Translate this page into:
Environmental friendly synthesis of carbon nanoplates supported ZnO nanorods for enhanced degradation of dyes and organic pollutants with visible light driven photocatalytic performance
⁎Corresponding author. malsalhi@ksu.edu.sa (Mohamad S. AlSalhi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nanorods-like ZnO particles was prepared by effective chemical synthesis. Materials were used for photocatalysts and remediation wastewater. Higher photo-stability and photocatalytic performance were noted.
Abstract
Herein, nanorods-like ZnO particles was prepared by effective chemical synthesis process and carbon nanoplates supported ZnO nanorods (ZnO/C) was prepared by wet chemical process. The synthesized materials were characterized by various instrumental analysis via power XRD, UV–vis DRS, FESEM and EDX spectroscopy. The synthesized samples were utilized as competent photocatalysts for the remediation wastewater. The visible light-driven photocatalytic performances of the samples was assessed by the photodegradation of methylene blue. Compared to pure ZnO, the ZnO/C photocatalyst showed an excellent photocatalytic performances by efficiently degrading (around 95%) of MB as targeted organic pollutants within the irradiation time period of 80 min. The improved photodegradation activity of ZnO/C might be due to an improved absorption of ZnO/C photocatalyst in the visible region as well as good structural morphology and surface state which was accredited to an efficient separation of charge carriers and hindered recombination at ZnO/C interfaces. The higher photo-stability and photocatalytic performance was exposed that ZnO/C samples can be a potential photocatalysts for the applications of environmental remediation. Finally, a plausible degradation pathway mechanism for an enhanced activity of ZnO/C has been proposed.
Keywords
Methylene blue
Photocatalysis
Photodegradation
Radicals trapping
Visible light
ZnO/carbon nanoplates
1 Introduction
Nowadays, organic dye contamination is a worldwide environmental issue and is hazardous to human and ecosystem. It is exceptionally important to remove organic pollutants from wastewater before its discharge from the industries such as pigments, paints, textiles, leather and paper into the environment (Theerthagiri et al., 2018). Different chemical, physical and biological methodologies including filtration, biodegradation, adsorption and electrochemical process are employed to remove the organic pollutants from the wastewater. Among the various methodologies, semiconductor based photocatalysis is the widely investigated and cost-effective methods for the degradation of organic pollutants by changing over them into less toxic molecules in the contaminated water (Michael et al., 2015). The investigation of semiconductor based materials is at the vanguard of researcher’s consideration in view of their potential applications. Predominantly II-VI semiconductor oxides, for example, ZnO-based materials have extraordinary properties like a broad band gap energy (∼3.37 eV), good chemical stability, good structural morphologies, less toxic and high surface area (Wu and Wang, 2019). Though, ZnO has some limitations because it experiences quick recombination and photocorrosion under UV light illumination and an acidic medium (Chen et al., 2017). In order to overcome these limitations of ZnO materials, some noteworthy attempts have been made such as adding of dopants/supporting materials, mixing with other semiconductor materials to produce a composites and all these attempts for intending to enhance the photocatalytic performance, stability and to enlarge the absorption in the more visible region (Mishra et al., 2018). Also, the good structural morphology of the ZnO photocatalysts such as nanoflakes, nanosphers, nanowires, flowers-like structure and nanorods are most favoured to progress the photocatalytic performances towards the removal organic pollutants. To the extent morphological points of view of ZnO are concerned, the preparation and manufacture route are likewise assumes an important part. The various parameters utilized for example, reaction temperature, surfactant and duration of the reaction, etc., are play a fundamental competence in the development of different kinds of structural morphology of the ZnO materials (Rahimi et al., 2019).
Recently, Chauhan et al. (2019) fabricated ZnO/GO nanoflowers on Si substrate and employed for the photodegradation of MB. The composite of ZnO/GO exhibited an enhanced activity than the pure ZnO, which might be due to the hindrance of recombining of electron-holes pairs. Furthermore, GO supply an excess of electrons to upgrading the photocatalytic degradation of MB by creating an excess of H+ radicals. In this investigation, ZnO nanorods was prepared by simple chemical process and carbon nanoplates supported ZnO photocatalysts by wet impregnation method. Carbon nanoplates chosen as supporting materials because of excellent properties such as flexibility, low-cost, good electrical conductivity and can easily coupled with other materials. Herein, carbon nanoflakes may simply play the work of a support to the ZnO photocatalysts. Carbon materials themselves have insignificant or no photocatalytic performance while as supporting materials they improve the photodegradation of the ZnO nanorods. The degradation activity of the ZnO/C composite was analysed for the photodegradation of MB under visible light. Also, the systematic experiments for radical trapping are carry out to trap the active species for the photodegradation of MB. Finally, a possible degradation mechanism of MB dye over ZnO/C photocatalyst has been discussed.
2 Experimental section
2.1 Materials
ZnCl2 and ethanol were provided by SDFCL, India. Carbon nanoplate was purchased from Cabot Corporation, USA. C6H15NO3 (TEOA), benzoquinone, isopropyl alcohol and MB dye were purchased from Sigma-Aldrich.
2.2 Preparation of carbon nanoplates supported ZnO nanorods
ZnO nanorods were prepared by the adopted and slight modified previous procedure (Shimpi et al., 2016). ZnO/C photocatalyst was produced by simple wet impregnation process. For the preparation, calculated amount of carbon nanoplates (3 wt% with respect to ZnO) and as-prepared ZnO nanorods was blended well into 30 mL of ethanol. Then, the blended material was kept for ultra-sonication for 30 min. Further, the reaction sample was stirred and subsequently the ethanol was evaporated at 60 °C. The final product of the attained ZnO/C after evaopration of ethanol was dried at 100 °C for 3 h.
2.3 Photocatalytic degradation
The photocatalytic degradation perofrmances of ZnO and ZnO/C samples were measured by the degradation of MB under 500 W Hg visible lamp illumination. The prepared samples were taken into 100 mL of aqueous MB solution (25 mg/L) in a 500 mL photocatalytic reactor. The suspension was magnetically stirred 15 min and then kept in a dark for 60 min. Then, the dye dispersions were subjected in to irradiation of visible light with continuous stirring. 2 mL of samples was extracted at 10 min of regular intervals followed by centrifugation prior to UV–vis analysis for reaction monitoring. After completion of the degradation process, prepared photoatalyst was taken for reuse by centrifugation. The as prepared catalysts were used for 3 cycles successively to completely examine the reusability of the catalysts and their applications in potential water treatment applications.
2.4 Radical trapping experiment
The trapping of radical experiments was carried out in order to find the possible photodegradation mechanism of the photocatalysis. For this scavenging activity OH• and O2•– radicals are trapped by TEOA, IPA and BQ separately. The trapping investigations were carried out with the addition of different scavenger in to the photocatalytic reaction. The catalysts was taken from the photo-reactor to measure their absorption spectra.
2.5 Insrumental charctaerization
The crystalline phase and structure of the synthesized pure ZnO and ZnO/C photocatalyst was examined using X-ray diffraction (XRD) instrument using Rigaku, Mini Flex II, Japan model with Cu-Kα (λ = 0.154 nm) radiations. UV–vis DRS spectra of the prepared materials were investigated via Shimadzu 2100 model spectrophotometer. Surface morphological image and elemental compositional study was investigated using FESEM (Hitachi, S-4800 model) equipped with EDX spectrometer (Bruker Nano GmbH, model 5010).
3 Results and discussion
3.1 XRD studies
The purity and formation of the prepared samples was examined via XRD and the observed results are depicted in Fig. 1. In case of pure ZnO (Fig. 1a), the diffraction peaks at 2θ = 32.0°, 34.67°, 36.57°, 47.77°, 56.83°, 63.13°, 66.58° and 68.26° are in well matched with the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) and (2 0 1) are related to the hexagonal crystalline structure of ZnO (JCPDS No. 13-1397). The XRD patterns of ZnO/C photocatalyst is displayed in Fig. 1b, which showing that the diffraction patterns of pristine ZnO was slightly shifted and reduced the peak intensity because of an addition of carbon nanoplates. The crystalline size of the samples was calculated using Scherrer formula (Theerthagiri et al., 2016). The estimated crystallite size of the pristine ZnO and ZnO/C sample was about 41.09, and 33.40 nm, respectively. The carbon nanoplates supported ZnO nanorods shows the smaller crystalline size than that of pure ZnO, which may expected to reveal an enhanced photocatalytic performances.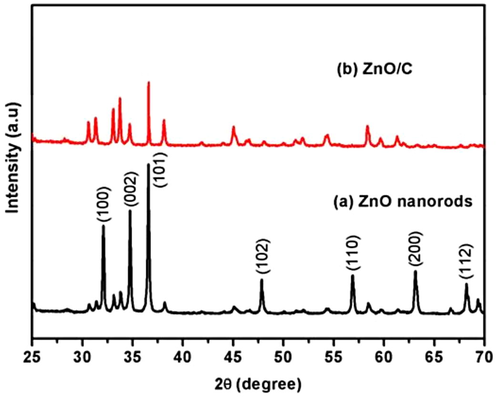
XRD diffraction patterns of (a) ZnO and (b) ZnO/C photocatalysts.
3.2 Optical absorption properties
The absorption behaviour of the synthesized ZnO and ZnO/C catalysts was studied by UV–vis DRS (Fig. 2a). It was noted that the edge of absorption for ZnO/C sample was marginally expanded to higher visible region upon the addition of carbon nanoplates. The improved absorption of ZnO/C sample in the visible region might be a result of bringing down the recombination rate between the photo generated electrons and holes (Malathi et al., 2017). Fig. 2b demonstrates the tauc plot, which gives the band energy (Eg) of the synthesized photocatalyst. The examined Eg value for the ZnO and ZnO/C photocatalysts are 3.22 eV and 2.76 eV. The Eg values of the prepared photocatalysts exposed that the ZnO/C gained a good activity in the visible light. As a consequence of optical absorption properties, it can be likely that the ZnO/C photocatalyst demonstrate a good catalytic activity under visible light.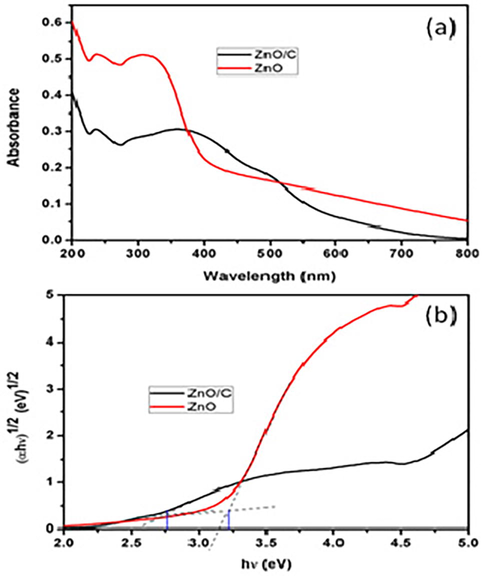
(a) UV–vis DRS spectra, and (b) the plot of (αhν)1/2 versus hν of ZnO and ZnO/C photocatalysts.
3.3 Surface morphology and elemental studies
The FESEM image of the pure ZnO, ZnO/C and carbon nanoplates are displayed in Fig. 3. The bar ZnO (Fig. 3a) showed the nanorods-like surface, whereas the carbon particles (Fig. 3c) exhibited a nanoplates shape. The surface morphology of the carbon nanoplates supported ZnO nanorods clearly illustrated that the ZnO and carbon materials were uniformly distributed (Fig. 3b). The good structural morphology and surface state of the photocatalysts may play an imperative role in inducing the catalytic performances (Liu et al., 2010). In view of this, the formation of nanorods-like surface morphology of ZnO particles and carbon nanoplates are favourable for the separation of charge carriers, which can expected to be a potential materials for photocatalytic degradation activity.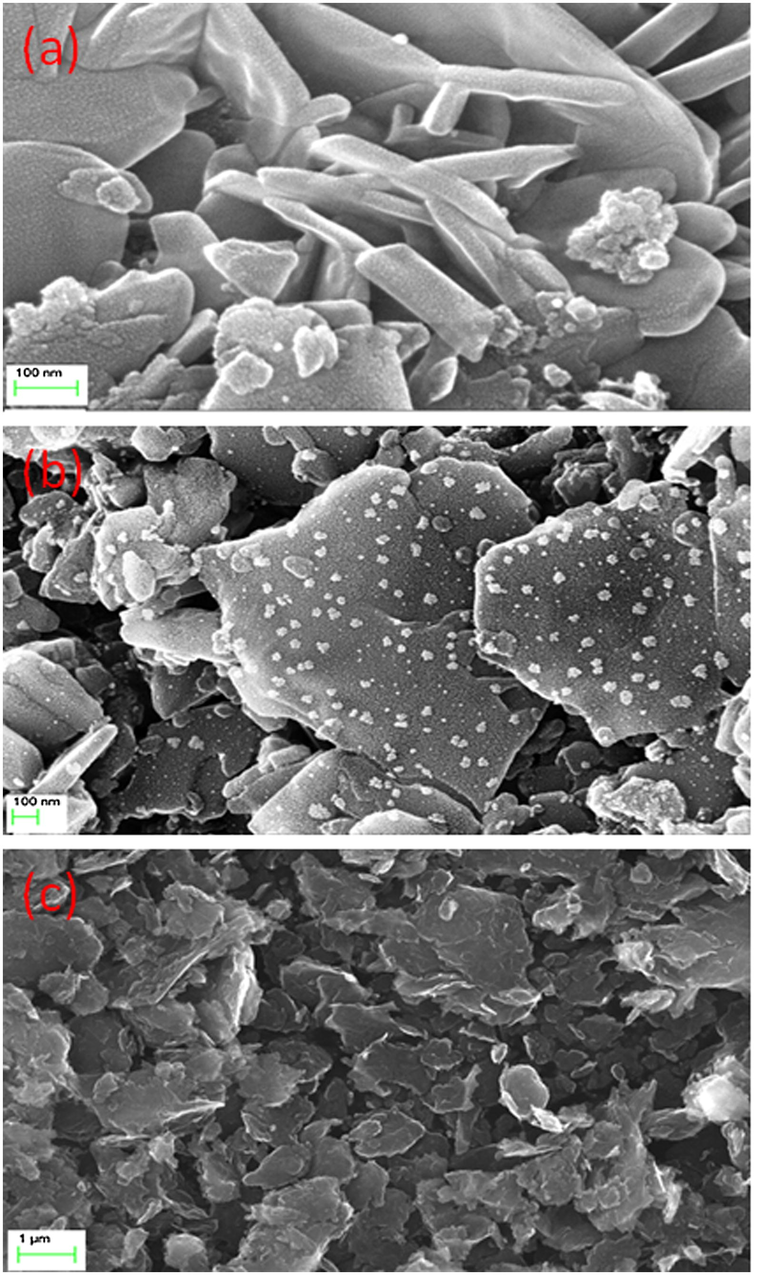
FESEM images of (a) pure ZnO, (b) ZnO/C and (c) carbon nanoplates.
The elemental composition studies of the ZnO and ZnO/C were find out using EDS and the accomplished spectra are depicted in Fig. 4(a, b). Fig. 4a showed the prepared ZnO was composed of Zn and O atoms, whereas Fig. 4b confirms the presence of zinc, oxygen and carbon atoms in ZnO/C sample. Furthermore, the elemental mapping of ZnO/C was illustrated in Fig. 4c which authenticated the coexistence of all elements of the ZnO/C sample.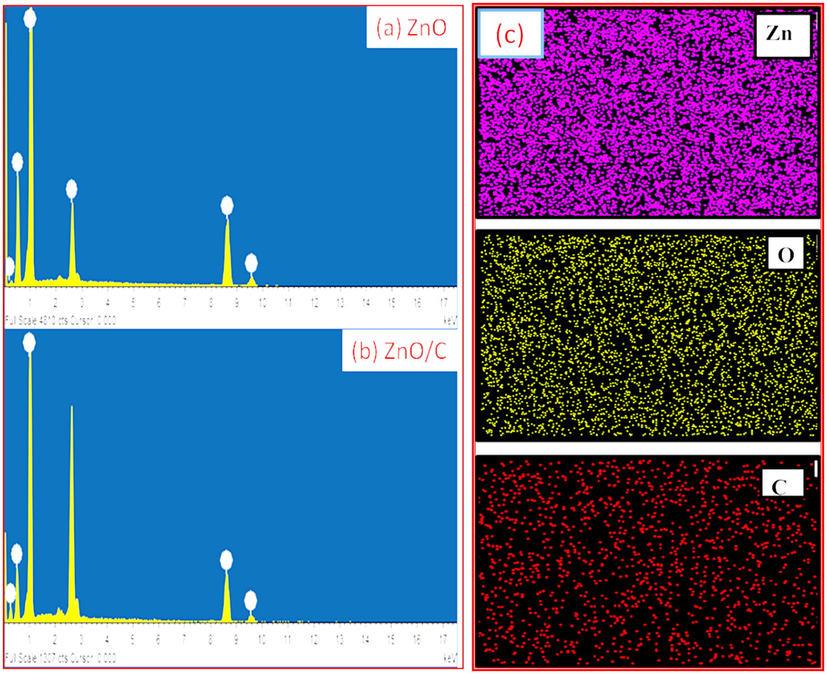
(a, b) EDS spectrum of ZnO and ZnO/C photocatalysts, and (c) elemental mapping showing the existence of Zn, O and C in ZnO/C sample.
3.4 Photocatalytic dye degradation
To evaluate the photocatalytic ability of the as-prepared materials were analysed the photodegradation of MB dye under visible-light. Fig. 5(a) and (b) represent the photodegradation absorption spectra of MB before and after a series of illumination with visible light whose degradation was measured at the characteristic wavelength around 664 nm by using UV–vis spectrophotometer. Under the visible light irradiation, self-degradation of the samples were also investigated, without any catalyst minute decrease observed in the dye concentration which may be due to the effect of light producing some thermal degradation in the aqueous medium. After the addition of photocatalysts, the concentration of MB dye decreased which because of the degradation. Fig. 5c shows the rate of photodegradation efficiency of photocatalysts towards the degradation of MB dyes. The photodegradation kinetics of the as-prepared samples was evaluated by pseudo-first order reaction kinetic and the rate constant (k) calculated to be 0.9945 and 0.9925 for ZnO and ZnO/C (Fig. 5(d)). The ZnO/C heterojunction formation was exhibit much more effective photocatalytic degradation than the pure ZnO nanorods.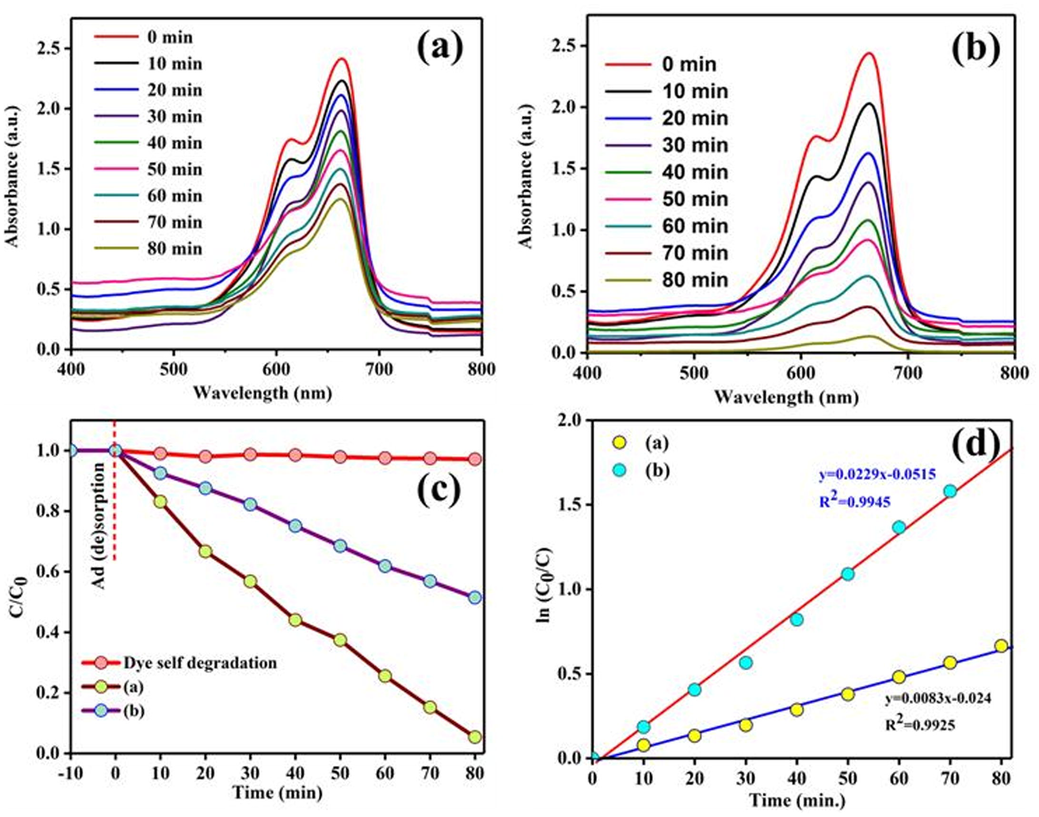
Photodegradation of MB dye over (a) Pure ZnO nanorods (b) ZnO/C hybrid (c) Rate of photodegradation, and (d) Pseudo-first-order kinetics linear simulation curves of MB over the catalysts (a = ZnO; b = ZnO/C).
The recyclability of the ZnO/C was analysed through the photodegradation of MB for the catalyst was recycled 3 times (Fig. 6). Consequently, the photodegradation efficiency of the ZnO/C photocatalyst for the 3 cycling reuse were 95.01, 91.7 and 84.2 after 80 min, respectively. The photodegradation show the minor deterioration in the reutilize experiments it may due to the minute loss and the deactivation of the photocatalyst in the experiment, finally the results show the high stability and durability of the as prepared sample.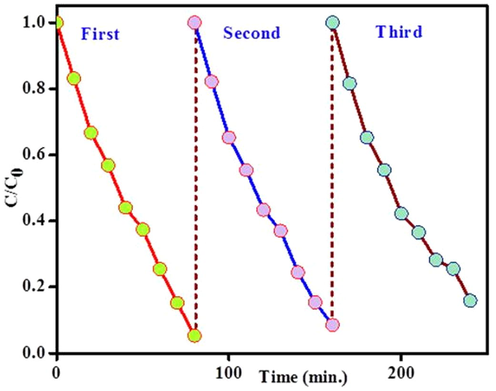
Cyclic runs in the photocatalytic degradation of MB using the ZnO/C under visible light irradiation.
3.5 Radical trapping
Holes, hydroxyl radicals and superoxide anions are possible active species in photodegradation of aqueous organic pollutants. To elucidate the catalytic process, active species produced during the reaction were confirmed by radical and scavenging of hole measurements. The photocatalytic dye degradation reaction starts with the initiation of prepared photocatalyst by radiation with energy higher (or) equal that of the band energy of photocatalyst. The dye with the scavenger of OH• and O2•– radicals exhibited certain degradation, but no excessive change in the absorption peak position. This result indicates that h+ active species has prominent role in this reaction. The h+ radicals were formed by visible light irradiation, and play significant roles in dye degradation process (Park et al., 2014). The addition of the scavengers for h+ was dramatically suppressed the photocatalytic reaction (Fig. 7). From the above investigation, it was concluded that hole (h+) radical show main role in the photocatlytic degradation MB.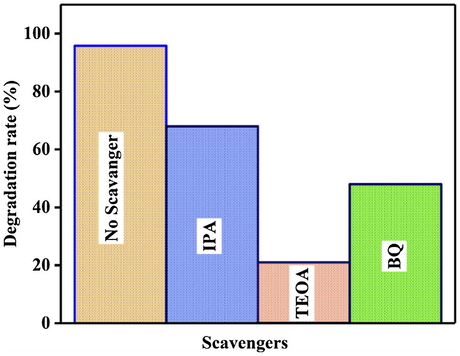
Effect of different radicals on the photocatalytic degradation of MB dye under visible light irradiation.
3.6 Photodegradation reaction mechanism
A possible photodegradation reaction mechanism for MB degradation over ZnO/C under visible light was represented in Fig. 8. Upon visible light irradiation, e− from the VB of ZnO was excited to CB which generates h+ in the VB and thus creating e−−h+ pairs. In the absence of carbon nanoflakes, only a little bit of e− and h+ took part in the photodegradation reaction because of their high recombination rate in pure ZnO which resulting in a low photodegradation performance. The carbon nanoflakes supported ZnO nanorods may sustain a proficient photo-generated electron transfer from ZnO to carbon matrix. The rate of recombination among the e−−h+ pairs on the ZnO surfaces in ZnO/C photocatalyst was effectively decreased. The e− positioned at CB might respond with O2 to generate O2•–, whereas the h+ located in VB might react with OH− to generate OH•. MB dye molecules as a targeted organic pollutant might be oxidized into CO2, H2O etc., by the O2•– and OH• radicals (Zhao et al., 2017).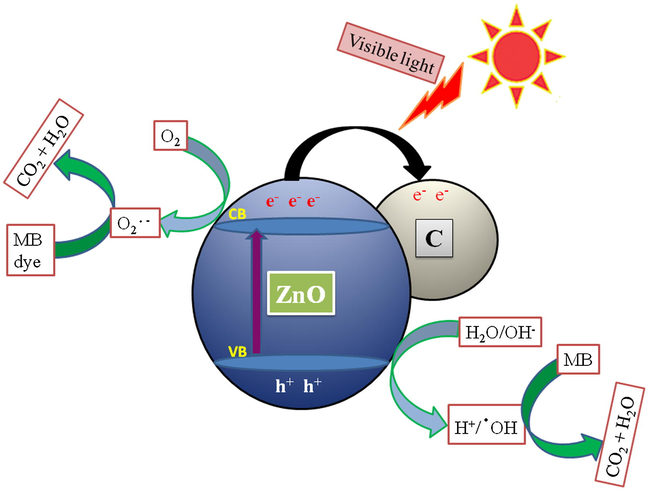
A possible photodegradation reaction mechanism for MB degradation over ZnO/C catalysts.
4 Conclusions
In conclusion, nanorods shaped ZnO was prepared by chemical process and carbon nanoplates supported ZnO nanorods was obtained by wet chemical route. The phase, structural morphology elemental composition of the prepared samples was analysed by XRD, FESEM, and EDS analysis. The optical absorption properties was obtained using UV–vis-DRS studies. The synthesized samples were utilized as competent catalysts for MB degradation as targeted organic pollutants. The visible light-driven photocatalytic degradation results illustrated that ZnO/C sample exhibited an improved photocatalytic efficiency. The ZnO/C sample was demonstrated 2 times higher photocatalytic degradation rates than the pristine ZnO sample towards the photodegradation of MB. The improved photodegradation activity of ZnO/C might be due to an improved absorption of ZnO/C photocatalyst in the visible region as well as good structural morphology and surface state and hindered recombination at ZnO/C interfaces. Furthermore, the ZnO/C photocatalyst showed a good reusability and photo-stability as observed from the experimental results. In this way, the ZnO/C samples are potential photocatalysts and provided an important insight of knowledge for building up effective novel visible light driven catalytic materials.
Acknowledgement
The authors are grateful to the Researchers Supporting Project Number (RSP-2019/68), King Saud University, Riyadh, Saudi Arabia.
References
- Facile synthesis of ZnO/GO nanoflowers over Si substrate for improved photocatalytic decolorization of MB dye and industrial wastewater under solar irradiation. Mater. Sci. Semicond. Process.. 2019;89:6-17.
- [Google Scholar]
- One-pot synthesis of ZnO/oligoaniline nanocomposites with improved removal of organic dyes in water: Effect of adsorption on photocatalytic degradation. Mater. Res. Bull.. 2017;95:459-467.
- [Google Scholar]
- Titania-based photocatalysts-crystal growth, doping and heterostructuring. J. Mater. Chem.. 2010;20:831-843.
- [Google Scholar]
- A robust visible-light driven BiFeWO6/BiOI nanohybrid with efficient photocatalytic and photoelectrochemical performance. Appl. Surf. Sci.. 2017;412:85-95.
- [Google Scholar]
- Cu2S-incorporated ZnS nanocomposites for photocatalytic hydrogen evolution. RSC Adv.. 2015;5:30175-30186.
- [Google Scholar]
- Room temperature synthesis of urea based imidazole functionalised ZnO nanorods and their photocatalytic application. Mater. Res. Bull.. 2018;102:311-318.
- [Google Scholar]
- Adsorption and UV/Visible photocatalytic performance of BiOI for methyl orange, Rhodamine B and methylene blue: Ag and Ti-loading effects. CrystEngComm. 2014;16:3155-3167.
- [Google Scholar]
- Enhancement of sunlight-induced photocatalytic activity of ZnO nanorods by few-layer MoS2 nanosheets. Mater. Lett.. 2019;234:134-137.
- [Google Scholar]
- Synthesis of ZnO nanopencils using wet chemical method and its investigation as LPG sensor. Appl. Surf. Sci.. 2016;390:17-24.
- [Google Scholar]
- Synthesis and characterization of (Ni1−xCox)Se2 based ternary selenides as electrocatalyst for triiodide reduction in dye-sensitized solar cells. J. Solid State Chem.. 2016;238:113-120.
- [Google Scholar]
- Recent developments of metal oxide based heterostructures for photocatalytic applications towards environmental remediation. J. Solid State Chem.. 2018;267:35-52.
- [Google Scholar]
- Graphene oxide (GO) doping hexagonal flower-like ZnO as potential enhancer of photocatalytic ability. Mater. Lett.. 2019;234:287-290.
- [Google Scholar]
- Synthesis of fireworks-shaped ZnO/graphite-like carbon nanowires with enhanced visible-light photocatalytic activity and anti-photocorrosion. Colloids Surf. A. 2017;518:57-63.
- [Google Scholar]







