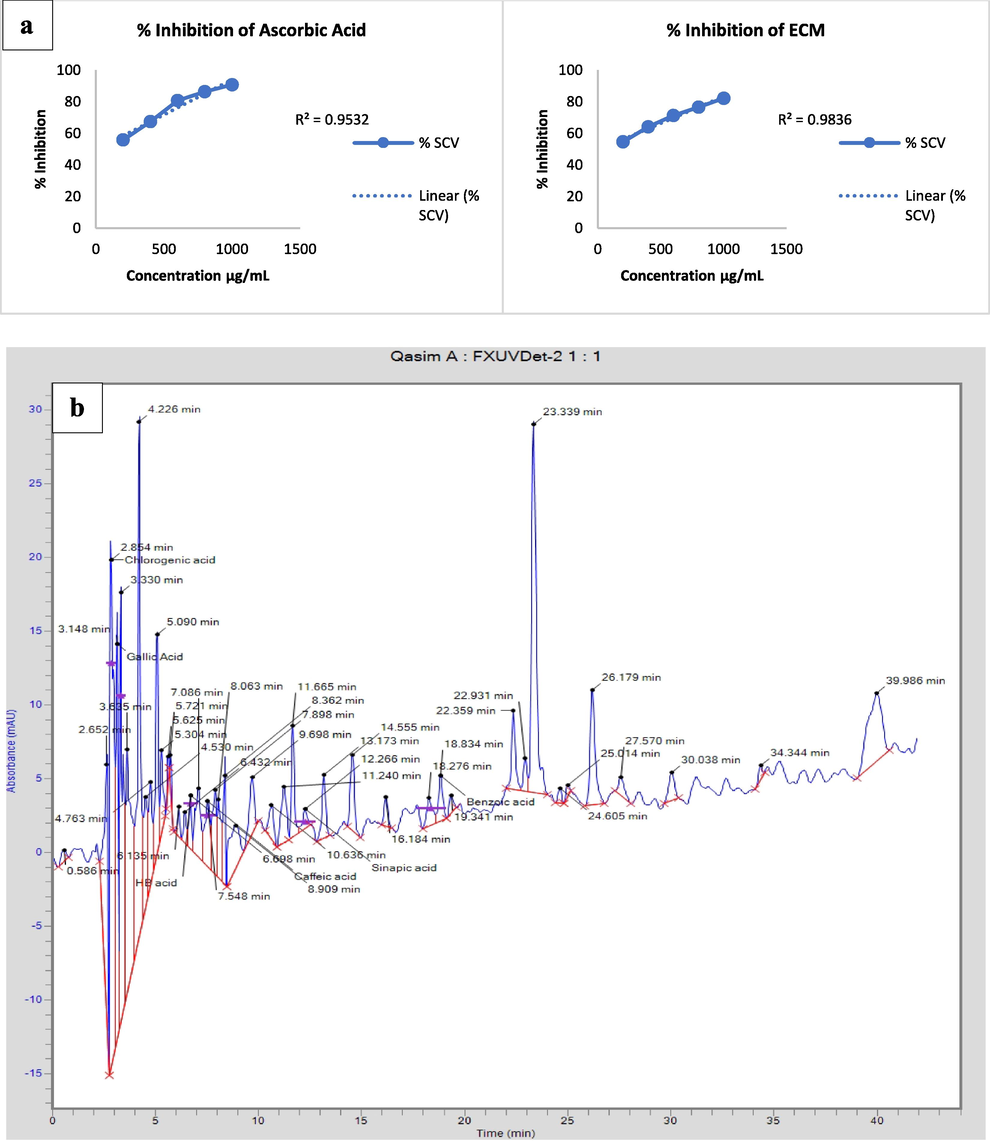
(a) Percentage-inhibition of ascorbic acid and ECM (b) HPLC chromatogram of ECM.
3.2 High-performance liquid chromatography
HPLC spectrum (Fig. 1b) of ECM revealed several important compounds present in E. cotinifolia. When HPLC spectrum was compared to compounds library acquired by the analysis of pure compounds in lab and then by comparing the sample peaks with that of pure compounds peak, many important compounds identified like chlorogenic, gallic, caffeic, sinapic, benzoic acid and quercetin, indicating the existence of phenolic and flavonoids. They all are a source of antioxidants in the food and pharmaceutical sectors.
3.3 Investigation of anti-Alzheimer’s properties
3.3.1 Behavioral analysis
In MWM test (Moris water maze), the escaping latency (seconds) of the diseased control increased significantly after training (p < 0.001) as compared to treated and the control groups. The ECM treated groups were better than the control groups in terms of escape delay. Animals in the AlCl3-induced model group reach the platform more slowly, and their contact behavior (time spent at the pool edge was an indicator of anxiety and platform seeking strategies) is more different from the ECM treatment group and the control group Table 2. Values indicated are mean (±SEM), (n = 6), ***(p < 0.001), **(p < 0.01) and *(p < 0.05) against control.
Group
Quadrant-I
Quadrant-II
Quadrant-II
Quadrant-IV
Escape latency (sec)
Control
23.11 ± 0.9
21.54 ± 1.3
21.24 ± 1.4
22.45 ± 1.3
Disease control
45.31 ± 1.1**
42.50 ± 2.1***
44.71 ± 1.3***
43.63 ± 1.6***
Standard group
22.45 ± 0.4 *
23.87 ± 0.7 *
21.90 ± 0.4 *
24.20 ± 0.4 *
100 mg/kg
39.80 ± 1.6***
38.60 ± 0.7**
40.00 ± 0.4***
37.50 ± 0.5**
300 mg/kg
35.45 ± 1.2**
32.30 ± 0.3**
24.50 ± 0.8 *
36.00 ± 0.4***
800 mg/kg
21.66 ± 0.6 *
19.62 ± 0.3 *
22.00 ± 1.1 *
24.45 ± 0.6 *
In both groups, neurobehavioral monitoring of the open field revealed that the time consumed at the peripheries was comparatively larger (p < 0.001) than the time consumed at the middle of the apparatus. In the disease control group, total travelled distance in apparatus, such as rearing, and time spent in middle were all reduced. In the disease-control model, the freezing, defecation, and stretching-postures were observed more commonly in comparison to control and ECM treated groups travelled a larger distance and passed larger lines at the chamber's edge as depicted in Table 3. Values indicated are mean (±SEM), (n = 6), ***(p < 0.001),**(p < 0.01) and *(p < 0.05) against control.
Groups
Freezing (Seconds)
Grooming/10 min
Rearing/10 min
Total No. of lines (Crossed)
Control
0.00 ± 0.0
4.00 ± 0.5
6.00 ± 0.2
29.00 ± 0.5
Disease control
89.0 ± 0.5***
0.00 ± 0.0***
0.00 ± 0.0***
8.00 ± 0.0***
Standard group
9.50 ± 0.8***
5.00 ± 0.5 *
5.30 ± 1.5 **
26.45 ± 0.6 *
100 mg/kg
45.0 ± 1.15***
3.66 ± 0.8 **
4.00 ± 1.2 *
18.00 ± 1.8***
300 mg/kg
28.00 ± 0.5***
4.55 ± 0.3 *
5.66 ± 0.3 ***
22.00 ± 0.4**
800 mg/kg
0.50 ± 0.0 *
3.00 ± 0.5 **
5.00 ± 0.4 ***
25.00 ± 0.4 *
Comparison to control, the stepdown-latency of the diseased-controls were reduced significantly (p < 0.05), while the stepdown-latency of the ECM treated groups and the standard group was greatly improved. In control, ECM treatment, and standard groups, the time spent on the platform was longer (Fig. 2a).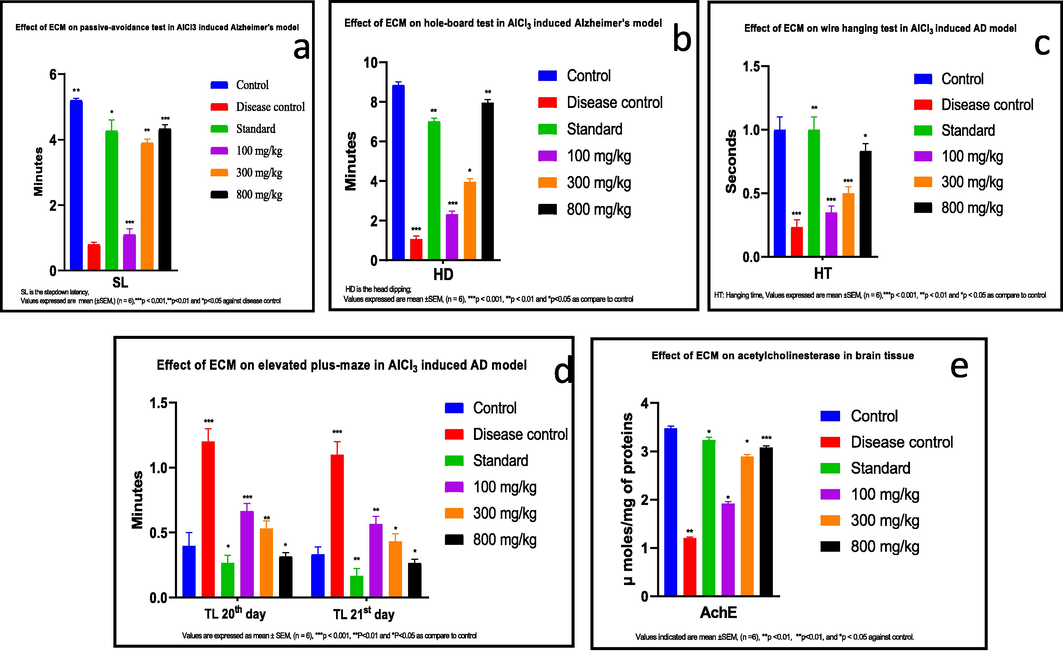
(a) ECM treatment in AlCl3 induced AD rats leads to improve the stepdown latency in passive avoidance test and memory improvement (b) ECM treatment in AlCl3-induced AD rats in holes board test improved the exploratory behavior or neophilia (c) ECM treatment showed a significant effect on wire-hanging test in AlCl3-induced AD rats. The muscle strength was improved significantly in a dose dependent manner (d) Prolongation of transfer latency was reduced by the administration of ECM in AlCl3-induced AD rats on elevated plus maze test. Reduction in transfer latency showed a significant improvement in memory (e) ECM treatment in AlCl3-induced AD rats showed a significant inhibitory effect on activity of acetylcholinesterase enzyme (μmol/mg of proteins) in brain tissues.
There are significant differences in the proportion of spontaneous changes in all subgroups in Y-maze. Comparison to control and ECM treatments, the proportion of spontaneous changes in the disease control-group was lower significantly than in the standard-group (p < 0.001) (Table 4). Values indicated are mean ± SD, (n = 3), ***(p < 0.001),**(p < 0.01), *(p < 0.05) against control.
Group
Total No of triads
% Spontaneous alteration
Total No of arm entries
Laterality-index
Control
4.00 ± 0.0
41.00 ± 0.5
12.00 ± 0.5
0.15
Disease control
0.00 ± 0.0**
0.00 ± 0.0***
3.00 ± 0.3***
−0.34
Standard group
3.66 ± 0.3 *
40.00 ± 0.5 *
11.00 ± 0.5 **
0.14
100 mg/kg
1.50 ± 0.0*
24.00 ± 0.8***
8.00 ± 0.5***
−0.3
300 mg/kg
5.00 ± 0.0 ***
37.66 ± 0.5***
6.88 ± 0.30***
0.1
800 mg/kg
3.10 ± 0.0 ***
39.50 ± 0.44 **
14.00 ± 1.7 **
0.2
Compared to control, the frequency of bending, moving, and exploring behaviors in the diseased control was lessened significantly (p < 0.05). In the standard and ECM treatment groups, reverence and exploratory behaviors were significantly improved in the following ways: 100.0 mg/kg < 300.0 mg/kg < 800.0 mg/kg (Fig. 2b).
The duration of hanging on the wire was significantly shortened (p < 0.05), and the dropdown was drastically high in diseased-control group juxtaposed to control, standard, and ECM-treated groups. The differences in duration of wire-hanging and falling-down time between the control and ECM treated-group was not significant (p > 0.05) (Fig. 2c).
The metastasis delay in control was lessen significantly (p < 0.001). In elevated-maze experiment, the diseased-control and treatment groups were tested on day 20 and day 21. In contrast to the disease control group, the metastasis latency of the ECM and standard treatment groups was significantly improved (Fig. 2d).
3.3.2 Biochemical evaluation
We discovered in the examination of biochemical markers that AlCl3 caused a significant reduction (p < 0.05) in SOD, GPx level, CAT level, and GSH level in the disease groups. ECM and the drug i.e., rivastigmine remarkably elevate the SOD level, GPx level, CAT level, and GSH level in the ECM treated group (800 mg/kg) and in a group which was kept standard. The level of Malondialdehyde (MDA) also improved remarkably (p < 0.05) in diseased-control as well as remarkably retained in ECM treated-groups (800 mg/kg) (Table 5). Values indicated are mean (±SEM), (n = 6), *** (p < 0.001), **(p < 0.01) and *(p < 0.05) against control.
Group
CAT
(IU/µL)
GSH
(µ/mg protein)
GPx
(µ/mg protein)
MDA
(TBA mg/mL)SOD
(IU/µL)
Control
0.81 ± 0.15
0.54 ± 0.5
6.83 ± 0.2
2.91 ± 0.17
0.24 ± 0.17
Disease control
0.59 ± 0.03***
0.33 ± 0.02***
4.97 ± 0.04***
4.16 ± 0.3***
0.09 ± 0.2***
Standard
0.72 ± 0.6**
0.51 ± 0.2*
6.51 ± 0.02**
2.89 ± 0.15**
0.19 ± 0.4*
100 mg/kg
0.65 ± 0.24***
0.39 ± 0.05***
5.29 ± 0.5***
3.90 ± 0.26***
0.11 ± 0.25***
300 mg/kg
0.71 ± 0.30***
0.48 ± 0.4**
6.79 ± 0.15**
3.12 ± 0.6**
0.20 ± 0.3*
800 mg/kg
0.79 ± 0.03*
0.55 ± 0.3*
6.73 ± 0.2*
2.89 ± 0.4*
0.25 ± 0.9**
We also noticed that the activity of acetylcholinesterase (AChE) was remarkably reduced (p < 0.05) in diseased control against the healthy one. AChE activity is remarkably retained in ECM-treated group (100.0 mg/kg < 300.0 mg/kg < 800.0 mg/kg) (Fig. 2e).
3.3.3 Effect of ECM on the mRNA expression of AlCl3 caused AD rats
Application of E. cotinifolia’s whole plant extract reduced the upregulation of mRNA expression of neuro-inflammatory and AD related indicators in a dose dependent pattern. In comparison to control, mRNA expressions of IL–1β, IL–1α, TNF–α and TNF–β secretase elevated significantly (p < 0.001) in disease controls, although AChE was determined to be non-significant (p > 0.05). Despite this, the AChE had significantly high value (p < 0.05) in ECM treatment group (800 mg/kg) juxtaposed to control group (Fig. 3).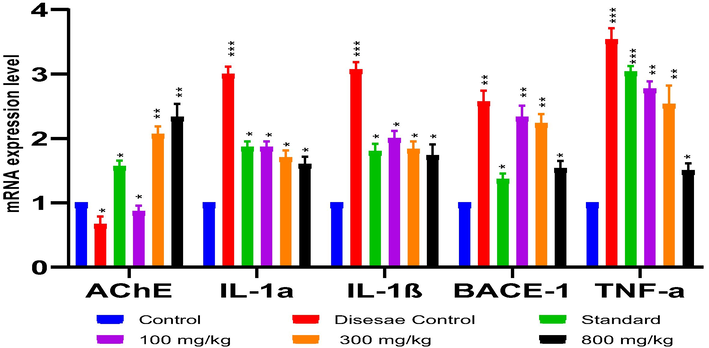
Effect of ECM (800 mg/kg) on mRNA expressions of AlCl3 inducing AD in rats showed a significant reduction in inflammatory cytokines and an inhibition of AChE (Values indicated are mean (±SEM), (n = 6), ***(p < 0.001), **(p < 0.01), *(p < 0.05) against control).
3.3.4 Effect of ECM on histopathology in AlCl3-caused AD rats
A cross-sectional examination of the control group's histopathology revealed a positive framework and healthy tissue architecture. In the disease control group, severe neurodegenerative pathological symptoms were observed, such as pigmentation, vacuolar cytoplasm, and NFT. The NFT and neuronal loss in the standard and ECM treated groups were significantly improved in a dose dependent manner (800 > 300 > 100 mg/kg (Fig. 4).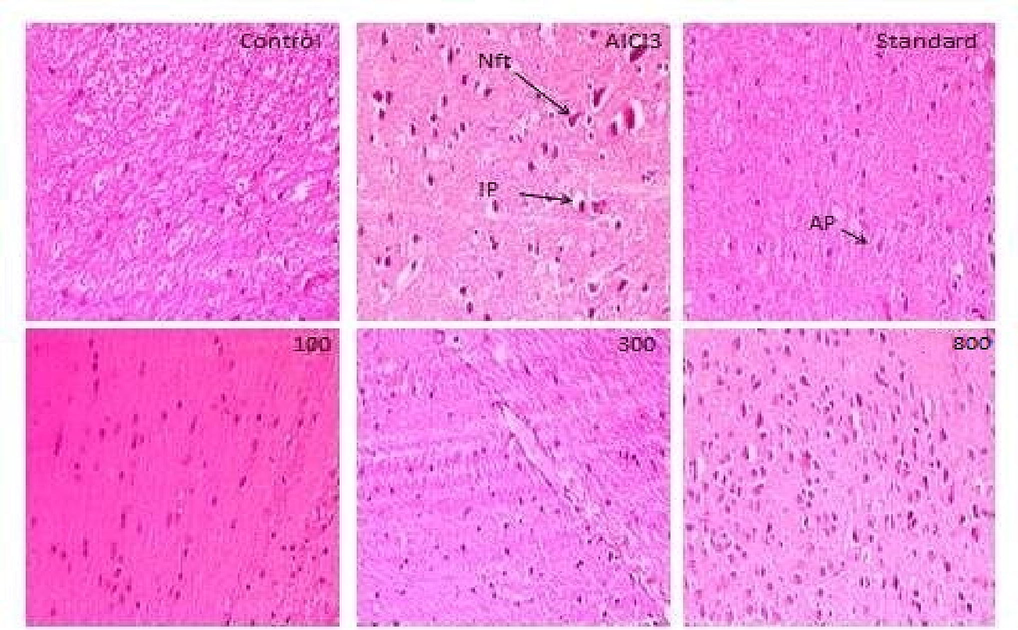
Eosin and Hematoxylin staining of brain sections. Nft: neurofibrillary tangles; AP: amyloid beta plaques; IP: intracellular plaques. Control exhibited normal control group architecture. With AlCl3, in disease control inflammation, neurofibrillary tangles, vacuolated cytoplasm and neurodegeneration were noticed. Neuronal deprivation and neurofibrillary tangles were reduced in the standard group. Remarkably ameliorated architecture was noticed in 800 mg/kg ECM treated group of transverse brain sections.
4 Discussion
AD is considered as the major root cause of dementia, and its prevalence is increasing with ages. Two pathological characteristics of neurodegenerative disease are β-amyloid plaque-formation and the other is neurofibrillary hyperphosphorylation (Behl et al., 2020). Cholinesterase inhibitors, such as tacrine, galantamine, rivastigmine, and donepezil, and many other herbal compounds (Table S3) are indicated for patients with AD and cognitive impairment. Aluminum chloride produces neurotoxicity by impairing glucose use, causing damage by peroxidation of proteins and lipids, altering phosphoinositol metabolism, altering proteins phosphorylation, and increasing free radicals release (Phaniendra et al., 2015). Evidence suggested that damage induced by stress (i.e. oxidative) performs an important part in the course of AD pathogenesis. The neurons are very sensitive to ROS as compared to other parts of human body. To overcome the oxidative damage antioxidants are the fundamental compounds as described by Moneim A. E. (2015) (Moneim 2015). In our study, E. cotinifolia possesses a variety of antioxidants, confirmed by the HPLC chromatogram. The previous studies also confirmed the occurrence of terpenoids, steroids, tannins, glycosides, and flavonoids in ethyl-acetate and methanolic extracts. Steroids, glycosides, carbohydrates were also found in chloroform and petroleum-ether extracts. The HPLC equipped photodiode-array (HPLC-DAD) detection assay confirms the occurrence of significant concentrations of caffeic acid and phenolic compounds. All these compounds are strong antioxidants in nature which may be used to combat the ROS (Arslan et al., 2020).
Behavioral analyses were carried out to estimate the ECM neuroprotective effects. The Morris test concluded that the elevated escape-latency and latency in disease control in the ECM treatment group are accordant to study by Petrasek. In open field, learning and exercise outcomes are accordant with antecedent studies. Long-term treatment with aluminum chloride reduced the rat’s short-term memory, sustained cognition, and inherent ability to change arms or neophilia in Y-maze task. The ECM treatment group revealed an improvement in performance in cognitive behavior (Kraeuter et al., 2019).
A study on the Y-maze showed that the long-term introduction of aluminum chloride caused the rats to change their habits or short-term enhancement of the arm, and the congenital and continuous cognitive abilities were destroyed (Wolf et al., 2016). Our research shows that the use of E. cotinifolia increases muscle strength in rats because of its multi-purpose pharmacological activity. Memory retention deficits were studied in the passive avoidance test. Disease-controls showed that aluminum chloride had the worst effect on memory retention, the shortest step latency, and lack of response to electrical shocks. Lakshmi's earlier study supported the findings (Lakshmi et al., 2015).
The results from holes-board setup for assessment of curiosity, neophilia, and the exploratory behaviour were consistent with the (Brown and Nemes, 2008) findings.
Acetylcholinesterases are enzymes that hydrolyze acetylcholine into acetyl CoA and choline. The aluminum induction increases acetylcholinesterases in body (Colović et al., 2013). In present study, we assessed the remarkable decrease in acetylcholinesterase levels in the ECM treatment group. The results of this experiment appear to be consistent with the Lakshmi’s recent study (Lakshmi et al., 2015).
Aluminum induction induces oxidative damage by generating radicals and damaging lipids through the mechanisms of peroxidation and protein production in the cerebral cortex as well as in hippocampus. Aluminum induces the Fenton response by altering iron homeostasis and ultimately leads to neurodegeneration. Aluminum-induced oxidative stress leads to lower the first-line enzymatic antioxidant i.e. catalase, reductase, superoxide dismutase, and glutathione peroxidase. Due to the destruction of the Golgi apparatus, decreased axonal mitochondrial production, and decreased synaptic vesicles, antioxidants are reduced, leading to the production of peroxynitrite, superoxide dismutase, carbonyl compounds, aldehyde, and other substances. Since oxidative damage and cognitive dysfunction are closely related, substances with antioxidant potential are believed to help treat neurodegenerative disorders, like AD (Phaniendra et al., 2015).
Our findings are relevant with previous findings because AlCl3 treatment in diseased-control resulted in a significant decrease of first-line antioxidant enzymes and an increase level of malondialdehyde. GSH, GPx, SOD and CAT levels in the ECM treatment group increased, while the MDA level decreased significantly due to the antioxidant properties of the plant. Low SOD levels are related to neurodegeneration and heart damage. Previously, many mental illnesses were found to be related to CAT deficiency (Kuyumcu and Aycan 2018).
GPx is an essential oxidation inhibitor enzyme found in mitochondria and cytoplasm. It inhibits oxidative stress and lipid peroxidation, and their absence is related to neurotoxicity (Ighodaro and Akinloye 2018). Antioxidant enzymes in the first line of defense play a key role in preventing ROS, mono-oxygen free radicals, and superoxide free radicals. Physical and chemical examination of E. cotinifolia showed that it contains high concentrations of flavonoids (Jayalakshmi et al., 2021) and can chelate metals with aluminum, zinc, cadmium, iron, and beryllium. Therefore, the flavonoids present in E. cotinifolia forms aluminum chelate, reducing the deposition of aluminum and increasing its elimination (Kumar and Pandey 2013).
The results showed that ECM improved the biochemical and behavioral parameters in the AD model and provided neuroprotection. Histopathological studies have shown that ECM intake reversed the pigmentation, neurofibrillary angle, neuronal loss, and neuro- inflammation caused by aluminum-chloride. We concluded that E. cotinifolia will be useful in future research on the treatment of neurodegenerative diseases due to its antioxidant capacity. Due to limited literature data available, an extensive study is required to explore the pharmacological properties of E. cotinifolia.
5 Conclusion
It is concluded that E. cotinifolia contains high amount of antioxidant compounds which was confirmed by the DPPH assay and HPLC spectrum. In vivo study demonstrates that it has significant effect against neurodegenerative disorders. Hence, E. cotinifolia can be classified as an abundant source of lead compounds that can be used for the development of neuroprotective drugs.
Acknowledgment
Researchers Supporting Project No. (RSP2023R397), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Early detection and prevention of Alzheimer’s disease: role of oxidative markers and natural antioxidants. Front Aging Neurosci.. 2020;12
- [CrossRef] [Google Scholar]
- Exploring the potential of therapeutic agents targeted towards mitigating the events associated with amyloid-β cascade in Alzheimer’s disease. Int. J. Mol. Sci.. 2020;21(20):7443.
- [Google Scholar]
- Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today. 2021;26(3):794-803.
- [CrossRef] [Google Scholar]
- The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behavioural processes. 2008;78(3):442-448.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol.. 2013;11(3):315-335.
- [CrossRef] [Google Scholar]
- Aggregation and neurotoxicity of α-synuclein and related peptides. Biochem. Soc. Trans.. 2002;30(4):559-565.
- [Google Scholar]
- Glevitzky, I., Dumitrel, G.-A., Mirel, G., et al., 2019. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Revista de Chimie -Bucharest- Original Edition-. 70, https://doi.org/10.37358/RC.19.9.7497.
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54(4):287-293.
- [CrossRef] [Google Scholar]
- Isolation and characterization of bioactive compounds from Euphorbia cotinifolia. Future J. Pharmaceut. Sci.. 2021;7(1):1-9.
- [Google Scholar]
- Isolation and characterization of bioactive compounds from Euphorbia cotinifolia. Future J. Pharmaceut. Sci.. 2021;7(1):9.
- [CrossRef] [Google Scholar]
- The Y-Maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. (Clifton, N.J.).. 2019;1916:105-111.
- [CrossRef] [Google Scholar]
- Chemistry and biological activities of flavonoids: an overview. Sci. World J.. 2013;2013:162750
- [CrossRef] [Google Scholar]
- Evaluation of oxidative stress levels and antioxidant enzyme activities in burst fractures. Med. Sci. Monit.. 2018;24:225-234.
- [CrossRef] [Google Scholar]
- Protective effect of selenium against aluminum chloride-induced Alzheimer's disease: behavioral and biochemical alterations in rats. Biol. Trace Elem. Res.. 2015;165(1):67-74.
- [CrossRef] [Google Scholar]
- Oxidant/Antioxidant imbalance and the risk of Alzheimer's disease. Curr. Alzheimer Res.. 2015;12(4):335-349.
- [CrossRef] [Google Scholar]
- Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem.. 2015;30(1):11-26.
- [CrossRef] [Google Scholar]
- Biomarkers of neurodegenerative disorders: how good are they? Cell Res.. 2004;14(5):349-358.
- [Google Scholar]
- Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules. 2019;9(8):337.
- [CrossRef] [Google Scholar]
- Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann. Transl. Med.. 2015;3(10) 136–136
- [CrossRef] [Google Scholar]
- A Comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 Mice. PLoS One. 2016;11(1):e0147733-e.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102785.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







