Translate this page into:
Empirical modeling of different viscosity and density behavior of biodiesel from chichá (Sterculia striata) with diesel versus temperature variation
⁎Corresponding author. rafaela.gabriel@ctec.ufal.br (R. Gabriel)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Currently, biodiesel is marketed with mineral diesel constituting blends of biodiesel in accordance with the National Program for the Production and Use of Biodiesel (Law 11,097/05) but depending on the composition of biodiesel/diesel blends, there will be the physico-chemical properties of the mixtures. Among the properties, the density and kinematic viscosity very significantly with the changes in the compositions of the mixtures and therefore deserve to be high lighted, besides representing two important parameters monitored by National Petroleum, Natural Gas and Biofuel Agency (ANP) resolutions. In this article, the biodiesel produced from chichá oil was mixed with diesel in different proportions where the viscosities and densities of the blends were analyzed at different temperatures. With the values obtained, a study of the influence of these variables was carried out and a model of a surface was proposed to adjust the curves that varied as a function of the biodiesel concentration and the temperature. The model was validated with an unused curve during its construction and showed good ability to represent the relationship between the variables. Experimental data showed that the density and viscosity of the mixture increase with increasing percentage of biodiesel in the mixtures and decreased with increasing temperature for all biodiesel mixtures studied.
Keywords
Biodiesel
Chichá
Modeling
Viscosity
Density
1 Introduction
The depletion of fossil fuel resources, the changes caused by local environmental degradation, associated with the production and high consumption of fossil fuels are among the most significant challenges facing the world. In anticipation of reducing the environmental impact caused by the emission of gaseous pollutants from fossil fuels, research has been stimulated to enable the use of alternative and economically viable sources of energy (Silitonga et al., 2017). Biodiesel is the most promising alternative for total or partial substitution of petroleum-based diesel because of its environmental benefits (Chaves et al., 2004; Lei et al., 2016).
Conventionally, biodiesel is produced from the transesterification of oils and fats (Silitonga et al., 2016). However, excessive use of edible vegetable oils for the production of biodiesel has not proved to be a viable solution in the long term, because this approach establishes a scenario of food insecurity causing fluctuations in plant prices, which in the last 10 years has increased significantly, thus affecting the viability of the biodiesel industry (Gülüm and Bilgin, 2016; Milano et al., 2018).
In this sense, chichá seed oil (Sterculia striata) appears as a viable triglyceride source that presents a strong potential for the production of biodiesel, the oil extracted from the seed have high concentrations of triglycerides derived from oestrical and malarial fatty acids whose structures have a cyclopropene ring (Mangas et al., 2012). On the use of chichá oil applied to biodiesel production, there are few studies that address this theme and these few are still in the exploration phase of choosing the best reactive process, such as the works developed by (Chaves et al., 2004; Filho et al., 2015).
In compliance with the legislation, diesel/biodiesel blends are currently being used in order to reduce pollutant emissions. Currently, the 8% blend (B8) with projections is used so that by March 2019 this percentage reaches 10% (Souza et al., 2013). Kozak et al. (2013) point out that due to the mixture, several changes in the properties of the fuels can be perceived, such as: viscosity, pour point, fog point, density, cetane number, among others. In this sense, kinematic viscosity and density are properties that deserve attention because they vary significantly with changes in mix compositions (Ramírez-verduzco et al., 2012).
The quality of biodiesel is verified through the determination of various parameters imposed by the standards, such as the American Society for Testing and Materials (ASTM) and the European Committee for Standardization (CEN) known as EN 14214. In Brazil, the National Agency of Gas, Petroleum and Biofuels (NPA), through Resolution N°. 45, of 08.25.2014, regulates the specifications of biodiesel for the quality control to be attended by the agents that commercialize the product throughout the national territory (Lobô et al., 2009; Mangas et al., 2012; Shimamoto and Tubino, 2016).
In order to guarantee reliability in the use of biodiesel/diesel mixtures, technical and scientific research in the area of simulation and optimization of processes has been adopted internationally for the development of prediction models that guarantee a rapid estimation of the parameters and through the analysis of the generated information , it is possible to guarantee greater robustness in the decision by raising the biodiesel content (Caires et al., 2012; Kozak et al., 2013; Silva et al., 2015; Zarska et al., 2014). This approach allows the assessment of whether the fuel resulting from the blending process meets the standards and specifications for diesel-powered fuels without compromising the use of the equipment and financial resources to calculate these properties directly (Fasina et al., 2006; Prieto et al., 2015).
The models of prediction of parameters are widely diffused and adopted in many research works, such as the works of Benjumea and Agudelo, 2008; Fasina et al., 2006; Geacai et al., 2015; Gülüm and Bilgin, 2018, 2016; Ramírez-verduzco et al., 2012, 2011; Tesfa et al., 2010 and Yuan et al., 2005 among others.
However, there is as yet no application of these studies to biodiesel produced from chichá oil which is relevant since the properties of biodiesel differ from one sample to another due to the varied composition of esters determined by the type of raw material (Geacai et al., 2015).
In this work, the kinematic viscosity and density data were modeled from empirical correlations to predict the fundamental properties of methyl and ethyl biodiesel produced from chichá oil, diesel and biodiesel/diesel blends: B0, B10, B20, B40, B60, B80 and B100. With the values of viscosity/density as a function of biodiesel concentration and temperature, a study of the influence of these variables was carried out and a model of a surface was proposed to adjust the obtained curves.
2 Methods
2.1 Characterization of chichá oil
-
-
Kinematic viscosity, determined on an Ostwald viscometer, according to ASTM D445;
-
-
Density, determined in an Anton Paar digital densimeter, model DMA 35n, according to ASTM D4052;
-
-
Iodine content, determined by the method of Wijs according to the procedure described in (IAL, 2011);
-
-
Acid value, determined according to the official AOCS method (Cd 3d-63);
-
-
Peroxide content, determined according to the procedure described in (IAL, 2011).
2.2 Production of chichá biodiesel
The biodiesel used was produced in the Laboratory of Separation and Process Optimization Systems (LASSOP) of the Federal University of Alagoas, from the reaction of transesterification of chichá oil, in a pilot unit composed of a jacketed reactor, a mechanical agitator and a thermostatic bath. The reaction was carried out with the following parameters: molar ratio of oil/methyl and ethyl alcohol of 1:10, amount of catalyst of 1.0% in relation to the oil mass, reaction time of 30 min and temperature of 70° C (Filho et al., 2015). In total two transesterifications were performed, one using ethyl alcohol and the other with methyl alcohol. After the transesterifications, the biodieses were purified with an acidic solution of hydrochloric acid having a pH of 2 and distilled water until the pH was equal. Dry magnesium sulfate P.A. was used to remove moisture from the biodiesel.
2.3 Characterization of methyl and ethyl chichá biodiesel
The physicochemical analyzes performed were acidity index, iodine index, density and viscosity for chichá biodiesel, using the same methodology already described in Section 2.1. In addition, assays were carried out to determine the conversion to the esters according to the methodology of Peiter et al. (2020).
2.3.1 Preparation of biodiesel/diesel blends
After the process of production and purification of the biodiesel of methyl and ethyl chichá, the blends of biodiesel and diesel S10 pure were realized, for the pre-established concentrations: B0, B10, B15, B20, B40, B40, B60, B80 and B100 in v/v as indicated by the ANP. The volumes were prepared in duplicate and collected in 10 mL beakers.
2.3.2 Determination of physical-chemical properties biodiesel/diesel mixtures
The samples composed of biodiesel, S10 diesel and the blends were analyzed according to the density and viscosity procedures indicated by NPA Resolution N° 45 of 08.25.2014 – DOU 07.26.2014 and NPA Resolution N° 30, of June 23, 2016 – DOU 06.24.2016 which assess the quality of biodiesel and biodiesel/diesel blends respectively, setting the maximum limits allowed by the legislation. The samples were analyzed on two aspects: variation of the biodiesel fraction in the mixture and temperature of the analysis.
2.3.3 Density analysis for biodiesel/diesel blends
The physico-chemical density analyzes were performed as described in Section 2.1 with the samples of diesel S10, biodiesel of methyl and ethyl chichá and their mixtures, in pre-established proportions. temperature curves were constructed with the biodiesel/diesel blend, the following temperatures were used: 283.15, 288.15, 293.15, 298.15, 303.15, 308.15 and 313.15 K.
2.3.4 Kinematic viscosity analysis for biodiesel/diesel blends
Physical and chemical analyzes of kinematic viscosity were performed as described in Section 2.1 with mixtures of chichá methyl and ethyl biodiesel with capillary used diesel of 100 cst.s−1 with manufacturer's constant of 0.0161 cst and temperature curves were constructed with the biodiesel/diesel blend, the following temperatures were used: 293.15, 298.15, 303.15, 313.15, 323.15, 333.15, 343.15 and 353.15 K.
3 3 Results and discussions
3.1 3.1 Physical-chemical analyzes of chichá oil
The Table 1 shows the results of the characterizations of the chichá oil sample. Fonte: *Chaves et al. (2004) **Filho et al. (2015).
Properties
Results
Literature
Oil content (%)
66 ± 0.01
–
Acidity level (mg KOH.g−1)
0.71 ± 0.00
0.81 ± 0.01*
Iodine index (g.100 g−1)
45.26 ± 0.41
66.30 ± 1.3*
Purity Index
0 ± 0.00
_
Kinematic viscosity (mm2.s−1)
63.04 ± 0.07
53.66 ± 0.04**
Density (kg.m−3)
927.50 ± 0.00
924 ± 0.001**
Analysis of the acidity index served as the basis of choice for the biodiesel production process, as chichá oil had low acidity, about 0.71 mg KOH.g−1 (<1.0 mg KOH.g−1), step neutralization was eliminated and only the transesterification step was used to convert the oil to the biodiesel esters. The iodine content found for the sample was 45 g iodine.100 g, presenting a value below those available in the specific literature, but still beneficial since the iodine index is used with a parameter to predict the degree of unsaturation of the sample serving as indicative of tendency to oxidation of vegetable oils. Peroxides were not detected in chichá oil. The presence of peroxides is not desirable in oils and fats, since it presupposes degradative processes and the values found for viscosity and density are in the range of the values quoted in the literature.
3.2 Physical-chemical analyzes and characterization of chichá biodiesel
For the chichá methyl and ethyl biodiesel, the physical-chemical characterizations were performed according to the ANP Resolution N° 45 of August 25, 2014 as shown in Table 2.
Properties
Chichá methyl biodiesel
Chichá ethyl biodiesel
ASTM D*
EN****
Acidity level (mg KOH.g−1)
0.25 ± 0.02
0.19 ± 0.02
0.50
0.50
Iodine index, max (g.100 g−1)
47.57 ± 0.13
45.23 ± 0.27
–
120
Density (kg.m−3)
888.5 ± 0.00
883.8 ± 0.00
850 a 900**
860 a 900***
Kinematic viscosity (40 °C) (mm2.s−1)
6.16 ± 0.00
6.21 ± 0.03
3.0 a 6.0
3.50 a 5
Ester content, min (% mass)
97.55 ± 1.15
99 ± 1.00
96.5
96.5
The results of the characterizations of chichá biodiesel were satisfactory, the only parameter that did not meet the criteria of the regulatory standards ASTM D and EN 14214 was the kinematic viscosity that was slightly above the allowed, but still showed that the process of biodiesel production by transesterification is feasible, one that this process was able to significantly reduce the initial oil viscosity of 63.04–6.16 mm2.s−1. In addition, the chromatographic analysis revealed that in a single step and with little reaction time it was possible to reach the quality specifications regarding the ester content established by NPA Resolution N° 45, dated 08.25.2014.
3.3 Empirical modeling
A model with empirical correlations was proposed to relate the experimental data obtained from the tests performed for kinematic viscosity and density of biodiesel blends and diesel blends using Matlab software (R2011a). The curves were plotted and the surfaces of the model were analyzed by means of polynomial adjustments, considering the values of the coefficient of determination, R2.
3.3.1 Empirical equations for predicting kinematic viscosity
The behavior of the kinematic viscosity as a function of each of the variables (temperature and composition of the mixture) was verified using quadratic polynomials. The kinematic viscosity data were plotted as a function of the volumetric fraction of the biodiesel in the BX mixtures collected in Tables S.1 and S.2. The correlations of the mixtures models were expressed through Eqs. (1) and (2), which represent the dependence of the kinematic viscosity with the temperature and with the volumetric fraction of the biodiesel of chichá methyl and ethyl, respectively. The generated model does not contain kinematic viscosity values for T = 313.15 K because they were used to carry out the validation.
The experimental data were adjusted by a grade 3 polynomial for temperature and grade 2 for the composition which provided values of R2 = 0.9989 for mixtures of methyl biodiesel and R2 = 0.9985 for ethyl biodiesel. Alternatively, smaller polynomial adjustments were tested, however, the errors associated to the experimental value and predicted by the generated models were significantly increased. The relative errors associated with the predicted experimental against the result can be found in Table S.3. Thus, the relation between the composition and the kinematic viscosity and the relation between the temperature and the kinematic, quadratic and cubic viscosity, respectively, were considered.
Table 3 shows the values of the curve fitting coefficients and the coefficient of determination (R2) for the samples studied.
Coefficients
Methyl chichá biodiesel blends
Ethyl chichá biodiesel blends
A
576.5
674.6
B
1.529
1.437
C
-5.117
-6.006
D
0.001604
0.001808
E
-0.009041
-0.008538
F
0.01526
0.01794
G
-0.000004476
-0.00000499
H
0.00001344
0.00001275
I
0.00001524
0.00001793
R2
0.9989
0.9985
Figs. 1 and 2 show the kinematic viscosity curve adjustments as a function of the volume fraction of B100 present in the blend for B0, B10, B20, B40, B60 and B80 in the temperature range of 293.15–353.15 K.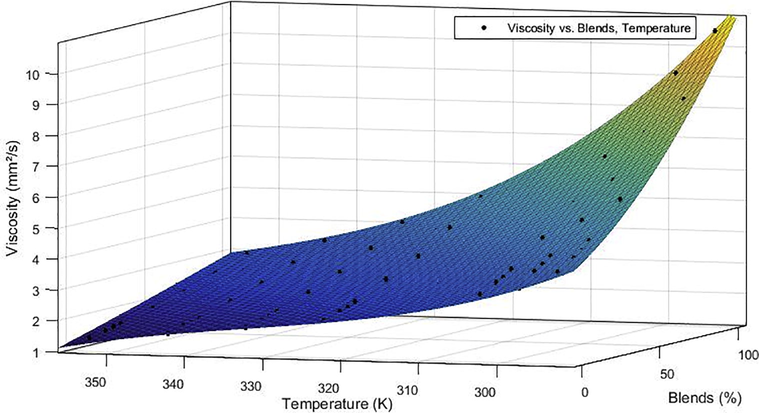
Curve adjustment for the kinematic viscosity of chichá/diesel biodiesel blends by the methyl route.
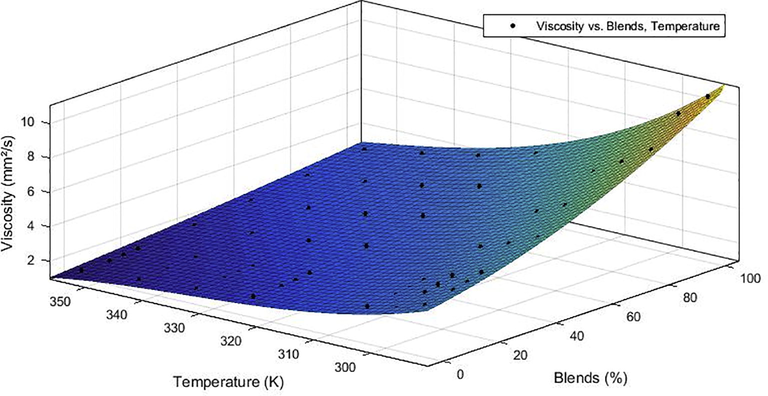
Curve adjustment for the kinematic viscosity of chichá/diesel biodiesel blends by the ethanol route.
The analysis of Figs. 1 and 2 shows that the experimental data showed reasonable agreement with the predicted trends, and that the kinematic viscosity-temperature relation for the tested fuels shows a tendency: the mixtures of both fuels presented decreasing behavior with increasing temperature. The analysis of the kinematic viscosity-composition ratio shows that for a fixed temperature, the increase in biodiesel concentration raises the kinematic viscosity of the mixture, thus evidencing the effect of the addition of biodiesel to the diesel. In general, kinematic viscosity decreases with increasing temperature and increases with the fraction of biodiesel in the biodiesel/diesel blend, which can harm diesel powered engines.
The curvature surface models are practically identical for ethyl and methyl mixtures, this behavior can be attributed to the fact that they are produced from the same source of triglycerides and the difference between the two fuels is only in the type of alcohol used (methyl or ethyl). Therefore, because of this characteristic, abrupt variation would not be justified since the properties of biodiesel are associated with the amounts of each fatty acid present in the oil and the transesterification alone does not alter the fatty acid in the composition of the raw material.
According to Mejía et al. (2013) structural characteristics are directly responsible for causing changes in the physical properties of the fuel, chain length, unsaturation and chain branching being the most notable characteristics. As regards kinematic viscosity, the number of double bonds and the chain length are the most relevant structural features. Data reported in the literature indicate that kinematic viscosity is reduced by shorter chain length of shorter fatty acids and by the presence of double bonds of methyl groups. The kinematic viscosity of 6.1682 ± 0.01 mm2.s−1 and 6.2105 mm2.s−1 ± 0.01 was obtained for the pure and ethylic chichá biodiesel at 313.15 K, respectively, according to Table S.1 and S.2. In this way, the ones consulted in the literature are respected because the biodiesel, which presents a lower chain of biodiesel esters than the ethylic biodiesel, has a lower kinematic viscosity.
For the validation of the model, the density data of the biodiesel/diesel blends were used for the temperature of 313.15 K. The result of the adjustment for validation is shown in Figs. 3 and 4.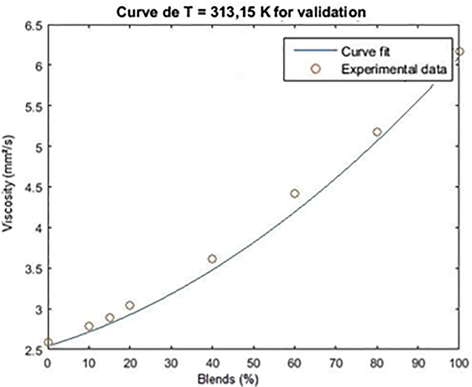
Adjustment curve for the validation of the kinematic viscosity empirical model as a function of the concentration of the methyl/diesel biodiesel blends for T = 313.15 K.
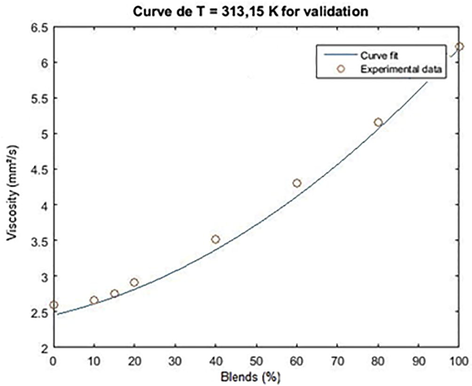
Adjustment curve for the validation of the kinematic viscosity empirical model as a function of the concentration of ethylene/diesel biodiesel blends for T = 313.15 K.
The mean square error associated with the validations for methyl and ethyl chichá biodiesel was 0.0081 and 0.0073, respectively, and it was acceptable for the kinematic viscosity range from 2.592 to 6.168 mm2.s−1 for the methyl and ethyl blends. 2.592–6.222 mm2s. −1 for the ethylic blends, at a temperature of 313.15 K.
3.3.2 Empirical equations for density prediction
The behavior of density as a function of each of the variables (temperature and composition of the mixture) was verified using 1st degree polynomials. The density data were plotted as a function of the volumetric fraction of the biodiesel in the BX mixtures collected in Tables S.4 and S.5. The correlations of the mixtures models were expressed through Eqs. (3) and (4), which represent the dependence of the density with the temperature and with the volumetric fraction of the biodiesel of chichá methyl and ethyl, respectively, according to the equation proposed by (Gülüm and Bilgin, 2015). The generated model does not contain the density values for T = 193.15 K because they were used to carry out the validation.
The experimental data were adjusted by a polynomial of first degree, evidencing that the mixture models for the density had a linear configuration, both for concentration and for temperature, other non-linear models were not tested since the experimental data fit the model satisfactorily created. Thus, the relation between density and temperature was considered linear for the two chichá biodieses. The relative errors associated with the predicted experimental against the result can be found in Table S.6.
Table 4 shows the values of the curve fitting coefficients and the coefficient of determination (R2) for the samples studied.
Coefficients
Methyl chichá biodiesel blends
Ethyl chichá biodiesel blends
A
1033
1040
B
0.5963
0.5503
C
−0.6995
−7.7202
R2
0.9941
0.9936
Figs. 5 and 6 present the density curve adjustments as a function of the volume fraction of B100 present in the blend for B0, B10, B15, B20, B40, B60 and B80 in the temperature range of 283.15–313.15 K. It is possible to note the linear behavior suggested by the blends models that relates the blends and the density varying with the temperature. The analysis of Figs. 5 and 6 shows that the experimental data showed reasonable agreement with predicted trends, the density of BX mixtures increases linearly with the volumetric fraction of B100 and decreases with increasing temperature.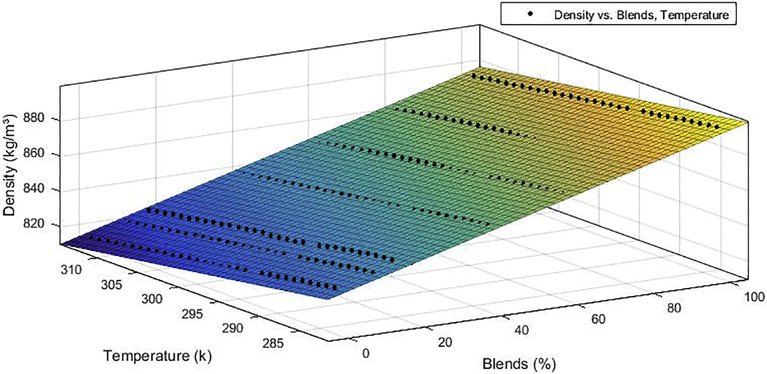
Curve adjustment for the density of blends of chichá/diesel biodiesel by the methyl route.
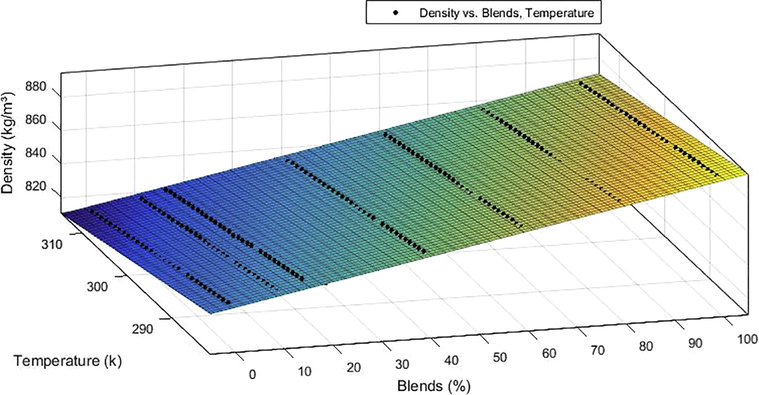
Curve adjustment for the density of the blends of chichá/diesel biodiesel by the ethanol route.
The density-temperature relationship presented a decreasing behavior with increasing temperature, while the density-composition relation presented increasing behavior, evidencing that the increase in the concentration of biodiesel increases the density of the mixture for the ethyl and methyl mixtures. This was expected behavior since, the density of biodiesel Chichá is slightly higher than the density of petroleum diesel. Therefore, increasing the percentage of biodiesel in the mixtures will result in a consequent increase in the density of the biodiesel/diesel blend.
The comparison between the two biodieses tested evidences that they have a similar relationship between them, presenting slight variations due to small differences in the densities of the pure fuels, being the biodiesel of chichá produced via the esterification of the lower density. This difference may be associated with different structures that make up the fuel, as previously explained. The molar mass and the degree of unsaturation are determinant factors in the quantification of the density, so data reported in the literature indicate that the density decreases as the molar mass increases and increases as the degree of unsaturation increases (Ramírez-verduzco et al., 2012). Thus, the data produced by this study are in agreement with the consulted literature, since ethyl chichá biodiesel, which has higher molar mass, presented a lower density (883.8 kg.m−3) than the biodiesel produced by route (888.5 kg.m−3) at the standard analysis temperature of 293.15 K.
The analyzes were performed according to ASTM D4052 which provides a method for obtaining the density for biodiesel (B100) and for mixing biodiesel in petroleum diesel through APN Resolutions No 45 of 25.08.2014 and NPA N° 30 of 06.23.2016, respectively, and that we also set standard limits of 850 and 900 kg.m−3 for the density of biodiesel (B100) and 817.8–865.0 kg.m−3 for blending biodiesel with petroleum diesel at 293.15 K. It is noted that biodiesel from methyl and ethyl chichá (B100) comply with the legislation, as well as all blends up to the B30 blend, for higher concentrations of biodiesel in the blend there is still no regulated resolution by NPA.
For the validation of the model, the density data of the biodiesel/diesel blends were used for the temperature of 293.15 K. The result of the adjustment for validation is shown in Figs. 7 and 8.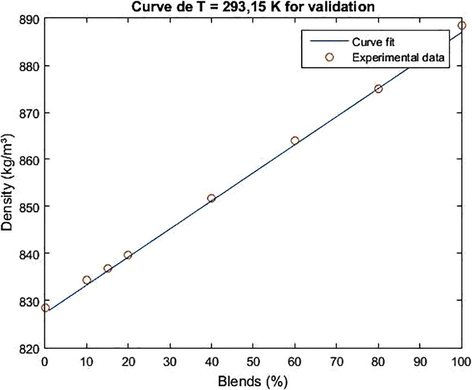
Adjustment curve for the validation of the empirical density model as a function of the concentration of the methyl/diesel biodiesel blends for T = 293.15.
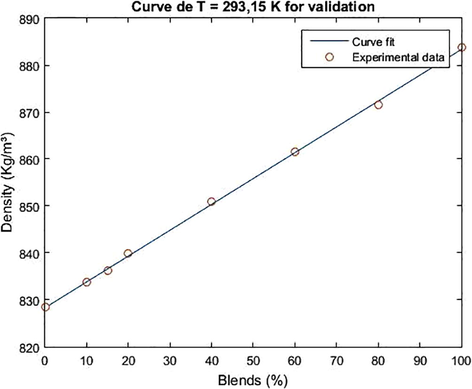
Adjustment curve for the validation of the empirical density model as a function of the concentration of ethylene/diesel biodiesel blends for T = 293.15.
The mean square error associated with the validations for the chichá methyl and ethylic biodiesel was 0.2503 and 0.4046 respectively, and it was acceptable for the density range taken 828.5–888.5 kg.m−3 for the methyl blends and 828.5–883.8 kg.m−3 for the ethyl blends, at the temperature of 293,15 K.
4 Conclusion
Empirical models were constructed using Matlab software that satisfactorily described the behavior of kinematic viscosity and density of the studied samples, valid for a study range with temperatures from 293.15 to 353.15 K for kinematic viscosity and 283, 15–313.15 K for the density. Cubic equations were generated for the viscosity analysis and linear equations for the analysis of the sample density of biodiesel and diesel blends: B0, B10, B15, B20. B40, B60, B80 and B100. The regression models were tested against the experimental data. We obtained maximum errors of about 0.22% with the density models and in the viscosity models we obtained maximum errors of 11.85%. Thus, the predictive density equations give better results than the predictive viscosity models in all the systems studied. Based on the analysis carried out, it is evident that the increase of the percentage of biodiesel in the diesel/biodiesel mixture should be studied in detail in order to ensure a proper functioning of the engine because, due to the mixing process, it was verified that the kinematic viscosity and density are directly affected by the increase in the percentage of biodiesel in the biodiesel/diesel blend and by the increase in temperature.
References
- Basic properties of palm oil biodiesel – diesel blends. Fuel. 2008;87:2069-2075.
- [CrossRef] [Google Scholar]
- Quantification of biodiesel content in dieselbiodiesel blends by fluorescence spectroscopy: evaluation of the dependence on biodiesel feedstock. Renewable Energy. 2012;46:137-140.
- [CrossRef] [Google Scholar]
- EN, 2008. EN14214 - Automotive fuels - Fattyacidmethylesters (FAME) for diesel engines - Requirements and test methods,” [Online]. Available: https://www.ds-bremen.com/tl_files/media/pdf/eng_biodiesel.pdf [Acedido em 04 May 2018].
- Caracterização química do óleo da amêndoa de sterculia striata st. hil. et naud. Quim. Nova. 2004;27:404-408.
- [Google Scholar]
- Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. J. Am. Oil Chem. Soc. 2006:83.
- [Google Scholar]
- Biodiesel production from Sterculia striata oil by ethyl transesterification method. Ind. Crop. Prod.. 2015;74:767-772.
- [CrossRef] [Google Scholar]
- Measurement, correlation and prediction of biodiesel blends viscosity. Fuel. 2015;143:268-274.
- [CrossRef] [Google Scholar]
- Density, fl ash point and heating value variations of corn oil biodiesel – diesel fuel blends. Fuel Process. Technol.. 2015;134:456-464.
- [CrossRef] [Google Scholar]
- Two-term power models for estimating kinematic viscosities of different biodiesel-diesel fuel blends. Fuel Process. Technol.. 2016;149:121-130.
- [CrossRef] [Google Scholar]
- A comprehensive study on measurement and prediction of viscosity of biodiesel-diesel-alcohol ternary blends. Energy. 2018;148:341-361.
- [CrossRef] [Google Scholar]
- The tandem analytical method of fl ow injection diode array spectrophotometry and fl ame atomic absorption spectrometry (FI-DAD(vis)-FAAS) in iron speciation studies using 1, 10-phenanthroline complexes. Microchem. J.. 2013;110:54-60.
- [CrossRef] [Google Scholar]
- Performance and emission characteristics of a diesel engine running on optimized ethyl levulinate e biodiesel e diesel blends. Energy. 2016;95:29-40.
- [CrossRef] [Google Scholar]
- Biodiesel: parâmetros de qualidade e métodos analíticos. Quim. Nov.. 2009;32:1596-1608.
- [Google Scholar]
- Characterization of biodiesel and bio-oil from Sterculia striata (chicha) oil n = 6: Malvalic acid n = 7: Sterculic acid. Ind. Crop. Prod.. 2012;36:349-354.
- [CrossRef] [Google Scholar]
- Effect of blends of diesel and palm-castor biodiesels on viscosity, cloud point and flash point. Ind. Crop. Prod.. 2013;43:791-797.
- [CrossRef] [Google Scholar]
- Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil- Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manage.. 2018;158:400-415.
- [CrossRef] [Google Scholar]
- Stirring and mixing in ethylic biodiesel production stirring and mixing in ethylic biodiesel production. J. King Saud Univ. - Sci.. 2020;32:54-59.
- [Google Scholar]
- Correlation and prediction of biodiesel density for extended ranges of temperature and pressure. Fuel. 2015;141:23-38.
- [CrossRef] [Google Scholar]
- Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel. 2012;91:102-111.
- [CrossRef] [Google Scholar]
- Prediction of the density and viscosity in biodiesel blends at various temperatures. Fuel. 2011;90:1751-1761.
- [CrossRef] [Google Scholar]
- Alternative methods to quantify biodiesel in standard diesel-biodiesel blends and samples adulterated with vegetable oil through UV – Visible spectroscopy. Fuel. 2016;186:199-203.
- [CrossRef] [Google Scholar]
- Synthesis and optimization of Hevea brasiliensis and Ricinus communis as feedstock for biodiesel production: a comparative study. Ind. Crop. Prod.. 2016;85:274-286.
- [CrossRef] [Google Scholar]
- A comparative study of biodiesel production methods for Reutealis trisperma biodiesel energy sources, Part A: recovery, utilization, and A comparative study of biodiesel production methods for Reutealis trisperma biodiesel. Energy Sources Part A Recover. Util. Environ. Eff.. 2017;00:1-9.
- [CrossRef] [Google Scholar]
- A new spectrophotometric method for determination of biodiesel content in biodieseldiesel blends. Fuel. 2015;143:16-20.
- [CrossRef] [Google Scholar]
- A close dielectric spectroscopic analysis of dieselbiodiesel blends and potential dielectric approaches for biodiesel content assessment. Fuel. 2013;105:705-710.
- [CrossRef] [Google Scholar]
- Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy. 2010;35:2752-2760.
- [CrossRef] [Google Scholar]
- Temperature-dependent kinematic viscosity of selected biodiesel fuels and blends with diesel fuel. J. Am. Oil Chem. Soc. 2005:195-199.
- [Google Scholar]
- High pressure physicochemical properties of biodiesel components derived from coconut oil or babassu oil. Fuel. 2014;125:144-151.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2018.08.009.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







