Translate this page into:
Evaluating the fate of agrochemical through adsorption and desorption studies of chlorfluazuron in selected agricultural soils
⁎Corresponding author. chemist.phd33@yahoo.com (Irum Shaheen), irumshaheen112@gamil.com (Irum Shaheen),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Around two millennia ago agrochemicals were introduced to enhance crop quality and quantity. Later on these pesticides were found to cause undesired environmental effects mainly contamination of water bodies and soil. Therefore it became necessary to study and understand the environmental behavior of pesticides. In the present study adsorption and desorption of benzoylphenylurea insecticide, Chlorfluazuron was investigated for 4 different types of soils (clay, Sandy clay loam, Sandy loam and Sandy clay). These 4 types of soils were collected from 10 different agricultural areas of Pakistan. The physiochemical properties of selected soil samples were analyzed according to standard test method (STM), and then their sorption study was investigated through batch equilibrium method involving UV–Visible spectrophotometer and HPLC techniques. The sorption data was fitted well into the Freundlich and linear equation. The Freundlich adsorption coefficients (Kf ads) ranged from 3.9 to 9.05 µg ml−1 and distribution adsorption coefficients (Kd ads) ranged from 4.7 to 12.0 µg ml−1 suggesting that chlorfluazuron was weakly adsorbed on the tested soils. The Freundlich desorption coefficients (Kf des) varied from 1.23 to 8.9 µg ml−1, and distribution desorption coefficients (Kd des) ranged from 4.09 to 9.2 µg ml−1 indicating that chlorfluazuron tend to be leached from soils thus there is significant potential for surface and groundwater contamination of tested soil samples with chlorfluazuron. These results were further confirmed by leachability/mobility index KOM, which classified chlorfluazuron in highly mobile group of pesticides.
Keywords
Agrochemical
Chlorfluazuron
Linear isothermal model
Freundlich isothermal model
Soil physicochemical properties
1 Introduction
Chlorfluazuron (CF) is one of the most important compounds in the benzoylphenylurea IGR insecticides (Otsuka, 2013). This insecticide was discovered and developed by ISK and was launched in the late 1980s (Ghimire and Woodward, 2013). Since then, chlorfluazuron has been internationally used under the trademark of Atabron for primarily controlling Lepidoptera on cotton, bean, vegetables and fruit trees, etc. Chlorfluazuron is an insect growth regulator inhibiting chitin synthesis and can provide a good control of a variety of pest insects, especially Lepidoptera at low dose rate. Chlorfluazuron has very little to no negative impact on non targeted specie (Huo et al., 2010; Huang et al., 2015). Therefore it can be used in integrated pest management (IPM) programs (Chakraborty and Sun, 2014; Fenner et al., 2013; O'Brien, 2014). Chlorfluazuron is being used to control Lepidoptera such as Helicoverpa, Spodoptera and Pseudoplusia on soybean and Plutella, Spodoptera and thrips on vegetables; Adoxophyes, Ascotis on tea and fruit trees and have also used on potatoes, ornamentals and turf. Application rate of CF is 10–100 g a.i./ha. Structure of chlorfluazuron is given in Fig. 1.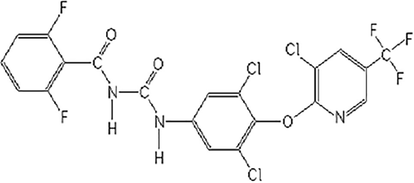
Structure of Chlorfluazuron (CF).
As disused above the use of chlorfluazuron is quite prevalent for protection of different fruits, vegetation etc, but there is very limited information available on its interaction with different environmental components particularly with soil, as each soil has different physiochemical properties which are responsible for different behavior of pesticides. Therefore present study is an attempt to understand interaction of chlorfluazuron with different agricultural soils through adsorption and desorption studies of chlorfluazuron. Pesticide adsorption-desorption has a significant role in predicting the fate and behavior of pesticides in soil and aquatic environment (Khooharo et al., 2008; Valavanidis and Vlachogianni, 2010; Liang et al., 2014). Pesticide adsorption-desorption investigation has been reported for number of pesticides e.g. Doretto et al. reported adsorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in different Brazilian soils and found out that their tested pesticides were easy leach out through sandy soils with low organic carbon contents can contaminate groundwater bodies (Doretto et al., 2014). Similarly Aslam et al. studies adsorption and desorption behavior of glyphosate, s-metolachlor and epoxiconazole and analyzed Kf, Kd, Kfoc and Koc for determining the interaction of selected pesticides (Aslam et al., 2013). Bermúdez-Couso et al. investigated different methods for adsorption and desorption of carbofuran insecticide, by comparing the results of different methods they concluded that in all tested methods carbofuran adsorption was higher in the soil with the higher SOM content while the opposite behavior was observed for the desorption of carbofuran (Bermúdez-Couso et al., 2011). In another study Singh and Cameotra investigated adsorption and desorption behavior of chlorotriazine herbicide in the agricultural soils. Results of their study indicated that the soil organic matter content and aqueous solubility play an important role in the adsorption–desorption behavior of chlorotriazine herbicides. Moreover they stated that chlorotriazine herbicide sorption–desorption process will help to determine the herbicide fate and availability in soil, biodegradation, runoff and leaching (Anil Kumar and Swaranjit, 2013). Similar studies of sorption have also been reported for different fungicides and insecticides. (Krishna et al., 2008; Bansal, 2010; Gebremariam et al., 2012; Shariff et al., 2012). However previous there is no literature available on adsorption-desorption of CF for agricultural soils.
Therefore the purpose of present study was to understand the interaction chlorfluazuron with different agricultural soils through sorption studies as well as determining the mobility, runoff and leaching of chlorfluazuron through selected soils. The present research focused on exploring effects of soil physiochemical properties on sorption of chlorfluazuron. The present work also intended to predict contamination of water bodies with chlorfluazuron through leaching and runoff based on sorption coefficients.
2 Experimental
Chemicals: All solvents and reagents were analytical grade. Sodium chloride and potassium chloride was supplied by Merck (Merck KG 64271Darmstdt, German. Fluka analytical grade Chlorfluazuron insecticide was supplied by Sigma-Aldrich chemie GmbH, Riedstr. 2, D-89555 Steinheim 49 7329 970, absolute ethanol and 99.9% Acetone were also supplied by Merck (Merck KGaA 64271Darmstdt, German Throughout the study, double distilled water was used and obtained from a Milli-Q purification system. Standard stock solutions (10 ppm) of chlorfluazuron were prepared by dissolving 10.3 mg of each CF in 1000 mL of distilled water. Working solutions were prepared through the appropriate dilution of a standard stock solution with 0.1 M NaCl and 0.1 M CaCl2.
2.1 Soil analysis
Ten agricultural regions from province of Punjab, Balochistan, Khyber Pukhtoon Khw and Azad Kashmir Pakistan were selected for sorption studies of pesticide. Therefore 10 different soil samples were collected from 10 different districts of Pakistan, which were classified into four types of soil based on their texture. Details of sampling and soil samples are given in Table 1. Considering the significance of the sampling depth for studying the soil-pesticide interaction, in the present research, all soils were sampled at 0–15 cm depth following random and composite sampling. The soil samples were air dried at room temperature (20–25 °C). Disaggregated first manually and then using a mortar and pestle. Homogenized soils samples were sieved (2 mm sieve) to obtained final prepared soils. The prepared soil samples were then investigated for the physiochemical parameters which were considered to be mainly responsible for adsorptive capacities of soils (Table 1) Clay Sandy clay Sandy loam Sandy clay loam
Types of soils
Sampling areas
Sampling Sites
Physicochemical parameter
pH
OM
Clay
Sand
CEC
Texture
Sample-1
Okara
6.57
2.5
57
27
8.2
Clay
Sample-2
Poonch
6.84
2.8
55
26
8.3
Clay
Sampe-3
Chakwal
6.9
1.78
43
29
6.3
Clay
Sample-4
Khushab
6.89
2.47
51
28
7.7
Clay
Sample-5
Sargodha
6.26
2.9
65
8
8.5
Clay
Sample-6
Mandi Bahaud Din
6.96
2.07
36
46
7.5
Sandy clay
Sample-7
Gujranwala
8.01
0.97
11
66.6
5.9
Sandy loam
Sample-8
Zhob
7.31
2.01
30
48
7.3
Sandy clay loam
Sample-9
Buner
7.49
1.82
22
50
7.0
Sandy clay loam
Sample-10
Abbottabad
7.95
1.52
20
58
6.1
Sandy clay loam
For sorption studies it is essential to find out texture of soils (OECD, 2005). In this research texture of selected soil samples was determined by Octagon digital sieve shaker and USDA textural triangle was used to delineate soil textural classes. Behavior of pesticides is greatly affected by soil pH which was determined by saturation extract methods (Wagh et al., 2013). 5 g of soil was mixed in 10 ml of re-distilled water (1:2 soil solution), after contact time of one hour pH of the slurry was measured by MM 40 + pH meter of Crison company (Wagh et al., 2013). For sorption studies most important component of soil is soil organic matter (SOM). To determine SOM Loss on Ignition (LOI) method was used by the loss of weight of the soil sample heated at a high temperature. Temperature of LOI method is enough to burn organic matter of soil but should not decompose carbonates present in the soil (Robertson et al., 2011). First soil samples were placed in oven at 105 °C for 24 h, and then the samples were placed in muffle furnace (of Ncycraft Company) for 2 h at 360 °C. %OM was calculated by comparing the weight of a sample before and after the soil has been ignited (Robertson et al., 2011; Onojake and Osuji, 2012; Hoogsteen et al., 2015).
2.2 Adsorption–desorption studies
10.3 ppm stock solution was prepared by dissolving 10.3 mg of chlorfluazuron in 1000 ml of deionized water on a magnetic stirrer for 12–24 h. Before adding distilled water few drops of analytical grade 99.9% acetone was added in order to enhance solubility of pesticide in water. The stock solutions were prepared just before application to tested soil samples, However extra amount of stock solutions were kept and stored at 4 °C. Other stock of 0.1 M sodium chloride and 0.1 M CaCl2 were also prepared.
When pesticide was completely dissolved in water, 16 concentrations (with duplicates) (0, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0 and 7.5 ppm) of chlorfluazuron were made in 10 ml of 0.1 M NaCl. The volume of each concentration was raised up to 100 ml by distilled water. 10 ml of each concentration was added into 15 ml falcon valves having 0.5 g of selected soil samples. One control sample having chlorfluazuron in 0.1 M NaCl solution was subjected to the same batch equilibrium procedure to check the stability of the CF in NaCl solution. One blank sample (soil + NaCl solution) was also run in order to detect contamination of soils or interfering compounds which could cause matrix effects. All the falcon valves, including controls and blanks were then continuously agitated in overhead orbital shaker of Irmeco Gmbh Germany at room temperature for 24 h at 90 rpm velocity. After achieving equilibrium, the falcon valves were centrifuged by Sigma 2-6E centrifuge of high-speed at 3500 rpm for 20 min at room temperature. Supernatant was then collected and filtered through 0.2 µm nylon filters. Clear aliquots were now ready for analysis by UV–Visible spectrophotometer and High-performance liquid chromatography (HPLC).
Desorption was conducted on the same soil pesticide solutions. Once the adsorption was done, the supernatant was discarded. The falcon valves with the contents the valves were reweighed and 9 ml of freshly prepared 0.1 M CaCl2 solution was added to the remaining soil in the falcon valves. The samples were again shaken, centrifuged and analyzed at same condition as for adsorption.
2.3 Analytical method
The analytical methods which were used for analysis of the pesticide in the present research were:
2.3.1 UV–Visible spectrophotometer
Sorption studies of chlorfluazuron of 14 concentrations including with duplicates were studied by BMS 1602 UV–Visible spectrophotometer. UV–Visible spectra of CF under study were recorded at 250 nm. The absorbance of the pesticide solution which was recorded at the lambda maximum was taken as peak height. To check the change in the intensity of lambda maximum (λmax) spectra of pesticide solution was scanned for a range of wavelength.
2.3.2 High-performance liquid chromatography (HPLC)
For chlorfluazuron analysis, the portion of solution (20 μl) was analyzed by HPLC (LC-20 AT UFLC by schinal 24, Japan). HPLC was equipped with two k-500 pumps, a UV–vis detector and a Hypersil C18 column. The mobile phase was 26:74 (v/v) acetonitryle/ci-trate buffers (pH 6.5). 0.5 cm3·min−1 was the flow rate of the mobile phase, with a run time of 6 min per sample and detection wavelength was 250 nm. The retention time was 2.9 min. The detection limit was 0.5 μg·dm−3, reproducibility of results with the relative standard deviation lower than 5%.
3 Data analysis
The quantity of the pesticide adsorbed (µg/g of soil) was calculated by using the following Eq. (1) (OECD, 2005).
Cs is the amount of the pesticide adsorbed on the soil, V is the volume of solution, m is weight of soil sample in grams, Cb is equilibrium concentration of blank sample and Ca is equilibrium concentration of pe sticide soil solution or treatment supernatant.
The distribution coefficient (Kd) of both adsorption and desorption was calculated by taking the ratio of Cs to Ce (ml/µg) (Shariff et al., 2012; Doretto et al., 2014).
Ce is equilibrium concentration of pesticide in solution (Gebremariam et al., 2012).
Adsorption isotherm parameters were calculated using the linear form of Freundlich equation (Doretto et al., 2014; Gebremariamet al., 2012).
-
Kf = Freundlich adsorption coefficient
-
1/n = Slope of Freundlich adsorption isotherm
-
n is a linearity factor, it is also known as adsorption intensity
-
log Kf is the intercept of the straight line resulting from the plot of log Cs versus log Ce while Cs and Ce are defined previously.
H can be determined by using the following equation.
The linear or distribution coefficient (Kd) is related to soil SOM by the Eq. (6) (Ahmad et al., 2014).
4 Results and Discussion
Physiochemical investigation of selected soil samples is given in Table 1. Based on textural assessment, fist five soils were identified as clay type soil, this type of soils found to have 43%–65% clay content in present study. Soil sample-6 and sample-7 was grouped into Sandy clay and Sandy loam soils, having 46% and 68% sand content respectively while soil sample-8, sample-9 and sample-10 was classified into Sandy clay loam soil with 48, 50 and 66.6% sand content. Therefore four distant soil types were investigated in present research. From data in table one it was depicted that the pH of clay type soil is 6.57–6.9 while sand clay revealed 6.9 pH. Sandy loam soil has 8.01 pH and Sandy clay loam has pH from 7.31 to 7.95. Similarly highest SOM was found for clay soil (sample-5) and comparatively lowest SOM was observed for Sandy loam (sample-7). CEC values were highest for clay soil and lowest Sandy loam as shown in Table 1.
4.1 Linear adsorption and desorption of chlorfluazuron
Chlorfluazuron sorption was studied by HPLC and UV Visible spectrophotometer. Linear isotherms of CF are as follow (Figs. 2 and 3).
Linear adsorption isotherms of chlorfluazuron by selected soils.

Linear desorption isotherms of chlorfluazuron by selected soils.
The Kd ads values of chlorfluazuron varied between 12 and 4.7 µg ml−1 while the regression coefficient R2 value ranging from 0.81 to 0.97 with standard error (S.E.) ranged between 0.059 and 2.5. The competitive effect of chlorfluazuron adsorption by each soil normally decreased with increasing initial insecticidal concentrations. This is because of the saturation of sorption sites in soils with increasing concentration of pesticides (Doretto et al., 2014). The desorption experiments were conducted with a nonionic surfactant, the comparative results of linear isotherms are shown in Table 2. According to this data Kd des varied between 4.09 and 9.2 µg ml−1 with R2 and S.E ranging between 0.96 to 0.80 and 2.04 to 0.065 respectively. Usually values of KOM below 500 specify minimum or no adsorption of a pesticide, this would indicates high probability of runoff or leaching (Onojake and Osuji, 2012). Pesticides have been categorized into three classes based on KOM i.e. very high mobility group having KOM values less than 50, high mobility group having KOM values 150–500 and low mobility group having KOM values more than 500 (Ahmad et al., 2014). According to data in Table 2 chlorfluazuron lies in high mobility class of pesticides as its KOM values are ranged from 272.13 to 403.82. chlorfluazuron exhibited less adsorption potential through all selected soil sample. From data in Table 2 it is predicted that chlorfluazuron has significant leaching potential for type C and D as these samples demonstrates less adsorption and more desorption. The calculated values of hysteresis for chlorfluazuron on the selected soil samples ranged from 0.24 to 1.549. H indicates greater or lesser irreversibility of adsorption in all samples, the highest values were corresponding for samples having highest adsorption constant i.e. sample-6, sample 5, sample 2, sample 9 and sample 10. Clay Sandy clay Sandy loam Sandy clay loam
Soils
Linear Model (Adsorption)
Linear Model (Desorption)
Hysteresis
Kd µg/ml
R2
S
KOM
Kd µg/ml
R2
S
H
Sample-1
10.2
0.96
1.6
376.3
5.50
0.80
0.4
0.950
Sample-2
10.8
0.90
0.059
388.6
4.2
0.92
0.15
0.95
Sample-3
5.87
0.96
0.8
314.9
8.8
0.86
0.36
0.631
Sample-4
10.2
0.92
2.4
342.7
6.5
0.91
0.65
0.731
Sample-5
12.0
0.90
2.5
403.8
4.09
0.85
2.04
1.549
Sample-6
7.79
0.81
0.06
328.4
6.9
0.81
0.065
0.63
Sample-7
4.94
0.96
0.3
272.1
9.2
0.96
0.49
0.485
Sample-8
6.5
0.88
0.05
321.8
6.7
0.74
0.42
0.53
Sample-9
5.9
0.97
0.31
314.2
7.6
0.95
0.3
0.485
Sample-10
4.7
0.97
0.28
312.1
8.05
0.94
0.29
0.24
4.2 Freundlich isotherms of chlorfluazuron
Chlorfluazuron for all soil samples were then analyzed for Freundlich kinetics. Figs. 4 and 5 are Freundlich adsorption and desorption isotherms of chlorfluazuron on all studies soils.
Freundlich adsorption isotherms of chlorfluazuron by selected soils.

Freundlich Desorption isotherms of chlorfluazuron by selected soils.
Highest value of Kf ads found for sample-5 (9.05 µg ml−1) and lowest value was observed for sample-7 (3.9 µg ml−1). Values of R2 were ranging between 0.81 and 0.99 with associated S.E. between 0.04 and 0.59. These results intensely proved that Freundlich is good fitted model for adsorption studies of chlorfluazuron. Kf was calculated from the regression equation demonstrated that Freundlich adsorption model effectively describes isotherms for the chlorfluazuron in all cases. Desorption isotherms of the chlorfluazuron were fitted to the liberalized form of the Freundlich equation. Table 3 is also depicting 1.23–8.69 µg ml−1 variations in Kf des values. Minimum value of R2 for desorption is 0.87 with associated maximum and minimum S.E. values are 0.88 and 0.22 respectively. According to Kf analysis chlorfluazuron illustrated comparatively enhanced adsorption for soil samples of type A while enhanced desorption Sandy loam and Sandy clay loam soils respectively. Clay Sandy clay Sandy loam Sandy clay loam
Type
Soils
Freundlich Model (Adsorption)
Freundlich Model (Desorption)
Energy change
Kf µg/ml
R2
S
Kfom
nads
1/n
Kf µg/ml
R2
S
n
1/n
ΔG
Sample-1
8.56
0.85
1.6
342.4
0.4
1.1
4.1
0.87
0.17
0.86
1.15
−14.4
Sample-2
8.5
0.95
0.059
305.41
0.336
1.79
1.23
0.87
0.33
1.39
1.69
−14.16
Sample-3
4.8
0.81
0.8
311.74
0.58
2.0
5.5
0.91
0.07
0.48
2.11
−14.24
Sample-4
7.7
0.88
2.4
312.06
0.5
1.7
5.1
0.9
0.19
0.64
1.78
−14.22
Sample-5
9.05
0.96
2.5
402.06
0.27
0.9
3.4
0.83
0.4
0.92
1.92
−13.8
Sample-6
6.9
0.94
0.06
334.896
0.4
2.36
4.01
0.91
0.02
1.19
1.25
−14.4
Sample-7
4.03
0.93
0.3
269.6
0.6
3.6
8.9
0.97
0.06
0.07
0.425
−14.80
Sample-8
5.75
0.96
0.05
286.069
0.424
1.34
5.4
0.85
0.13
0.79
0.88
−14.01
Sample-9
5.04
0.88
0.31
276.96
0.56
1.27
6.4
0.99
0.05
0.78
2.47
−13.9
Sample-10
3.9
0.99
0.28
265.96
0.745
0.71
8.6
0.93
0.88
0.59
2.97
−13.83
Like Kf 1/n was also calculated from same regression equation. Values of 1/n ads were following the range of 0.71 to 3.6 and 1/n des from 0.425 to 2.97. nads follow the range of 0.33 to 0.745. ndes shown range of values varied between 0.714 and 2.36. Vales of nads and ndes are indicating the greater or lesser irreversibility of adsorption in all samples, the highest values corresponded for soil which is showing highest adsorption constant i.e. .type A soils. ΔG of chlorfluazuron for selected soils followed the range from −14.8 to −13.8 Kj mol−1. In present research ΔG demonstrated week physical adsorption as well as exothermic interaction of chlorfluazuron with selected soil samples.
Like Kom, Kfom also identified chlorfluazuron as highly mobile insecticide as Kfom values ranged from 265.96 to 402.06.
4.3 Effects of soil parameters on sorption behavior of chlorfluazuron
By comparing Tables 1–3 it was revealed that except sample 3, all samples of type A (Clay soil) depicted higher adsorption of CF while type B, C and D demonstrated higher desorption of chlorfluazuron. This is because of chemical properties of soils e.g. Clay content and SOM facilitate adsorption of pesticide (Krishna et al., 2008; Bansal, 2010; Shariff et al., 2012), therefore adsorption is higher for type A soil samples, similarly Type B, Type C and Type D soils have more sand content which results in higher desorption and enhance mobility of CF.
According to data in Table 1, highest values of SOM, Clay content, and CEC were found for Sample-5, thus Sample-5 depicted higher values of all adsorption coefficients. When adsorption coefficients were compared with pH of selected soil samples it was revealed that pH is negatively correlated with selected soil samples. Clay soils (sample 1 to sample 5) have comparatively lower pH and their adsorption coefficients (Kd ads and Kf ads) are higher as compared to rest of tested soils. From data in Tables 1–3 it was verified that SOM has positive correlation with Kd ads and Kf ads. SOM have greater potential of binding particles together and retaining pesticides in soils by providing adsorption sites for pesticides to adsorb. In present research chlorfluazuron illustrated relatively higher affinity for sample for Clay soils because these samples have comparatively higher SOM. Clay being smaller particle, facilitates adsorption of chlorfluazuron by providing greater surface area and significant adsorption sites (Onojake and Osuji, 2012; Khairatul et al., 2013). On other hand chlorfluazuron indicated less mobility and less desorption through samples having more clay content and SOM. These two soil parameters are strongly restricted the mobility and desorption of pesticide. For desorption and mobility there must be more sand content and less clay, SOM and CEC (Chen, 2015; Ahmad et al., 2014; Shariff, 2012; OECD, 2005). In Table 1 Sandy clay loam (sample-8, 9 and 10) and Sandy loam (sample-7) exhibited more sand content, therefore revealed higher values of desorption coefficients as well as higher mobility of chlorfluazuron (predicted from data in Tables 2 and 3). Sandy soil like sample-7 and sample- 10 has fewer pores or adsorption sites which result in minimum adsorption and enhanced mobility of pesticide (Gebremariam et al., 2012; Ahmad et al., 2014). Because of enhanced mobility and leaching, chlorfluazuron tend to contaminate ground water bodies of sandy clay loam (sample-8, 9 and 10) and Sandy loam (sample-7) while for clay soils risk of contamination of water bodies is negligible. However there is slight leaching of chlorfluazuron was assessed for clay soils.
However soil sample-3 depicted different behavior from other clay type soils (sample 1, 2, 4, 5). Sample-3 although a clay soil, yet it indicated less adsorption because of lower (1.78%) SOM content than other clay type soils. Sample-1, sample-2, sample-4, and sample-5 have high clay as well as SOM which is responsible for higher adsorption while sample-3 have higher clay content but lower SOM thus sample-3 revealed lower adsorption potential.
5 Conclusion
This study has quantitatively assessed the adsorption- desorption of Chlorfluazuron to four characteristic soils of the Pakistan. The investigated soils of the textural classes (clay, Sandy clay loam, Sandy loam and Sandy clay) cover maximum area of the country. The sorption studies indicated that, chlorfluazuron has a low sorption potential on the Sandy clay loam and Sandy loam. The adsorption is directly related to the SOM of soils and an increase in adsorption was verified in soil with a higher SOM. Moreover, the adsorption capacities of chlorfluazuron were larger in clay soils than in the sandy soil. The adsorption capacity followed the sequence of sample-5, sample-2, sample-1, sample-4, sample-6, sample-8, sample-9, smaple-3, sample-7 and sample-10 based on the Kd and Kf values. The Freundlich desorption coefficients for tested soils were similar to the corresponding adsorption coefficients, showing a reversible adsorption/desorption process on these soils. The relatively low sorption coefficients of chlorfluazuron found in this study indicated the weak interactions between the chlorfluazuron molecules and the binding sites on the soils. Moreover it was revealed that chlorfluazuron tend to leach from soils with high sand content and low SOM and low CEC exchange capacity as demonstrated for sample-7, sample-10, sample-9 and sample-3. Significant contamination of ground water bodies was predicted for sample-7, sample-10, sample-9 and sample-3 as chlorfluazuron exhibited high mobility for these soil samples respectively.
Acknowledgement
The authors acknowledge faculty of Department of Environmental Sciences, Fatima Jinnah Women University Rawalpindi Pakistan.
References
- Sorption-desorption characteristics of benzimidazole based fungicide 2-(4-fluorophenyl)-1H-benzimidazole on physicochemical properties of selected Pakistani soils. J. Chem. Soc. Pak.. 2014;36:31-36.
- [Google Scholar]
- Adsorption and desorption behavior of chlorotriazine herbicides in the agricultural soils. J. Pet. Environ. Biotechnol.. 2013;4:154.
- [Google Scholar]
- Adsorption and desorption behavior of selected pesticides as influenced by decomposition of maize mulch. Chemosphere. 2013;91:1447-1455.
- [Google Scholar]
- The Effects of composts on adsorption-desorption of three carbamate pesticides in different soils of Aligarh district. J. Appl. Sci. Environ. Manage.. 2010;14:4.
- [Google Scholar]
- Adsorption and desorption kinetics of carbofuran in acid soils. J. Hazard. Mater.. 2011;190:159-167.
- [Google Scholar]
- An adsorption isotherm equation for multi-types adsorption with thermodynamic correctness. Appl. Therm. Eng.. 2014;72:190-199.
- [Google Scholar]
- Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils. Sci. Total Environ.. 2014;476:406-414.
- [Google Scholar]
- Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science. 2013;341:752-758.
- [Google Scholar]
- Adsorption and desorption of chlorpyrifos to soils and sediments. In: Reviews of Environmental Contamination and Toxicology. New York: Springer; 2012. p. :123-175.
- [Google Scholar]
- Under-and over-use of pesticides: an international analysis. Ecol. Econ.. 2013;89:73-78.
- [Google Scholar]
- Estimating soil organic carbon through loss on ignition: effects of ignition conditions and structural water loss. Eur. J. Soil Sci.. 2015;66:320-328.
- [Google Scholar]
- Comparison of the cytotoxic impact of chlorfluazuron on selected insect and human cell lines. Environ. Toxicol. Chem.. 2015;34:1675-1682.
- [Google Scholar]
- Residue detection and degradation of chlorfluazuron in rice, soil and field water. J. Saf. Environ.. 2010;29:686-691.
- [Google Scholar]
- Adsorption and leaching studies of molinate, carbofuran and propiconazole in Muda agricultural soils. J. Trop. Agric. Food Sci.. 2013;41:127-136.
- [Google Scholar]
- An empirical analysis of pesticide marketing in Pakistan. Pak. Econ. Soc. Rev.. 2008;46:57-74.
- [Google Scholar]
- Adsorption and desorption characteristics of lindane, carbofuran and methyl parathion on various Indian soils. J. Hazard. Mater.. 2008;160:559-567.
- [Google Scholar]
- Insecticides: Action and Metabolism. Academic Press; 2014.
- OECD, 2005. Guideline for the Testing of Chemicals. Adsorption-Desorption Using a Batch Equilibrium Method.
- Assessment of the physico-chemical properties of hydrocarbon contaminated soil. Arch. Appl. Sci. Res.. 2012;4:48-58.
- [Google Scholar]
- Food insecurity, income inequality, and the changing comparative advantage in world agriculture. Agric. Econ.. 2013;44:7-18.
- [Google Scholar]
- Plant and soil properties determine microbial community structure of shared Pinus-Vaccinium rhizospheres in petroleum hydrocarbon contaminated forest soils. Plant Soil. 2011;346:121.
- [Google Scholar]
- Effect of co-pesticide on adsorption–desorption process on agricultural soils. Int. J. Eng. Res. Dev.. 2012;1:55-69.
- [Google Scholar]
- Valavanidis, A., Vlachogianni, T., 2010. Agricultural pesticides ecotoxicological studies and environmental risk assessment. Sci. Adv. Environ. Toxicol. Ecotoxicol. issues Link: http://chem-tox-ecotox.org/wp.
- Physicochemical analysis of soils from Eastern Part of Pune City. Univ. J. Environ. Res. Technol.. 2013;3:1.
- [Google Scholar]







