Translate this page into:
Evaluation of antimicrobial, anticancer, antidiabetic, antioxidant activities and silver nanoparticles synthesized from Indian Clove- Syzygium aromaticum leaf extract
⁎Corresponding authors at: Department of General Science, Ibn Sina National College for Medical Studies, Al Mahajar Street: 31906, Jeddah 21418, Saudi Arabia (S.M. Shakeel Iqubal). KLE Technological University, Hubballi, Karnataka 580031, India (Uday M. Muddapur). muddapur@kletech.ac.in (Uday M. Muddapur), shakeeliqubal@ibnsina.edu.sa (S.M. Shakeel Iqubal) shakeeliqubal@gmail.com (S.M. Shakeel Iqubal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The Indian Clove (Syzygium aromaticum) is a naturally occurring spice with significant biological properties. It is primarily found in India and Indonesia, but can also be cultivated in other coastal areas. Clove is commonly used as a natural preservative and in the production of natural medicines. One of the notable benefits of clove is its ability to help treat diseases caused by various bacteria and fungi, including E. coli, P. aeruginosa, S. aureus, Z. mobilis, and C. albicans. Additionally, clove possesses antioxidant properties that can help reduce cell damage caused by free radicals. Clove has also shown potential in inhibiting the growth of cancer cells and reducing the activity of enzymes such as amylase, glucosidase, and lipase, which can be beneficial for managing diabetes. The study demonstrated that Silver nanoparticles (AgNPs) synthesized from Indian clove leaf extract exhibited promising antimicrobial, anticancer, antidiabetic, and antioxidant activities. This suggests that these nanoparticles could have versatile applications in antimicrobial therapy, cancer treatment, diabetes management, and antioxidant supplementation. However, further research is needed to understand the underlying mechanisms of these activities and to evaluate the safety and efficacy of these nanoparticles in more complex biological systems. In summary, clove possesses diverse biological properties and has potential applications in various therapeutic areas. The synthesis of AgNPs from clove leaf extract shows promise in multiple fields, but more research is required for a deeper understanding of its mechanisms and broader applications.
Keywords
Syzygium aromaticum
Silver nanoparticles
Anticancer
Phytochemicals
Antioxidant
UV vis
GC–MS
1 Introduction
The use of medicinal plants in medicine has been growing in importance due to their various applications in the development of drugs. With the misuse of antibacterial and antifungal drugs, and the emergence of drug-resistant microbial strains, infectious diseases have become a significant public health problem (Hiwandika et al., 2021; David et al., 2019). Therefore, there is a need to explore alternatives, such as developing drugs using medicinal plants, where different parts of plants can be utilized to treat certain diseases. Medicinal plants have been found to possess anti-inflammatory, antidiarrheal, antimicrobial, antioxidant, and antidiabetic properties (Osuntogun et al., 2004; Parthasarathy et al., 2008; Shaikh et al., 2022; Awadh et al., 2022; Deshpande et al., 2023).
Clove (Syzygium aromaticum) is a dried unopened flower bud that has been used as a spice since ancient times. It belongs to the family Myrtaceae and has various synonyms, including Caryophyllus aromaticus, Eugenia caryophyllus, and Myrtus caryophyllus (Ali et al., 2012; Rabeena et al., 2016). Clove is an evergreen woody plant that grows to a height of 8–12 m. The flowers of the clove are small in size, and the cloves themselves are approximately 2.5 cm in length and 1.25 cm wide (Ortes-Rojas et al., 2014; Ali et al., 2012). Clove plants thrive in well-drained soil with sufficient moisture content. Cloves are primarily harvested in India, Indonesia, Pakistan, and Tanzania, with the states of Karnataka, Tamil Nadu, and Kerala being the main cultivation areas in India (Mishra et al., 2014).
Clove is widely known for its strong antioxidant properties, and it has been traditionally used for dental care, as a flavoring spice, and as a natural preservative. The leaves of the clove plant contain phytochemicals such as alkaloids, flavonoids, and saponins. Clove essential oil is used in traditional Chinese medicine and has been found to have antiviral, antifungal, antibacterial, antiparasitic, analgesic, antioxidant, antimutagenic, antithrombic, and anticonvulsant activities. The antioxidants in clove help reduce the propagation of free radicals in the human body and protect cell membranes (Abdel-Wahhab et al., 2005; Arora et al., 1999; Muddapur et al., 2022; Muddapur Uday et al., 2022). It has been used to combat microorganisms causing diseases like cholera, malaria, and tuberculosis (Sulieman et al., 2007). The main constituents of clove are β-caryophyllene and eugenol acetate. Several studies have reported the fungicidal activity of clove oil against Candida (Razafimamonjison et al., 2016; Yashida et al., 1987).
The aim of the current study was to extract phytochemicals from clove leaves and identify their constituents, considering the widespread use of clove in medicinal preparations. The study also aimed to assess the importance of these phytochemicals as anticancer, antimicrobial, antifibrinolytic, anthelmintic, antidiabetic, and antioxidant agents. Additionally, the researchers aimed to purify and characterize the extract using thin layer chromatography and gas chromatography-mass spectrometry (GC–MS), as well as synthesize silver nanoparticles and assess its antimicrobial properties.
2 Methods
2.1 Collection of plant material
Clove leaves (S. aromaticum L.) were collected from the University of Agricultural Science Dharwad, Karnataka, India.
2.2 Preparation of extract
In the study, after collecting the clove leaves, they were dried for approximately 15 days in a dark and cool place to ensure proper dehydration. Once the leaves were completely dried, they were ground into a fine powder. In the extraction process, 150 mL of ethyl acetate, which acts as the solvent, was added to the round-bottom flask containing the powdered leaves. The setup was then heated in a boiler, and the temperature was maintained at 77 °C, which is the boiling point of ethyl acetate by the process known as refluxing.
The extraction process was monitored until the extract obtained turned light in color, indicating that the phytochemicals had been successfully extracted from the clove leaves (Fig. 1). By evaporating the ethyl acetate, the researchers obtained the extract containing the phytochemicals from the clove leaves, which could then be further analyzed, purified, and characterized for their various biological activities (Gupta et al., 2012; Alqahtani et al., 2022).
Leaves of S. aromaticum.
2.3 Initial phytochemical tests
The ethyl acetate extract of clove leaves was subjected to a preliminary phytochemical study to verify the existence of the extract's bioactive secondary metabolites, phytochemicals using conventional methods (Shaikh, J. R. et al., 2020; Alqahtani et al., 2022).
2.3.1 Quantitative phytochemical analysis
In the current study, we performed the phytochemical analysis by following the procedure done by Mir et al. (2013), wherein, the quantification of specific phytochemicals, including alkaloids, flavonoids, and phenols, was performed using standard curves generated with atropine, quercetin, and gallic acid, respectively.
2.3.2 Antimicrobial activity of phytochemicals
In the current study, we conducted the antimicrobial activity by following the methodology of Sulieman et al. and Byakodi et al., wherein, the antimicrobial activity of plant extracts was evaluated using the agar well diffusion method (Sulieman et al., 2007; Byakodi et al., 2018).
2.3.3 The MIC and MBC of phytochemicals
The Broth dilution method was used to determine the minimal concentration of plant extract required to inhibit the activity of microbial strains. A series of plant extract concentrations ranging from 20 to 160 µg/mL were made and then mixed with pathogens in nutritional broth. The mixture was incubated at 37 °C for 24 h, and the turbidity at 600 nm was evaluated using a UV visible spectrophotometer. Subsequently, it was inoculated into a nutrient agar plate to assess its growth and ascertain the minimal bactericidal concentration. An alternative approach, known as the ELISA method, can also be employed to ascertain the minimum inhibitory concentration (MIC). The growth of strains was assessed using an ELISA reader in a 96 well plate (Hemalatha et al., 2016, Sulieman et al., 2007).
2.4 Purification and characterization of phytochemicals
Thin layer chromatography (TLC) is a method used to separate and identify phytochemicals by their capacity to interact with either the stationary phase (silica) or the mobile phase (solvent). Silica plates were prepared and activated. A mark was created 1 cm from the bottom. Approximately 10 µl of sample was inserted at this point. The setup was then placed vertically in a developing chamber containing solvent (Rastogi et al., 2008; Fathoni, et al., 2017).
The UV spectrum analysis was conducted throughout the wavelength range of 200–600 nm. The peaks observed at specific wavelengths were compared to a standard in order to identify the phytochemicals present in the extract.
The chemical contents found in the extract were determined by GC–MS tests, which were conducted by Cytxon bio solution Pvt. Ltd. (Hema et al., 2010).
2.5 Application of phytochemicals as therapeutics
2.5.1 Anticancer activity of phytochemicals
The A549 cells were trypsinized and centrifuged at 300 rpm before being added to a 96-well microplate reader. The cell number was adjusted accordingly. The plate was then placed in a CO2 incubator with 5 % CO2 supply. Afterward, the medium was aspirated and 200 μl of medium containing 10 % MTT reagent was added to each well, resulting in a final concentration of 0.5 mg/ml. The plate was incubated for 3 h in the CO2 incubator with 5 % CO2. The medium was fully extracted. Finally, the absorbance was measured at 570 nm and 630 nm. The results were analyzed by calculating the IC50 value, which represents the concentration at which cell growth is inhibited by 50 % (Dwivedi, V et al., 2011).
2.5.2 Anthelmintic activity of phytochemicals
Eisenia fetida worms (earthworms) were used for assessing anthelminthic activity. Live and recently collected earthworms were gathered and approximately 2–3 worms of similar size were placed in sterile petri dishes. Different concentrations of plant extract (ranging from 100 to 1000 µg/mL) were added to the respective petri dishes. The time it took for the earthworms to die was recorded and compared to a control group that did not receive any plant extract.
2.5.3 Antidiabetic activity of phytochemicals
The invitro alpha amylase inhibitory assay was conducted using the standard DNS assay method. A volume of 1 mL of plant extract was used, and the absorbance was measured at 540 nm. Similarly, the invitro alpha glucosidase inhibitory assay was performed with absorbance measured at 405 nm. Lastly, the invitro alpha lipase inhibitory assay involved the addition of 0.5 mL of lipase and p-nitrophenyl palmitate, with absorbance measured at 405 nm.
2.5.4 Antioxidant activity of phytochemicals
Antioxidant activity was assessed by invitro DPPH inhibitory assay, 1 mL of DPPH was added to 1 mL of plant extract, further the mixture was kept at dark room for 30 mins and absorbance is taken at 635 nm (Da silva et al., 2018).
2.6 Nanoparticle synthesis
The synthesis of AgNPs involved the addition of 2 g of powdered clove leaves to 20 mL of distilled water, followed by filtration to obtain the crude extract, also known as the aqueous extract. Next, introduce 20 mL of the precursor solution (containing silver nitrate), subject it to a temperature of 80°C for a duration of 65 min, and maintain continuous stirring during the process. Subsequently, let the solution to remain in darkness for a duration of 24 h, and subsequently analyze any alterations in coloration, as this will serve as an indication of the existence of nanoparticles through the utilization of plant extract. To confirm the existence of nanoparticles, additional analysis of the UV spectrum was conducted by examining the peaks. (Venugopal et al., 2017, Ajitha et al., 2019; Shaikh et al., 2022; Alqahtani et al., 2022).
2.7 Statistical analysis
The data represents the average of three replicates along with the standard deviation (SD). The results underwent one-way ANOVA, and the mean comparisons were conducted using Tukey's post-hoc test with SPSS version 20.0 (Statistical Package for the Social Sciences, Inc., Chicago, IL, United States). Statistical significance was determined for differences between means at a p-value of less than 0.05.
3 Results and discussion
3.1 Phytochemical screening and analysis of alkaloids, flavonoids and phenols from S. aromaticum leaves extract
Table S1 (supplementary file) indicates the presence of alkaloids, flavonoids, saponins, glycosides, anthraquinones, coumarins, diterpenes, resins and oils. The presence of the phytochemicals in the plant extract indicates its potential therapeutic value and it use in medicine and as nutraceuticals.
3.2 Anti-bacterial and anti-fungal activity of S. aromaticum leaf extract
Table S2, Fig. 2 shows the zone of inhibition by plant extract on various bacterial and fungal strains. For E. coli, the extract exhibited a zone of inhibition (ZOI) of 19 mm, while the standard antibiotic (Streptomycin) had a slightly larger zone of 22 mm. When the plant extract was combined with the antibiotic, the zone of inhibition remained the same as the antibiotic alone (22 mm). In the case of B. subtilis, the extract showed a ZOI of 18 mm, which was slightly smaller than the standard antibiotic (Streptomycin) with a zone of 22 mm. However, when the plant extract was combined with the antibiotic, the ZOI increased to 23 mm, exceeding the antibiotic alone. P. aeruginosa and S. aureus both exhibited a ZOI of 13 mm. The standard antibiotics showed larger zones of inhibition, both measuring 23 mm. In case of Z. mobilis, the extract demonstrated a ZOI of 17 mm, whereas the standard antibiotic (Cefixime) resulted in a ZOI of 20 mm. When the plant extract was combined with the antibiotic, the ZOI remained the same as the antibiotic alone (20 mm). B. nakamurai exhibited a zone of inhibition of 14 mm when treated with the extract, while the standard antibiotic (Cefixime) had a slightly smaller zone of 13 mm. When the plant extract was combined with the antibiotic, the ZOI remained the same as the antibiotic alone (13 mm). In summary, the results indicate that the S. aromaticum leaf extract possesses antibacterial and antifungal properties, as evidenced by the zones of inhibition observed against various pathogens. While the effectiveness of the plant extract varied across different pathogens, it generally demonstrated good antibacterial activity. The combination of the plant extract with standard antibiotics did not significantly enhance the inhibitory effects compared to the antibiotics alone (Fig. S1).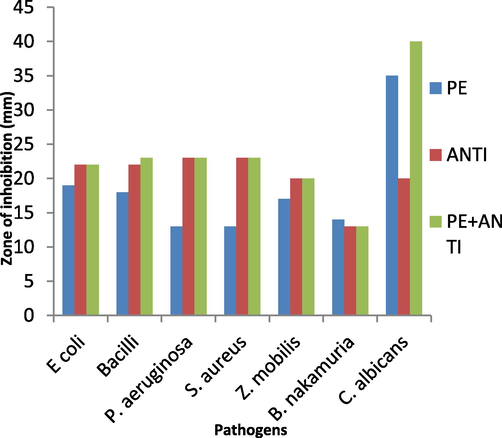
Zone of inhibition against bacteria and fungi by S. aromaticum leaves extract. PE = Plant Extract; ANTI = Standard Antibiotic.
Fig. 3 shows 339 µg/ mL of phenols, 376.5 µg/ mL of flavonoids and 213 µg/ mL of alkaloids in the plant extract.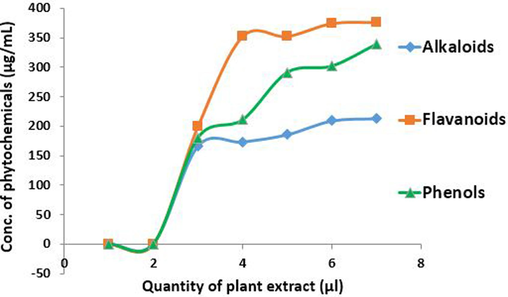
Quantitative analysis of S. aromaticum leaves extract.
3.3 Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) of S. aromaticum leaves extract
Plant extract inhibits E coli, B. subtilis, P. aeruginosa, S. aureus, Z. mobilis, B. nakamuria, C. albicans activity by 50 % when 90, 85, 64, 120, 80, 124 and 83 µg/mL of plant extract is added to each of strains. (Figs. 4a, 4b, 4c) (Table S3).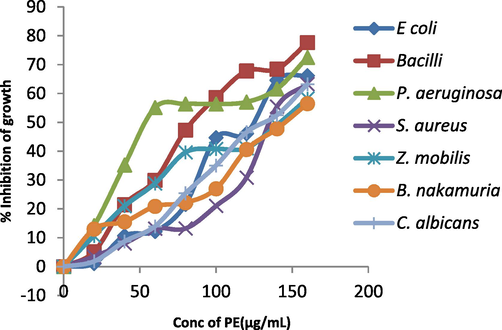
Percentage (% ) inhibition of growth caused by S. aromaticum leaf extract against pathogenic microorganisms.
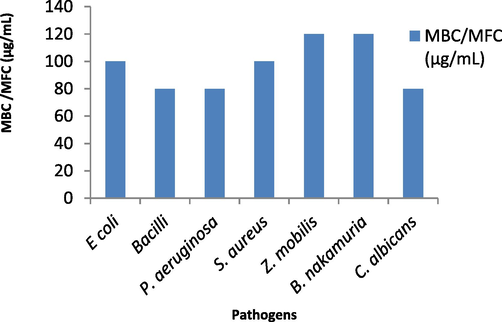
MBC and MFC of S. aromaticum leaf extract against pathogenic microorganisms.
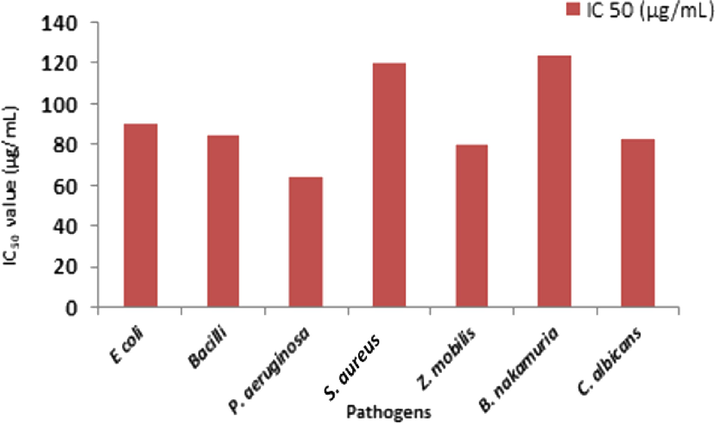
IC50 value of S. aromaticum leaf extract against pathogenic microorganisms.
3.4 Purification of plant extract by thin layer chromatography (TLC)
TLC of S. aromaticum plant extract shows 8 distinct bands (Fig S2) that indicates the presence of 8 compounds in the extract, Table S4 shows the distance of bands and Rf value of each band.
3.5 UV spectrum analysis of plant extract
Peak at 250 nm, 270 nm, 335 nm, 295 nm indicates the presence of flavonoids, phenols, glycosides and anthraquinones (Fig. 5a), the peaks were compared with standard to conclude about the type of compound present at particular wavelength (Table S5).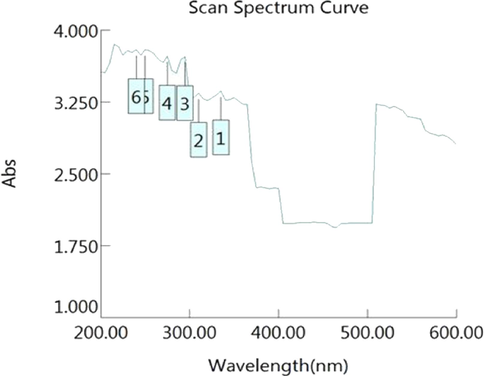
UV spectrum of S. aromaticum leaves extract.
3.6 GC–MS analysis of S. aromaticum leaves extract
GC–MS studies indicated the presence of chemical constituents such as beta-caryophyllene, germacrene-D, neophytadiene, phytol and squalene that shows significant role in preparation of medicine for curing various diseases, total of 10 volatile compounds were observed (Table S6), (Fig. 5b.).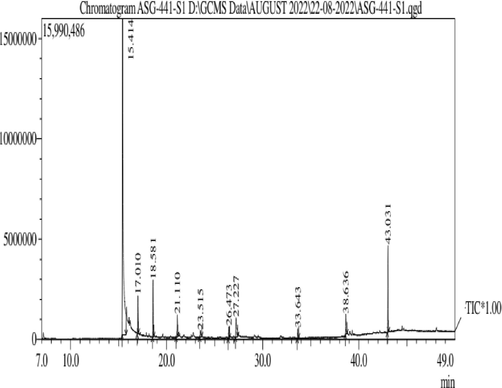
Chromatogram of S. aromaticum leaves extract.
3.7 Anticancer activity (MTT assay), in vitro antidiabetic, antioxidant, anthelmintic activity of S. aromaticum leaves extract
S. aromaticum leaves extract shows 31 % reduction in cell viability of A549 lung cancer cell lines when 100 µg/mL of plant extract is added in MTT assay (Fig. 6a). 96.83 %, 83.75 %, 63.46 % reduction in glucosidase, lipase and amylase activity respectively was observed when 100 µg/mL of plant extract was added in assay (Fig. 6b). Fig. 6c, indicates 62.48 % reduction in DPPH activity by 100 µg/mL plant extract. When 1000 µg/mL of plant extract was added to earthworms, we observed the death of worms in 5 mins following the treatment (Fig. 6d). Polyphenols, tannins, flavonoids, and saponins are some of the phytochemicals found in plants with anthelmintic properties (Ndjonka et al., 2014, Samje et al., 2014) that may work together to eradicate worms.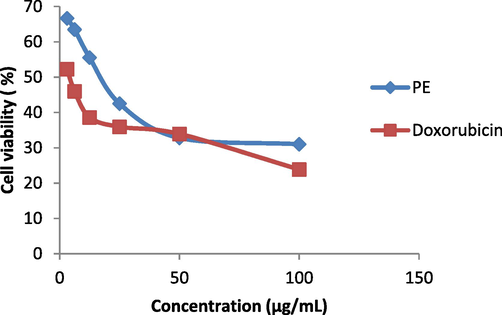
Percentage (%) cell viability (A549 cell lines) against S. aromaticum leaves extract. PE= Plant extract
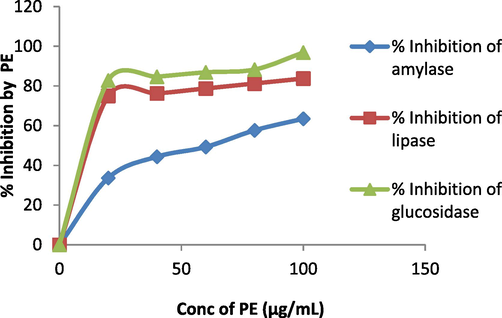
Anti-diabetic activity of S. aromaticum leaves extract. PE= Plant extract.
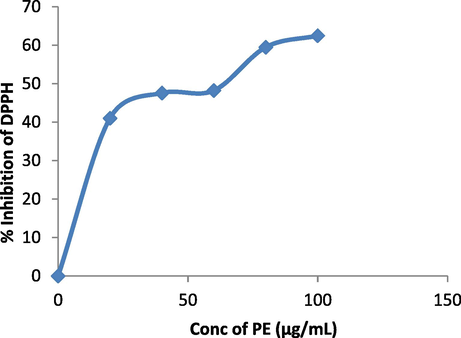
Antioxidant activity of S. aromaticum leaves extract.
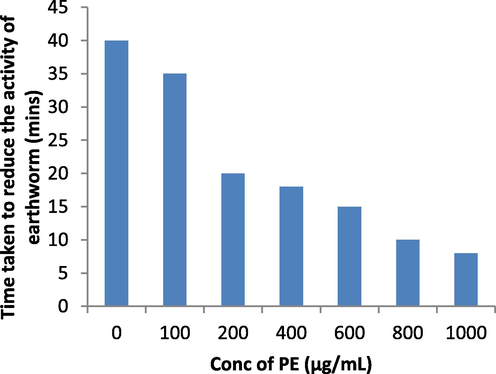
Anthelmintic activity of S. aromaticum leaves extract against Eisenia fetida worms.
3.8 Synthesis of AgNPs from S. aromaticum leaves extract
Silver nitrate was added to plant extract. Silver nitrate, being colorless in nature, reacts with plant extract and gives brownish yellow color solution which indicates the synthesis of AgNPs.
3.9 UV spectrum of silver nanoparticles from S. aromaticum leaves extract
The spectrum of AgNPs shows peak at 423 nm that indicates the presence of silver nanoparticles in plant extract when compared to the standard (only with silver nitrate) (Fig. 7).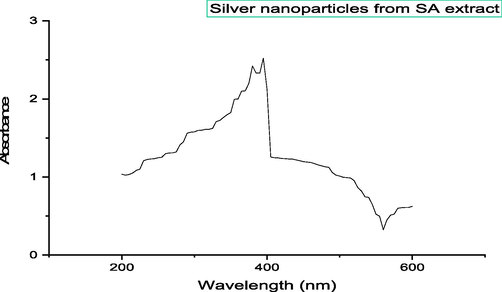
UV spectrum of silver nanoparticles synthesized from S. aromaticum leaves extract.
3.10 Antimicrobial activity of AgNPs from S. aromaticum leaves extract
AgNPs at 50 mg/mL were tested for antimicrobial activity which showed 25 mm and 23 mm zone of inhibition against P. aeruginosa and B. subtilis, respectively. Zone of inhibition by only plant extract was low compared to AgNPs i.e., 13 mm and 18 mm zone of inhibition against P. aeruginosa and B. subtilis, respectively shown in Fig. 8.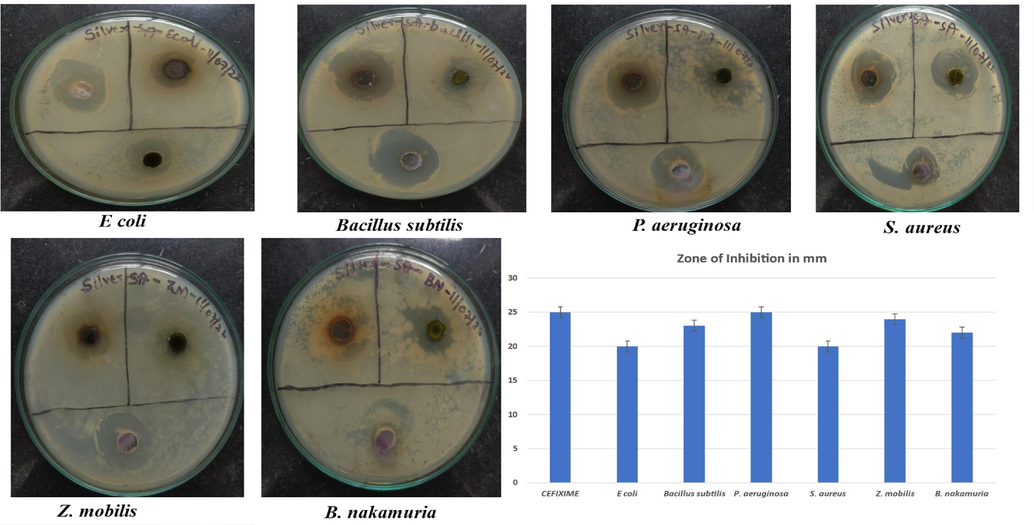
Antimicrobial activity of AgNPs from S. aromaticum leaves extract against pathogenic microorganisms.
According to the study conducted by Wankhede et al. in 2015, they found that S. aromaticum contains alkaloids, flavonoids, saponins, and glycosides (Wankhede et al., 2015). They also tested the ethanol extract of this plant against S. aureus and observed a zone of inhibition of 13 mm. In our study, we also observed a 13 mm zone of inhibition using the ethyl acetate extract. The study conducted by Pundir et al found that a concentration of 10 mg/ml of ethanolic clove extract was effective in inhibiting the growth of E. coli, while a concentration of 5 mg/mL was effective against S. aureus. This suggests that the addition of clove extract can help reduce the presence of bacteria that cause food-borne diseases (Pundir et al., 2010). In the current study, we found that a concentration of 0.1 mg/ml of ethyl acetate extract was sufficient to inhibit the growth of both E. coli and S. aureus. This indicates that the ethyl acetate extract has a lower minimum inhibitory concentration (MIC) compared to other extracts. Soliman and Walid conducted a study using gas chromatography-mass spectrometry (GC–MS) to analyze the ethanolic crude extract of clove. Their findings revealed the presence of various chemical compounds, including Phenol, 2-methoxy-4-(2 propenyl)-acetate, trans-Caryophyllene, Dimethyl Sulfoxide, and 18 others (Soliman et al., 2019). In our study, we also identified 10 constituents in the extract, one of which is Phenol, 2-methoxy-4-(2 propenyl)-acetate.
In the study conducted by Linn et al. in 2015, they investigated the thin-layer chromatography (TLC) of methanolic and chloroform extracts of clove. They observed five bands. In our study, we observed eight bands with distinct properties. Additionally, we measured the quantity of flavonoids and phenols in the extracts. Linn et al. found 17.91 µg/ mL of flavonoids and 66.92 µg/ mL of phenols, while we observed 376.5 µg/ mL of flavonoids and 339 µg/ml of phenols (Linn et al., 2015). The research reported on the inhibitory activity of alpha amylase by ethyl acetate extract. It reported that the addition of 1000 µg/ mL of plant extract resulted in 20 % inhibition. In the present study, we noticed a higher inhibition rate of 66.46 % for alpha amylase activity.
Jimoh et al., 2017 conducted a study on antioxidant activity using the DPPH scavenging assay. They used methanolic and distilled water extracts, the methanolic extract, resulted in a 90 % inhibition of DPPH activity (Jimoh et al., 2017). In comparison, our study found that adding 1 mg/ mL of the ethyl acetate plant extract resulted in a 62.48 % inhibition. Venugopal and colleagues conducted a study on the suppression of A549 lung cancer using both crude plant extract and AgNPs produced. They found that at a concentration of 100 µg/ mL of the plant extract, there was a 40 % cell viability (Venugopal et al., 2017). In the present study, we observed a cell viability of 31.26 %. When comparing the results in our situation, specifically when considering the ethyl acetate extract, there is a significant decrease in cell viability as determined by the MTT experiment.
According to Venugopal et al., 2017, they analyzed the UV-spectrum of AgNPs and found a peak at 540 nm. However, in our investigation, we observed a peak between 400 and 410 nm (Venugopal et al., 2017). Jayashree et al., reported that AgNPs exhibited antibacterial efficacy at a concentration of 20 mg/mL, resulting in a zone of inhibition measuring 17 mm against both E. coli and B. subtilis (Jayashree et al., 2021). In the present study, the addition of 50 mg/mL of AgNPs resulted in a zone of inhibition of 20 mm for E. coli and 23 mm for B. subtilis.
The primary compound found in S. aromaticum is phenol, 2-methoxy-4-(2 propenyl) -acetate. Beta-caryophyllene contributes to the antimicrobial and antioxidant properties. Phytol is a component of chlorophyll and aids in the synthesis of vitamin E and vitamin K1. Squalene assists in lowering cholesterol levels (Monica et al., 2015).
The activity of AgNPs can be attributed to both the silver (Ag) component and the nanoparticle (NP) form. Silver nanoparticles possess unique physical and chemical properties that make them effective in various applications, including antimicrobial, anticancer, antidiabetic, and antioxidant activities. The high surface area-to-volume ratio of nanoparticles allows for increased interactions with biological systems, which can enhance their activity compared to bulk silver. The small size of nanoparticles enables them to penetrate cells and tissues more efficiently, facilitating their biological effects(Muddapur et al., 2023).
The silver component of AgNPs contributes to their antimicrobial activity as well. Silver ions released from the nanoparticles can interact with microbial cells, disrupting cell membranes and inhibiting their growth. This mechanism of action makes AgNPs effective against a broad spectrum of microorganisms, including bacteria, fungi, and viruses.
AgNPs also show promise in managing diabetes due to their antidiabetic activity. They can enhance insulin sensitivity, improve glucose uptake by cells, and protect pancreatic beta cells. These effects contribute to better glycemic control and may have therapeutic implications for diabetes management.
Furthermore, AgNPs possess antioxidant properties, which can help neutralize harmful free radicals in the body. Free radicals are known to contribute to oxidative stress and various diseases. The antioxidant activity of AgNPs can help reduce oxidative damage and promote overall cellular health.
4 Conclusions
The findings indicated that the leaf extract of S. aromaticum contains alkaloids, flavonoids, phenols, and other phytochemicals. This extract demonstrated promising antimicrobial properties against a range of harmful bacteria and fungi. The GC–MS analysis revealed the presence of significant components such as phytol and Phenol,2-methoxy-4-(2 propenyl) -acetate. Furthermore, peaks observed in the UV spectrum indicated the presence of important phytochemicals. Therapeutic applications encompass the ability to combat cancer in A549 cell lines, resulting in a 31 % reduction in cell viability. Additionally, these applications include the capacity to act as an antioxidant. Furthermore, it exhibits a remarkable 96.83 % inhibition of glucosidase. Lastly, it demonstrates anthelmintic activity by reducing the earthworm's activity. Another significant application involves the synthesis of AgNPs. The color of the AgNPs was a brownish yellow, and the UV spectra showed a peak at 410 nm. The AgNPs showed potent antimicrobial activity against several foodborne pathogens. This study suggests that the plant extract from S. aromaticum contains phytochemicals with diverse chemical elements that have a substantial therapeutic role as biological agents. Overall, these findings contribute to the existing knowledge in the field by highlighting the multifaceted potential of AgNPs synthesized from Indian clove leaf extract. The study not only expands our understanding of the biological activities of AgNPs but also underscores the significance of utilizing green synthesis methods and natural sources for nanoparticle production. The potential implications for antimicrobial therapy, cancer treatment, diabetes management, and antioxidant supplementation pave the way for further research to explore the mechanisms of action, optimize synthesis protocols, and investigate potential clinical applications of these AgNPs.
5 Future implications
The current study has significant future implications, necessitating further research in several key areas. Understanding the mechanisms of action behind the antimicrobial, anticancer, antidiabetic, and antioxidant activities of AgNPs is crucial for optimizing their therapeutic potential. Additionally, evaluating the toxicity and safety profiles of AgNPs is essential to ensure their responsible use. Exploring synergistic effects and combination therapies with existing agents can enhance treatment outcomes and reduce resistance development. Translating the findings to in vivo models and conducting clinical trials will validate the efficacy and safety of AgNPs in human healthcare. Furthermore, research on formulation and delivery systems can optimize the bioavailability and targeted delivery of AgNPs.
Acknowledgements
We thank Prof. Tejraj M. Aminabhavi, Prof. N. Y Ayachit, and the RPC cell of KLE Technological University for their research support. The authors are thankful to the Deanship of Scientific Research at Najran University, Saudi Arabia for funding this work under the General Research Funding Program grant code (NU/DRP/MRC/12/23).
Funding
The authors are thankful to the Deanship of Scientific Research at Najran University, Saudi Arabia for funding this work under the General Research Funding Program grant code (NU/DRP/MRC/12/23).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
- Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J. Appl. Toxicol.. 2005;25:218-223.
- [CrossRef] [Google Scholar]
- Biomimetic synthesis of silver nanoparticles using Syzygium aromaticum (clove) extract: catalytic and antimicrobial effects: synthesis of silver nanoparticles: catalytic and antimicrobial effects. Appl. Organomet. Chem.. 2019;33:e4867.
- [Google Scholar]
- Ali, M., 2012. CBS publishers and distributors. 4596/1 11 Darya Ganj, New Delhi-110002 433–435.
- In vitro antibacterial activity of green synthesized silver nanoparticles using Mangifera indica aqueous leaf extract against multidrug-resistant pathogens. Antibiotics (basel). 2022;11:1503.
- [CrossRef] [Google Scholar]
- In vitro antibacterial activity of green synthesized silver nanoparticles using Azadirachta indica aqueous leaf extract against MDR pathogens. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Antimicrobial activity of spices. Int. J. Antimicrob. Agents. 1999;12:257-262.
- [CrossRef] [Google Scholar]
- Sustainable synthesis and characterization of zinc oxide nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals. 2022;12
- [Google Scholar]
- Phytoconstituents profiling and evaluation of antimicrobial and antioxidant attributes of methanolic extract of Centella asiatica. Res. J. Pharm. Biol. Chem. Sci.. 2018;9:493-500.
- [Google Scholar]
- Eugenol derivatives: synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J.. 2018;12
- [CrossRef] [Google Scholar]
- Antimicrobial properties of chlorocardium rodiei on Pseudomonas aeruginosa, bacillus spp. and Candida albicans. Nusant. Biosci.. 2019;11
- [CrossRef] [Google Scholar]
- Exploring the potential of phytocompounds for targeting epigenetic mechanisms in rheumatoid arthritis: an in silico study using similarity indexing. Molecules. 2023;28
- [CrossRef] [Google Scholar]
- Comparative anticancer potential of clove (Syzygium aromaticum) -an indian spice-against cancer cell lines of various anatomical origin. Asian Pacific J. Cancer Prevention. 2011;2:1989-1993.
- [Google Scholar]
- Identification of nonvolatile compounds in clove (Syzygium aromaticum) In: From Manado, in: AIP Conference Proceedings. Author(s). 2017.
- [Google Scholar]
- Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J. Food Sci. Technol.. 2016;53:1189-1198.
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activity of clove extract (Syzygium aromaticum): review. Eu Herb Ina. 2021;2:93-103.
- [CrossRef] [Google Scholar]
- Phytochemical screening and antimicrobial evaluation of Syzygium aromaticum extract and essential oil. Int. J. Curr. Microbiol. Appl. Sci.. 2017;6:4557-4567.
- [CrossRef] [Google Scholar]
- Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharm.. 2013;2:1-5.
- [Google Scholar]
- Antimicrobial activity of Syzygium aromaticum L. (clove) Int. Res. J. Biol. Sci.. 2014;3:22-25.
- [Google Scholar]
- GCMS analysis and identifications of chemical constituents of Syzygium aromaticum, brassica compestris and cow ghee. J. Chem. Pharm. Res.. 2015;7:568-572.
- [Google Scholar]
- Plant-based synthesis of gold nanoparticles and theranostic applications: a review. Molecules. 2022;2
- [CrossRef] [Google Scholar]
- Evaluation of green synthesized gold nanoparticles from abrus precatorius seeds for their antibacterial, anti-inflammatory, anti-proliferative and antidiabetic properties. Latin Am. J. Pharm.. 2022;41:17-45.
- [Google Scholar]
- Phytochemical screening of Bixa Orellana and preliminary antidiabetic, antibacterial, antifibrinolytic, anthelmintic, antioxidant, and cytotoxic activity against lung cancer (A549) cell lines. J. King Saud Univ. Sci.. 2023;35:102683
- [CrossRef] [Google Scholar]
- Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J. Helminthol.. 2014;88:481-488.
- [CrossRef] [Google Scholar]
- Clove (Syzygium aromaticum): a precious spice. Asian Pacific J. Tropical Biomedical. 2014;4:90-96.
- [Google Scholar]
- The effect of seasoning salts and local condiments on mineral availability from two nigerian vegetables. Pakistan Nutrition. 2004;3:146-153.
- [Google Scholar]
- Chemistry of spices. Spi, Pondicherry, India: Biddles Ltd, King’s Lynn; 2008.
- Antimicrobial activity of ethanolic extracts of Syzygium aromaticum and Allium sativum against food associated bacteria and fungi. Ethnobotanical Leaflets. 2010
- [Google Scholar]
- ‘Chemical composition and anti-bacterial effects of clove (Syzygium aromaticum) flowers. Int. J. Curr. Microbiol. Appl.. 2016;5:483-489.
- [Google Scholar]
- High-performance thin-layer chromatography densitometric method for the simultaneous determination of three phenolic acids in Syzygium aromaticum (L.) merr. & Perry. J. AOAC Int.. 2008;91:1169-1173.
- [CrossRef] [Google Scholar]
- In vitro anti-onchocerca ochengi activities of extracts and chromatographic fractions of craterispermum laurinum and Morinda lucida. BMC Complement. Altern. Med.. 2014;14:325.
- [CrossRef] [Google Scholar]
- Characterization of bioactive compounds from Acacia concinna and Citrus Limon, silver nanoparticles’ production by a. concinna extract, and their biological properties. Molecules. 2022;27:2715.
- [CrossRef] [Google Scholar]
- Nutritive value of clove (Syzygium aromaticum) detection of antimicrobial effect of its bud oil. Res. J. Microbiol. 2007;2:266-271.
- [Google Scholar]
- Synthesis of silver nanoparticles (ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B. 2017;167:282-289.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and antimicrobial activity of the indian clove Syzygium aromaticum L. merr. and perr. Int Res J Sci Eng. 2015;3:166-172.
- [Google Scholar]
- Antifungal activity of ajoene derived from garlic. Appl. Environ. Microbiol.. 1987;53:615-617.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103142.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







