Translate this page into:
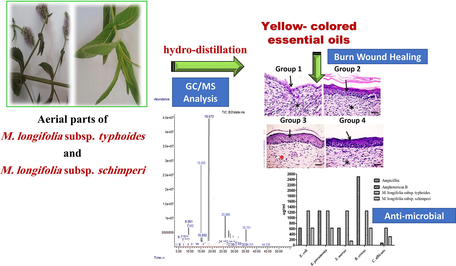
Essential oils from wild Mentha longifolia subspecies typhoides and subspecies schimperi: Burn wound healing and antimicrobial candidates
⁎Corresponding authors. melneketi@mans.edu.eg (Mona El-Neketi), gohar@mans.edu.eg (Ahmed A. Gohar) ahmedgohar99@yahoo.com (Ahmed A. Gohar) gohar@mans.edu.eg (Ahmed A. Gohar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
A comparative study was conducted on the essential oils (EOs) chemical composition from two subspecies of wild Mentha longifolia typhoides and schimperi, growing in Egypt, followed by biological investigation of EOs as antibacterial, antiquorum sensing and burn wound healing agents. Gas chromatography/mass spectrometry analysis of hydro-distillated EOs led to identification of 99 % of oil components. Schimperi oil revealed broadspectrum antibacterial activity with MIC values of 156 ∼ 625 μg/ml, lower than or close to ampicillin. The oil from typhoides exhibited a higher antiquorum-sensing effect. The potential of oils to heal burn injuries was assessed by applying the oils in ointment form to second-degree burn injury in mice for 21 days. Interestingly, skin healing activity in group treated with typhoides oil was more effective than that of the positive control (Silver sulfadiazine 1 %). These results suggest a promising candidate in the area of burn wound healing therapy.
Keywords
Mentha longifolia
Lamiaceae
Essential oils
Burn wound healing activity
Antiquorum-sensing activity
Antimicrobial activity
- LB broth
-
Luria-Bertani medium (lysogeny broth)
- MIC
-
Minimum inhibitory concentration
- QS
-
Quorum-sensing
Abbreviations
1 Introduction
Burn injuries are considered as one of the well-known morbidities worldwide and are associated with serious complications (Tiwari, 2012; He et al., 2017). About 2.4 million burn injuries are recorded annually, of which about 650,000 need treatment (Hashemi et al., 2014). Incidence and mortality rates vary according to different parameters of injuries such as their degree, area affected, depth, level of burns, and depending on treatment. Physiologically, burn wound healing occurs in three phases: inflammation reduction, proliferation and skin regeneration in the wounded area. Any drug that reduces the time for these phases can lead to better wound repair (Sood and Achauer, 2006).
Burn injuries are highly susceptible to infection leading to serious problems and these septic conditions may cause death among burn patients (Mokaddas et al., 1998). The extracts and essential oils of Mentha aerial parts were used as an effective disinfectant. They accelerate wound healing and tissue regeneration upon 2nd degree burn therapy in rats i.e. the application of M. pulegium extract in the form of dressings or oils (Habibi et al., 2018; Vaghardoost et al., 2019).
M. longifolia (L.) L. family Lamiaceae has different accepted synonymous names such as M. longifolia subsp. lavandulacea (Willd.) Briq.; M. longifolia (L.) Huds.; M. longifolia var. microphylla (Leij & Coutois) Rouy; M. longifolia subsp. schimperi (Briq.) Briq.; M. longifolia subsp. typhoides (Briq.) Harley.; and others. The last two names are among the infra-specific taxa in Mentha (WCSP, 2021; African Plant Database, 2021). These different names and diversity of Mentha varieties or subspecies are attributed to their geographical distribution and habitat (Lawrence 2006; Tucker and Naczi 2007). In Egypt, M. longifolia Huds. subsp. typhoides is called Habaq El-Mayya, Habaq El-Barr and Felaiyya while, M. longifolia Huds. subsp. schimperi is called Habaq (Abd El-Maksoud and Azer, 2013). It is noteworthy that these two investigated plants are known worldwide as wild mint or horse mint (Lawrence 2006; Verma et al., 2015).
The two titled plants are among the most promising medicinal plants that show diverse biological activities such as antioxidant, antimicrobial (Habibi et al., 2018; Hajlaoui et al., 2009), cytotoxic (Orhan et al., 2012), anti-diarrheal, antispasmodic and calcium channel blocking (Shah et al., 2010). Mints are used in tradtional medicine for bronchitis, nausea, flatulence, ulcerative colitis, liver complaints and anorexia. The Egyptians are using the wild mint plants to relief pain, fever, various inflammatory disorders, as well as to improve digestion, sedation and wound-healing (Eissa et al., 2014). Worldwide, M. longifolia is used traditionally as antiparasitic, anti-inflammatory, antimicrobial and / or antiseptic agents. It is also used for gastrointestinal disorders as peptic ulcer, anti-emetic, diarrhea, ulcerative colitis and liver diseases (Verma et al., 2015; Kozan et al., 2006; Aghili, 2009; Darwish and Aburjai, 2010). M. longifolia L. has been reported to contain different classes of secondary metabolites mainly flavonoids, essential oil, in addition to many other phenolic constituents (Farzaei et al., 2017). In a previous publication, we reported the presence of triterpenes, steroids, flavonoids, and phenolic acids in the titled plant (Haikal et al., 2021).
These findings along with the growing global need for new candidates in the area of wound healing especially from natural sources, encouraged us to assess the potential role of essential oils from the two wild Egyptian M. longifolia subspecies: typhoides and schimperi as promising wound healing agents and to demonstrate their antibacterial as well as antiqurum-sensing effects.
2 Materials and methods
2.1 Essential oils preparation and analysis
Fresh aerial parts of two subspecies of the wild Egyptian M. longifolia: typhoides and schimperi (250 g, each) were crushed into small pieces and subjected to hydro-distillation to a constant oil volume (8 h) using a Clevenjer-type apparatus to produce yellow-colored oils. Oils were dehydrated using anhydrous sodium sulfate and stored at low temperature for running GC/MS analysis (for the program, please see the supporting information) and biological studies. The oil components were identified by measuremants of component retention indices (Van den Dool and Kratz, 1963), their mass spectral fragmentation patterns (Adams, 2007; Halim et al., 1990) and/or stored data on the mass spectral database NIST/ ChemStation data system.
2.2 Biological activities of essential oils
2.2.1 Antimicrobial assay
The antimicrobial activity of the distilled essential oils was investigated using broth microdilution method. For a detailed procedure, please see the supporting information.
2.2.2 Antiquorum-sensing assay
The anti-quorum sensing activity was assessed for distilled essential oils employing El-Gohary and Shaaban, 2015 procedure. For detailed description, please refere to Haikal et al., 2021.
2.2.3 Burn wound healing activity
2.2.3.1 Preparation of the test samples and burn wound healing bioassay
Essential oils are incorporated into an ointment base formed of glycol stearate: propylene glycol: liquid paraffin (3:6:1); 1 % concentration (De Villiers, 2009; Süntar et al., 2012).
Wister albino mice weighing approximately 25–35 g (40 mice) were used in the experiment. All mice were in good health and had been checked for animal diseases by a veterinarian. Mice were separated in different shelves in sterilized containers in the animal laboratory, Faculty of Veterinary medicine, Mansoura university, at temperature about 22 °C. The experimental procedure were adopted by the Research Ethics Committee, Faculty of Pharmacy, Mansoura University, Egypt (Approval date: November 28th, 2017 to May 25th, 2021 – Approval number: 2021–263).
Forty mice were divided into 4 groups (n = 10). Groups were treated as follow: Group 1: with plain ointment base, group 2: with silver sulfadiazine 1 % ointment, group 3: with typhoides subsp. essential oil ointment and group 4: with schimperi subsp. essential oil ointment. Mice were anaesthetized with xylazine (10 mg/kg) and ketamine (60 mg/kg) as I.M injection. Their backs were shaved with a blade. Then, a second-degree burn wound (1.5 cm2) was generated with a metal cube (2 × 3 × 1 cm) heated to 105 °C for 15 s. Mice were resuscitated with a 1 ml injection of intraperitoneal normal saline solution, and then ointments were applied daily for 21 days to the wound-areas with insufficient amount. All wounds were not dressed in order to increase the visibility of wound conditions (Edraki et al., 2014). A digital camera (Canon power-shot D10) was used to photograph the burned areas on days 3–21.
The wound areas was recorded as 100 % on the first experimental day to which areas on the subsequent days were compared.
2.2.3.2 Histopathological examination
Skin tissue samples were taken for histopathological examination to assess wound re-epithelialization on days 3, 7, 14 and 21 (the end of treatment period). Small excisions containing part of the wound area were done, and then tissues were fixed in 10 % formalin and processed until immersed in paraffin blocks. Paraffin sections with a thickness of 5-μm were cut using a microtome and routinely stained with hematoxylin and eosin. These tissue sections were examined microscopically.
Angiogenesis (neovascularization) and infiltration with inflammatory cells were assessed in tissue sections on the third day after burn injury by giving a score as follows: zero or (-) score was given when vessels and macrophage cells are absent in each high-power field. Mild score 1 or (+) was set when 1–2 vessels or 1–2 macrophages cells were identified. The moderate score was 2 or (++), indicating 3–4 vessels for angiogenesis and 3–4 cells for cell study in each high-power field. The severe score was 3 or (+++) when 5 or more cells or vessels were detected in the high-power field.
A modified scoring system for histopathological criteria to assess surgical wound healing on the 7th, 14th and 21st days was defined according to Edraki et al., 2014; Pereira et al., 2012. The detailed description of histological scoring system are descriped in the supporting information.
3 Results and discussion
3.1 Components of the essential oils
Aerial parts of M. longifolia subsp. typhoides yielded 0.8 % v/w of a clear faint yellow, lighter than water essential oil. The oil components together with the percentage and retention indices are shown in Table 1. Forty-two compounds (99.865 %) were identified in the oil. Monoterpenes and sesquiterpenes are the main components, accounting for 88.980 % and 10.885 %, respectively. The monoterpenes are represented by piperitenone oxide (55.443 %), piperitone oxide (12.180 %), 1, 8 cineole (6.230 %), d-limonene (3.739 %), α- terpineol (1.858 %), (-) β-pinene (1.737 %) and (+)-α–pinene (1.123 %) as the main constituents. While, sesquiterpenes are represented by caryophyllene (7.127 %) and (+) epi-bicyclosesquiphellandrene (1.273 %) as the major constituents. Aerial parts of M. longifolia subsp. schimperi yielded 0.9 % v/w of a clear, faint yellow, lighter than water essential oil. Oil components with percentage and retention indices are shown in Table 1. Thirty compounds (99.671 %) were identified in the oil. Monoterpenes and sesquiterpenes, are the main constituents, accounting for 90.855 % and 8.816 %, respectively. The main constituents of monoterpenes are represented by pulegone (56.493 %), menthone (26.593 %), d-limonene (1.894 %), isopulegone (1.270 %) and 1, 8 cineole (1.244 %), and the main constituents of sesquiterpenes are represented by caryophyllene (2.075 %), humulene (1.099 %), (+)-epi-bicyclosesquiphellandrene (1.081 %) and cedrelanol (1.498 %). aBold values point out the major components; (–): constituent is absent. bRt = retention time, RI = retention index.
No.
Identified compounds
Rtb
RI
M+ peak
Base peak
% in subsp. typhoides
% in subsp. schimperi
1
α- Thujene
5.988
922
136.1
93.0
0.078
0.024
2
(+)-α–pinene
6.194
931
136.1
93.1
1.123
0.485
3
Camphene
6.640
943
136.1
93.0
0.025
0.013
4
Sabinene
7.418
968
136.1
93.1
0.714
0.339
5
(−) β-Pinene
7.527
980
136.1
93.1
1.737
0.599
6
(+)-β-Pinene
7.991
989
136.1
93.0
–
0.391
7
β–Myrcene
8.002
999
136.1
93.1
0.847
–
8
P-Mentha- 1(7),8-diene
8.443
1001
136.1
93.0
0.368
0.019
9
(+)-4- Carene
8.912
1007
136.1
93.0
0.021
–
10
Cymene
9.221
1017
134.1
119.0
0.018
0.021
11
d-Limonene
9.387
1029
136.1
68.1
3.739
1.894
12
1, 8 cineole
9.513
1030
154.1
81.1
6.230
1.244
13
Trans- β- Ocimene
9.724
1036
136.0
93.0
0.244
–
14
Cis-β-Ocimene
10.119
1041
136.1
93.0
–
0.075
15
γ- Terpinene
10.531
1051
136.1
93.0
0.041
0.075
16
4- Thujanol
10.943
1075
154.1
93.0
0.070
0.131
17
Terpinolene
11.716
1079
136.1
93.0
0.134
0.193
18
Linalool
12.276
1084
154.0
70.1
0.321
–
19
1,3,8-P-Menthatriene
12.700
1101
134.1
119.0
0.935
–
20
Menthonea
5.006
156
54.1
12.1
–
26.593
21
Isopulegone
15.504
1161
152.1
67.1
–
1.270
22
4- Carvomenthol (Terpinene 4-ol)
15.566
1162
154.1
71.0
0.556
–
23
α- Terpineol
16.202
1172
154.0
59.0
1.858
0.529
24
3-Carene
17.855
1201
136.1
93.0
0.297
–
25
Pulegone
18.273
1233
152.1
81.0
0.075
56.493
26
(-)-Carvone
18.485
1240
150.0
82.0
0.089
–
27
Piperitone oxide
19.022
1266
168.1
69.1
12.180
–
28
Bornyl acetate
20.270
1283
196.0
95.0
0.151
–
29
Diosphenol
20.888
1285
168.1
126.0
0.290
–
30
Thymol
21.288
1289
150.1
135.0
0.581
–
31
Eucarvone
22.713
1320
150.1
107.1
0.815
–
32
Piperitenone oxide
24.338
1360
166.1
67.1
55.443
–
33
Jasmone
25.168
1388
164.1
166.1
–
0.467
Monoterpenes
88.98
90.855
Oxygenated monoterpene
78.659
86.727
II. Sesquiterpenes
34
Caryophyllene
25.952
1399
204.1
93.1
7.127
2.075
35
δ–Cadinene
27.010
1431
204.2
161.1
0.033
–
36
Humulene
27.291
1448
204.2
93.0
0.641
1.099
37
Trans-β- Farnesene
27.480
1452
204.1
69.1
0.143
–
38
(+) epi-Bicycloses-quiphell-andrene
27.674
1471
204.2
161.1
1.273
1.081
39
(-)-d-Germacrene
28.407
1483
204.1
161.1
0.639
0.964
40
(+) Valencene
28.658
1495
204.2
161.1
0.014
–
41
Bicyclogermacrene
29.019
1502
204.1
93.1
0.150
–
42
Eremophilene
29.156
1507
204.2
161.1
0.039
–
43
γ- Cadinene
29.728
1512
204.1
161.1
0.335
0.808
44
(-)-Calamenene
30.089
1533
202.2
159.0
0.055
0.062
45
γ- Murolene
30.644
1553
204.1
161.0
0.089
–
46
Caryophyllene oxide
32.418
1588
221.1
79.0
0.258
0.769
47
Cadine-1,4-diene
33.659
1594
204.1
119.1
0.089
0.346
48
Cedrelanol
35.153
1644
222.0
161.1
–
1.498
49
(-)-α-Himachalene
36.314
1650
204.1
93.1
–
0.023
50
Farnesyl acetone
44.136
1820
262.1
69.1
–
0.091
Sesquiterpene
10.885
8.816
Oxygenated sesquiterpenes
0.258
2.358
Total oil content
99.865
99.671
3.2 Biological activities of essential oils
3.2.1 Antimicrobial assay
Essential oils isolated from both plants were assessed for their antimicrobial potential using the broth microdilution assay. The results (Table 2) proved that both oils exhibited broad-spectrum antibacterial activity. Oil from subsp. schimperi was more active than that from typhoides. The former revealed higher antibacterial activities against the tested Gram-positive and Gram-negative bacteria strains with MIC values in the range of 156–625 μg/ml lower than or close to that of ampicillin (MIC 625–2500 μg/ml). Whereas, the oil from typhoides subsp. exhibited relatively lower antibacterial activity. Both oils are inactive against the fungus C. albicans. aSample concentration: 5 mg/ml, Sample volume 100 μL /well, Results are calculated after subtraction of DMSO activity, nt: not tested.
Micro-organism
Ampicillin
Amphotericin B
M. longifolia typhoides oil
M. longifolia schimperi oil
Gram-negative
E. coli
625
nt
1250
625
K. pneumoniae
1250
nt
1250
625
Gram-positive
S. aureus
625
nt
1250
156.25
B. cereus
2500
nt
1250
625
Fungus
C. albicans
nt
78.125
625
312.5
High antibacterial activity of M. longifolia subsp. schimperi oil can be attributed to its content of pulegone as a major oxygenated compound, while the major oxygenated compound of M. longifolia subsp. typhoides oil is piperitenone oxide that has lower antibacterial activity compared to pulegone. This explains the lower antibacterial potency of M. longifolia subsp. typhoides oil incomparison to schimperi subsp. (Oumzil et al., 2002; Božović et al., 2015).
3.2.2 Antiquorum-sensing activity
Quorum-sensing (QS) in Ch. violaceum ATCC 12,472 secretes a purple pigment named violacein in response to the acyl HSLs auto-inducer molecules (Chu et al., 2011; McLean et al., 2004). Consequently, any medicine inhibiting acyl HSL-mediated QS activity in Ch. violaceum will stop violacein secretion. The QS activity of both oils was inferred from measurement of the pigment inhibition radius of essential oils. M. longifolia subsp. typhoides is relatively active (r = 13 mm) than M. longifolia subsp. schimperi (r = 11 mm) (Fig. 1).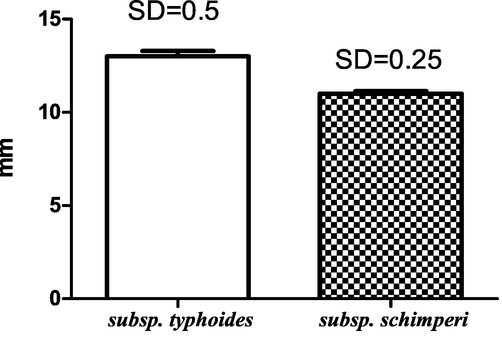
Antiquorum-sensing activity of M. longifolia subspecies essential oils.
3.2.3 Burn wound healing activity
Macroscopic examination of skin burn wounds at the beginning of therapy (3rd day) and at the end of therapy period (21st day) were demonstrated in (Fig. 1S&2S). At the end of this research, the appearance of regenerated skin with the naked eye seems normal and most of the skin appendages are totally repaired. The quality and quantity of burn wound healing and wound infection are directly related to treatment. The average burned area was significantly diminished in group 3 compared to other groups.
Microscopic examination of the skin from all groups on the third post-burn day showed the presence of necrotic epidermal cells, hyaline degeneration of collagenous fibers in the superficial dermis, a loose dermal structure, acute inflammatory reaction characterized by oedema, congested blood vessels and intense inflammatory cells infiltration (mainly neutrophils) with few lymphocyte and macrophages (Fig. 2). Inflammation intensity and prevalence was highest and deepest in group 1, however it was lowest and most superficial in group 3. Whereas, angiogenesis was highest in group 3 and was lowest in group 1 as shown in Table 3. Therefore, inflammation is inhibited due to angiogenesis in the tissues (Ribatti, 2017).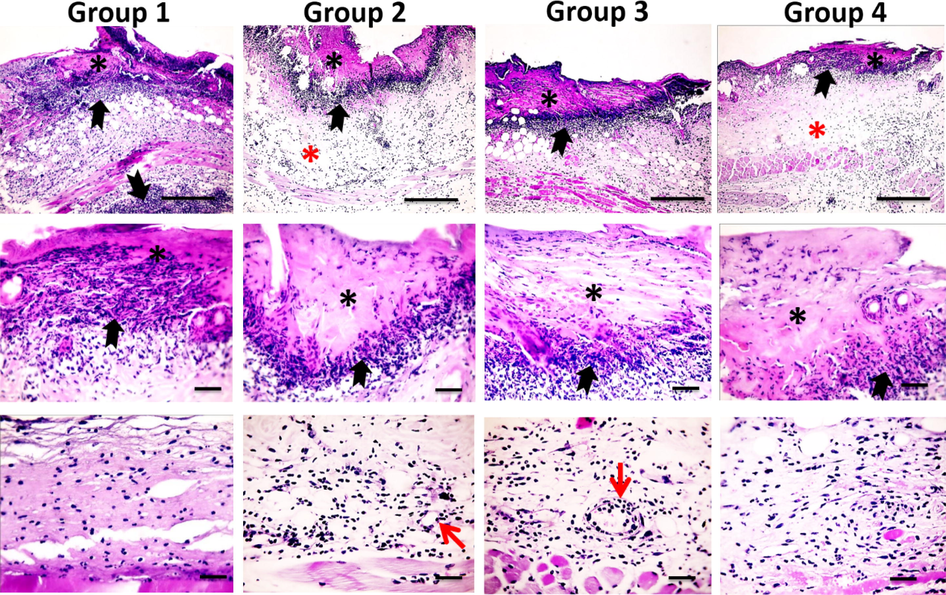
Microscopic pictures of H&E stained skin sections from four groups on the 3rd day after burn injury showing epiderma necrosis (*) with intense inflammatory reaction (black arrows) in all groups, stronger and deeper inflammation in Group 1, edema (*) in groups 2&4 (X: 100 bar 100 in 1st row). By higher magnification, the inflammatory reaction is mainly neutrophils infiltration (black arrows). Neovascularization (red arrows) appears in Groups 2&3 (X:400 bar 50 in 2nd and 3rd rows).
Components/Groups
Group 1
Group 2
Group 3
Group 4
P value
Degrees of inflammation and angiogenesis on the 3rd day after burn
Macrophage histocytic infiltration
2.5 ± 0.082
1.8 ± 0.013
0.8 ± 0.005
1.5 ± 0.096
0.0026
Angiogenesis
0.3 ± 0.014
1.3 ± 0.018
2.5 ± 0.018
0.8 ± 0.008
0.0027
Degrees of granulation tissue formation and its components on the 7th day after burn
Macrophage histocytic infiltration (0–3)
Neovascularization (0–3)
Fibroblastic proliferation (0–3)
Matrix mucopolisacharide deposition (0–3)
Degree of inflammation (0–3)
Extent of bacterial colonization [(-3)-0]
Degree of granulation tissue formation (0–3)
Total (-3–18)8.0 ± 0.07
10.2 ± 0.37
13.0 ± 0.35
8.9 ± 0.14
0.0004
Degrees of re-epithelialization parameters on the 7th day after burn
Epidermal thickness (0–3)
Thickness of granular cell layer (0–3)
Maturation organization of squamous cells (0–3)
Extent of keratin layer (0–3)
Orthokeratin (0–3)
Parakeratosis (0–3)
Total (0–18)
2.8 ± 0.58
7 ± 0.44
13.20 ± 0.60
5.8 ± 0.37
0.0007
Degrees of granulation tissue formation and its components on the 14th day after burn
Macrophage histocytic infiltration (0–3)
Neovascularization (0–3)
Fibroblastic proliferation (0–3)
Matrix mucopolisacharide deposition (0–3)
Degree of inflammation (0–3)
Extent of bacterial colonization [(-3)-0]
Degree of granulation tissue formation (0–3)
Total (-3–18)
10 ± 0.70
11 ± 0.54
12.8 ± 0.48
10 ± 0.50
0.03
Degrees of re-epithelialization parameters on the 14th day after burn
Epidermal thickness (0–3)
Thickness of granular cell layer (0–3)
Maturation organization of squamous cells (0–3)
Extent of keratin layer (0–3)
Orthokeratin (0–3)
Parakeratosis (0–3)
Total (0–18)
1 ± 0.30
7.8 ± 0.50
13.8 ± 0.24
7.4 ± 0.20
0.0009
Degrees of new dermis formation parameters on the 21st day after burn
Degree of scar formation (0–3)
Organization of collagen formation (0–3)
Extent of hair follicles (0–3)
Extent of lymphatic ducts (0–3)
Degree of innervations (0–3)
Total (0–15)
5.4 ± 0.74
9.4 ± 0.40
11.4 ± 0.42
7.2 ± 0.37
0.0009
On the 7th post-burn day, microscopic examination of untreated group 1 revealed persistent epidermal and dermal necrosis, neutrophils and sometimes lymphocyte and macrophages infiltration and formation of granulation tissue. The treated groups showed re-epithelialization, fibroblast infiltration, and angiogenesis. The area of granulation tissue formation, maturation, tissue organization and re-epithelialization were significantly higher in group 3 compared to group 1 (Table 3). Intense inflammatory cellular infiltration was detected in untreated group 1. Inflammation was markedly decreased in the treated groups particularly in group 3 (Fig. 3). These microscopic features indicate an early healing process in group 3.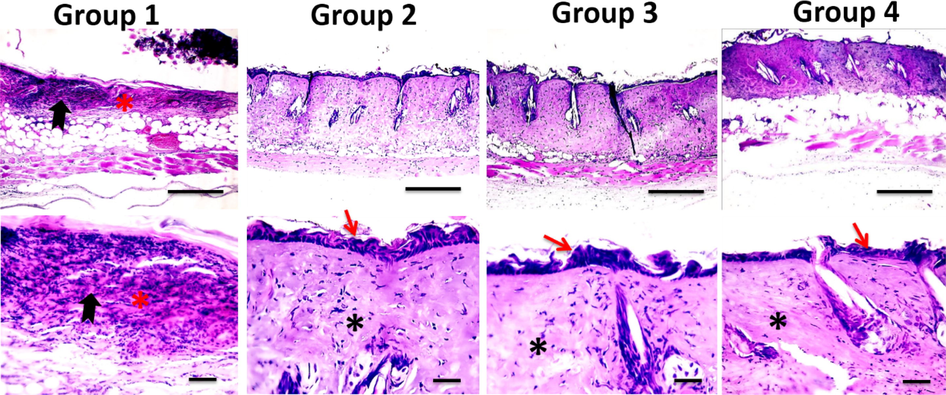
Microscopic pictures of H&E stained skin sections from four groups on the 7th day after burn injury showing the persistence of epidermal necrosis (*) with intense neutrophilic infiltration (black arrows) in Group 1 (X: 100 bar 100 in 1st row), re-epithelization (red arrows) and deposition of collagen (*) in treated groups 2–3 in higher magnification (X:400 bar 50 in 2nd and 3rd rows).
On the 14th day post-injury, skin from group 1 showed few squamous and granular epidermal cells with no orthokeratin. In this stage, the inflammatory exudates replaced the necrotic layer showing intense neutrophilic and few macrophages histiocytic infiltrations with few blood vessels. Skin sections of group 2 showed the appearance of a few layers of squamous and granular epidermal cells with parakeratosis. Many macrophages histiocytic infiltration with few blood vessels were noticed. Skin sections from group 3 showed the appearance of several layers of squamous and granular epidermal cells with the presence of orthokeratin many macrophages histiocytic infiltration and few blood vessels. Skin sections from group 4 showed few layers of squamous epidermal cells with parakeratosis, few neutrophilic infiltration, some macrophages histiocytic infiltration and few blood vessels. The appearance of new epithelium was observed in all groups with different thickness. The regenerated “epithelial islands” grow vertically, then migrate towards the surface and cover the wound. Neoformed capillaries are observed and granulation tissues are generated. Group 3 revealed significant extended granulation tissue formation, maturation, tissue organization and re-epithelialization compared to group 1 (Fig. 4) (Table 3).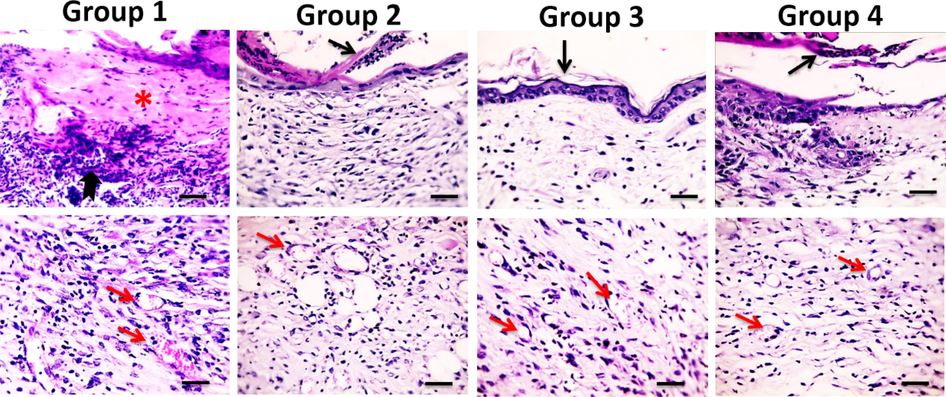
Microscopic pictures of H&E stained skin sections from four groups on the 14th day after burn injury showing the persistence of epidermal necrosis (*) with intense neutrophilic infiltration (black arrows) in Group 1, parakeratosis (black arrows) in Groups 2&4, orthokeratin in (black arrow) in Group 3, neovascularization (red arrows) in all groups (thick blue arrows) X:400 bar 50.
On the 21st day post-burn injury, skin sections from group 1 showed persistence of inflammatory exudate covering few layers of epidermal cells with an excessive amount of granulation tissue and severe inflammatory cells infiltration (mainly lymphocytes and a few macrophages). Skin sections in group 2 showed normal epidermis with moderate amount of granulation tissue with persistent intense inflammatory reaction. Skin sections from group 3 showed complete healing where the regenerated skin seemed to be normal with new skin appendages formation, increased deposition of well-organized collagen bundles and number of fibroblasts infiltrated with very few inflammatory cells. Skin sections in group 4 showed normal epidermis with early maturation of granulation tissue infiltrated with few inflammatory cells (Fig. 5). Histopathological findings showed that the new dermis formation was the best in group 3 followed by group 2 and then group 4 (Table 3).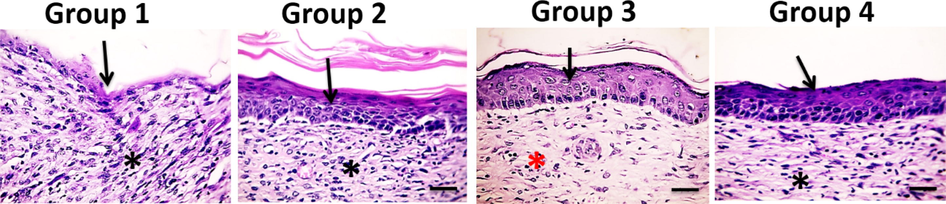
Microscopic pictures of H&E stained skin sections from four groups on the 21st day after burn injury showing few squamous epidermal cells in Group 1, completed new epithelization in Groups 2–4, inflamed granulation tissue in Groups 1, 2&4 (*), well-organized bands of collagen in Group 3 (*) X:400 bar 50.
Sum of the three histopathological component scales of burn wound healing showed that group 3 treated with M. longifolia subsp. typhoides essential oil had good healing followed by group 2 treated with the positive control (silver sulfadiazine) followed by group 4 treated with M. longifolia subsp. schimperi essential oil (Table 4).
Group
Re-epithelialization [0–15]
Extent of granulation tissue [(-3)-18]
New dermis formation [0–15]
Sum of scales [(-3)-58]
Group 1 B
1 ± 0.30
10 ± 0.70
5.4 ± 0.74
16.40 ± 1.28
Group 2 D
7.8 ± 0.50
11 ± 0.54
9.4 ± 0.40
28.2 ± 0.86
Group 3 O1
13.6 ± 0.24
12.8 ± 0.48
10.40 ± 0.24
38 ± 0.70
Group 4 O2
7.4 ± 0.20
10 ± 0.50
7.2 ± 0.37
25.2 ± 0.80
P value
0.0006
Histopathologically, the skin of control group 1 had a large region of ulceration, a low degree of scar development, and various new dermis components. When compared to silver sulfadiazine and untreated groups, the results demonstrated that M. longifolia subsp. typhoides essential oil substantially enhanced various phases of the deep second burn wound and histological components of healing. On day 21, the development of new dermis in group 3 was much higher than in the other groups. The fact that the control groups had a slower rate of wound healing than the other treatments could be due to the presence of bacteria in the wounds or their histopathological abnormalities. (Nasiri et al., 2015).
Wound healing is a complicated process that involves four stages: coagulation, inflammation, debridement, and re-epithelialization. Proliferation, migration, and differentiation of epidermal squamous epithelial cells play critical roles. Collagen deposition and remodelling occur intradermally at the end of the healing phase (Mekonnen et al., 2013).
Essential oil of M. longifolia subsp. typhoides reduced inflammation, therefore it may successfully prevent edoema, erythema, secretion, and other burn sequelae. On day 7 after the burn, all treated groups began re-epithelialization earlier than group 1 on day 14 after the burn. Previous research has suggested that some natural ingredients can speed up the re-epithelialization of a burn wound (Nasiri et al., 2015). On day 8 following burn injury, the re-epithelialization components of Malva sylvestris creams had a good epithelization. However, after 15 days of Hypericum perforatum treatment and 16.5 days of Calendula (as herbal medicine) treatment, re-epithelialization happened (Sayar et al., 2014). re-epithelialization is influenced by the thickness of the granular cell layer, epidermal thickness, squamous cell maturation and organization, and epithelial cell migration (Sayar et al., 2014). Collagen is the most abundant protein in the extracellular matrix produced by fibroblasts, and it gives the dermis its strength and integrity (Pirbalouti and Koohpyeh, 2011; Razavi et al., 2011). M. longifolia subsp. typhoides essential oil can increase the amount of well-organized collagen bundles. Similarly, some Malvacea and Boraginaceae species' anti-inflammatory effects enhanced collagen fibre production, epithelium regeneration, and epithelium thickness (Razavi et al., 2011).
The extent of granulation tissue, re-epithelialization and formation of new dermis, support the idea that topical application of M. longifolia subsp. typhoides essential oil was more effective in the treatment of second-degree burn wounds than standard common burn wound care. The histologically enhanced and accelerated wound healing may be explained by the anti-inflammatory and antimicrobial properties of M. longifolia subsp. typhoides essential oil.
4 Conclusion
This study was devoted to evaluate the effect of two subspecies of wild Egyptian Mentha longifolia essential oils on burn wound healing in mice. The antimicrobial activity of the Mentha sp. oils shown in our results, contributes to the burn wound healing. These findings can be attributed to the chemical composition of the essential oils especially the oxygenated fractions (Habibi et al., 2018; Oumzil et al., 2002; Božović et al., 2015; Shahverdi et al., 2004). However, the healing potential produced by M. longifolia subsp. typhoides oil is more significant when compared to that produced by M. longifolia subsp. schimperi oil. This difference is attributed to the high percentage composition of piperitenone oxide (55.443 %) and piperitone oxide (12.180 %) in M. longifolia subsp. typhoides essential oil, and we suggest them as good candidates for further study in the area of wound healing.
5 Contributorsʼ statement
Abdullah Haikal: Operating the hydrodistillation of plant materials, participated in the identification of the oils components and in the biological part.
Mona El-Neketi: Participated in the identification of the oils components, writing and revision of the manuscript, followed up the biological part and took over the publishing process.
Walaa F. Awadin: Participated in the biological part.
Madiha A. Hassan:Participated in the supervision of the research and revised the manuscript.
Ahmed A. Gohar: Suggested the research point, supervised the progress of the research, wrote and revised the manuscript and took over the publishing process.
Funding
This study did not receive any grant from public, private or non-profit funding agencies.
Acknowledgements
Dr. Soha El-Shaer (Department of Microbiology, Faculty of Pharmacy, Mansoura University) is acknowledged for carrying out the antiquorum–sensing screening assay.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Taxonomical and comparative studies on some wild and cultivated species of genus Mentha in Egypt. J. Appl. Sci. Res.. 2013;9(13):6567-6573.
- [Google Scholar]
- Identification of essential oil components by gas chromatography/mass spectrometry. Vol Vol. 456:. IL: Allured publishing corporation Carol Stream; 2007.
- Aghili, M., 2009. Makhzan-al-Advia. Edited by Rahimi R, Shams Ardekani MR, Farjadmand F. Tehran University of Medical Sciences, 258-259.
- Mentha suaveolens Ehrh. (Lamiaceae) essential oil and its main constituent piperitenone oxide: biological activities and chemistry. Molecules. 2015;20(5):8605-8633.
- [CrossRef] [Google Scholar]
- Bioassays of quorum sensing compounds using Agrobacterium tumefaciens and Chromobacterium violaceum. In: Rumbaugh K., ed. Quorum Sensing. Humana Press; 2011. p. :3-19.
- [CrossRef] [Google Scholar]
- Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC complementary and alternative medicine. 2010;10(1):9.
- [CrossRef] [Google Scholar]
- African Plant Database., 2021. Mentha longifolia (L.) L. http://www.ville-ge.ch/musinfo/bd/cjb/africa/details.php?langue=an&id=117478. On 24/1/2021 3:00 pm.
- De Villiers, M., 2009. Ointment Bases. A Practical Guide to Contemporary Pharmacy Practice, Edition, 3, 277-290.
- Healing effect of sea buckthorn, olive oil, and their mixture on full-thickness burn wounds. Adv. Skin Wound Care. 2014;27(7):317-323.
- [CrossRef] [Google Scholar]
- Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacology. 2014;151(1):317-332.
- [CrossRef] [Google Scholar]
- Synthesis, antimicrobial, antiquorum-sensing, and cytotoxic activities of new series of isoindoline-1, 3-dione, pyrazolo [5, 1-a]-isoindole, and pyridine derivatives. Arch. Pharm.. 2015;348(11):835.
- [CrossRef] [Google Scholar]
- Pharmacological activity of Mentha longifolia and its phytoconstituents. J. Tradit. Chin. Med.. 2017;37(5):710-720.
- [CrossRef] [Google Scholar]
- Topical effect of mentha pulegium essential oil on burn wounds in wistar rats. J. Res. Med. Dental Sci.. 2018;6(6):154-157.
- [Google Scholar]
- Mentha longifolia subsp. typhoides and subsp. schimperi: Antimicrobial and Antiquorum-Sensing Bioactivities. Chem. Nat. Compd.. 2021;57(5):933-938.
- [CrossRef] [Google Scholar]
- Biological activities of the essential oils and methanol extract of tow cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J. Microbiol. Biotechnol.. 2009;25(12):2227-2238.
- [CrossRef] [Google Scholar]
- Constituents of the essential oil of Mentha microphylla C. Koch. Egyptian Journal of Pharmaceutical Sciences. 1990;31(1–4):437-441.
- [Google Scholar]
- Effect of Orem Self-Care program on the life quality of burn patients referred to Ghotb-al-Din-e-Shirazi burn center, Shiraz, Iran: a randomized controlled trial. Int. J. Community Based Nursing Midwifery. 2014;2(1):40.
- [Google Scholar]
- Epidemiology of burns in rural Bangladesh: An update. Int. J. Environ. Res. Public Health. 2017;14(4):381.
- [Google Scholar]
- Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J. Ethnopharmacol.. 2006;108(2):211-216.
- [CrossRef] [Google Scholar]
- Mint: the genus Mentha. CRC Press; 2006. p. :21-28.
- A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods. 2004;58(3):351-360.
- [CrossRef] [Google Scholar]
- In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalancho petitiana A. Rich (Crassulaceae) leaves in mice. J. Ethnopharmacol.. 2013;145:638-646.
- [CrossRef] [Google Scholar]
- In vitro activity of Piperacillini/tazobactam versus other broad antibiotics against nosocomial Gram negative pathogens isolated from burn patients. J. Chemother.. 1998;10:208-214.
- [CrossRef] [Google Scholar]
- Effect of Malva sylvestris cream on burn injury and wounds in rats. Avicenna Journal of Phytomedicine. 2015;5(4):341-354.
- [Google Scholar]
- Isolation of some luteolin derivatives from Mentha longifolia (L.) Hudson subsp. longifolia and determination of their genotoxic potencies. Food Chem.. 2012;135(2):764-769.
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytotherapy Research. 2002;16(8):727-731.
- [CrossRef] [Google Scholar]
- Development of animal model for studying deep second-degree thermal burns. Biomed Res. Int.. 2012;2012
- [CrossRef] [Google Scholar]
- Wound Healing Activity of Extracts of Malva sylvestris and Stachys lavandulifolia. Int. J. Biol.. 2011;3:174-179.
- [CrossRef] [Google Scholar]
- Bioactivity of Malva Sylvestris L., a Medicinal Plant from Iran. Iranian Journal of Basic Medical Sciences. 2011;14:574.
- [Google Scholar]
- Ribatti, D., 2017. Inflammation and Angiogenesis. In: Inflammation and Angiogenesis. Springer, Cham. DOI: https://doi.org/10.1007/978-3-319-68448-2_6.
- Comparison of efficacy of topical phenytoin with hypericin in second-degree burn wound healing: An experimental study in rats. Med. Sci. Monit. Basic Res.. 2014;20:36-46.
- [CrossRef] [Google Scholar]
- Calcium channel blocking activity of Mentha longifolia L. explains its medicinal use in diarrhoea and gut spasm. Phytother. Res.. 2010;24(9):1392-1397.
- [CrossRef] [Google Scholar]
- Piperitone from Mentha longifolia var. chorodictya Rech F. reduces the nitrofurantoin resistance of strains of enterobacteriaceae. Phytotherapy Research. 2004;18(11):911-914.
- [CrossRef] [Google Scholar]
- Achauer and Sood's burn surgery: reconstruction and rehabilitation. PA: Saunders Elsevier Philadelphia; 2006.
- Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol.. 2012;139(2):533-540.
- [CrossRef] [Google Scholar]
- Burn wound: how it differs from other wounds? Indian J. Plastic Surgery. 2012;45(02):364-373.
- [Google Scholar]
- Mentha: an overview of its classification and relationships. In: Press C.R.C., ed. Mint: the Genus Mentha. Boca Rotan; 2007. p. :1-39.
- [Google Scholar]
- The effect of Mentha Pulegium on healing of burn wound injuries in rat. World J. Plastic Surgery. 2019;8(1):43.
- [CrossRef] [Google Scholar]
- A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr.. 1963;11:463-471.
- [CrossRef] [Google Scholar]
- Essential oil composition of Mentha longifolia (L.) L. collected from Garhwal Region of Western-Himalaya. J. Essential Oil Bearing Plants. 2015;18(4):957-966.
- [CrossRef] [Google Scholar]
- World Checklist of Selected Plant Families (WCSP)., 2021. Mentha longifolia (L.) in the plant list. http://www.theplantlist.org/tpl1.1/record/kew-124916.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102356.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







