Translate this page into:
Efficacy of chemical and bio-pesticides on cowpea aphid, Aphis craccivora, and their residues on the productivity of fennel plants (Foeniculum vulgare)
⁎Corresponding authors. moataz.moustafa79@gmail.com (Moataz A.M. Moustafa), samy_mahmoud@hotmail.com (samy sayed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The residual of pesticides after applications and their effects on plant parameters are very important to be estimated. Therefore, this study investigated the efficacy of six insecticides (acetamiprid, thiamethoxam, imidacloprid, azadirachtin, thiocyclam, and sulfoxaflor) against Aphis craccivora adults and their residue in fennel plants (Foeniculum vulgare) in two successive seasons. The physiological and phytochemical responses of fennel plants after exposure to these insecticides were studied. The tested insecticides were able to keep the reduction percentage of A. craccivora adults above 90% except of azadirachtin, which reached 52% only. Thiocyclam and sulfoxaflor were more effective in decreasing the number of A. craccivora adults than other treatments until 7th day after application. Significant effects were observed among different treatments compared to control for all the vegetative parameters of fennel plants. In the first season, the highest plant height (151.83 cm) and number of umbel/plant (77.63) were obtained in plants treated with thiocyclam with the values from fruit yield (2504 kg/fed) followed by azadirachtin (2281 kg/fed). The same trend for these parameters was obtained in the 2nd season. Azadirachtin demonstrated the best value of oil yield (2.99 and 3.77 ml/plant) for 2017 and 2018 seasons, respectively). The main chemical compound of fennel seed essential oils was estragole (methyl chavicol) and ranged from 68.10 (with Azadirachtin) to 79.90% (with control), the undesirable component, with increasing the amount of anethole (10.06%). It can be concluded that, thiocyclam, sulfoxaflor with neonicotinoid group could be included as a useful agent in a comprehensive aphid management programs in Egypt.
Keywords
Aphis craccivora
Thiocyclam
Sulfoxaflor
Azadirachtin
Fennel plant
Residues
1 Introduction
Fennel plant, Foeniculum vulgare Mill (Family: Apiaceae) is a native medicinal plant in the Mediterranean region. The fruits of the plant are used as antispasmodic, stomachic, sedative, balsamic, cardiotonic, digestive, diuretic, antispasmodic, anti-inflammatory, secretomotor, secretolytic, lactogogue, eye lotion and tonic properties (Goriz et al., 2012). Essential oils (Eos) obtained from the dried fruit of F. vulgare subsp. piperitum showed the presence of main compounds as methyl chavicol, fenchone and (E)-anethole (Cavaleiro et al., 1993), while D-limonene and β-myrcene have been shown to have hepato protective effects on liver (Özbek et al., 2003).
Several fennel parts are edible for infestation by pests such as leaves, stalks, and fruits (Bishr et al., 2012). Among the different pests, which attack the fennel plant, cowpea aphid, Aphis craccivora causes the most significant damage to the fennel crop as both nymph and adults suck the cell sap from leaves, stem and umbels and as a result plant becomes weak and stunted (de Sousa Ramalho et al., 2015). In addition, it exudes copious quantity of honeydew, which favor the growth of sooty mould and results into retarded growth of the plant (Ferreira and Sousa-Silva, 2004). Therefore, aphid could transmit pathogens of plant diseases through transfer of 14 legume viruses (Mweke et al., 2020). Generally, aphid control heavily relies on the use of synthetic insecticides such as carbamates, organophosphates and pyrethroids, which led to aphid resistance to these insecticides (Shetlar, 2001), therefore, another group of insecticides are required to be used for aphid management. One of these insecticides is neonicotinoid group including; imidacloprid, acetamiprid, and thiamethoxam, which have been used globally over the last decade because of their high impact against a wide range of dangerous aphid species (Jeschke et al., 2011) including A. craccivora. In contrast, there was a lack of information on the efficacy of some new insecticides including; sulfoxaflor, and thiocyclam on aphids infesting fennel plants under field conditions. Pesticides of neonicotinoids are registered in more than 120 countries with a share of more than 25% of total global insecticides sales (Bass et al., 2015). The classification scheme of the Insecticide Resistance Action Committee (IRAC) lists 7 commercial neonicotinoids in Group 4A (IRAC, 2019), which act as agonists of the nicotinic acetylcholine receptors (nAChR) in insects and other invertebrates. This action disrupts neuromuscular signaling pathways, which causing death of target insect pests. Sulfoxaflor is an insecticide belonging to the family sulfoximine, which was used effectively against piercing-sucking insect pests as whiteflies and aphids (Bacci et al., 2018). Sulfoxaflor acts on the same receptor as the neonicotinoids, but it is classified in subgroup of 4C (IRAC, 2019). While, thiocyclam is a nereistoxin analogue insecticide, which metabolically converted to nereistoxin in the insect to effect on nAChR (Ware and Whitacre, 2004). Thiocyclam is classified in group 14 (IRAC, 2019), which blocking the cholinergic transmission resulting in paralysis and insect death. Azadirachtin is extracted from neem tree (Azadirachta indica), that used effectively against mosquito larvae, aphids, and whitefly (Morgan, 2009). Considering the above facts, the main aim of this investigation was to assess the efficacy of acetamiprid, thiamethoxam, imidacloprid, azadirachtin, thiocyclam, and sulfoxaflor at their recommended rates of application against A. craccivora adults and their residues in fennel plant (F. vulgare Mill) under field conditions. In addition, the physiological and phytochemical changes in the treated plants due to the exposure to the proposed insecticides were investigated.
2 Materials and methods
2.1 Fennel plant cultivation
Fennel plants (F. vulgare, Mill) were cultivated in a Randomized Complete Block Design (RCBD) with 4 replicates at the farm of Medicinal and Aromatic Plants Research Department, Kalubeia, Egypt “30°19′ N, 31°13′ E, 16.9 m above sea level rise” on 25th October 2017 and on 30th October 2018. Fruits were planted in five rows of 3 m in length with a distance of 35 cm between plants in the rows and 50 cm between rows.
2.2 Tested pesticides
Six insecticides (Table 1) were evaluated against A. craccivora in field trials during two successive seasons.
Common name
Trade name
Group
Concentration/100 L water
Acetamiprid
Mospilan 20%SP
Neonicotinoid
25 g
Thiamethoxam
Actara 25%WG
Neonicotinoid
20 g
Imidacloprid
Imipower 35%SC
Neonicotinoid
75 cm3
Azadirachtin
Neemix 4.5%EC
Plant extract
75 cm3
Thiocyclam
Leafguard 75%WG
Nereistoxin
125 g
Sulfoxaflor
Closer 24%SC
Sulfoxamine
25 cm3
2.3 Field experiments
The experimental area was divided according to the abovementioned design including four replicates for each treatment and the control (6 × 7 m). Knapsack sprayer cp-3 was used for applying the insecticides as foliar treatment. The chemical application started when aphid infestation reached 5 adults/leaf.
The recommended concentrations of the tested insecticides were applied and leaf samples were collected randomly before applying the pesticides and then 1st, 3rd, 5th, and 7th day after application. One hundred leaves (25 leaves from each replicate) were investigated from each treatment and the control to calculate the reduction % of A. craccivora adult after spraying.
2.4 Analytical procedures
2.4.1 Preparation of samples
Each Sample from fennel leaves (10 ± 0.1 g) was transferred into a 50 ml Teflon centrifuge tube with10 mL acetonitrile to be prepared according to the QuEChERS method (Bojacá et al., 2013; Kowalskam, 2020). While, for oil samples, 2 g were taken and 10 ml of water were added then 10 ml acetonitrile was also added for each sample. The tubes were capped and vortexed vigorously for 1 min at maximum speed. Then, QuEChERS extraction salts mixture was added and then centrifuged for 5 min at >3000 g. For clean-up 1 ml of upper layer (acetonitrile) was transferred into the dispersive-SPE tubes containing150 mg anhydrous MgSO4, 25 mg primary secondary amine (PSA) and 25 mg C18. The supernatants were filtered through 0.22 mm Nylon syringe filters into an auto sampler vials for determination (Muhammed et al., 2020).
2.4.2 Determination of thiocyclam using gas chromatograph coupled micro electron-capture detector
Separation was performed on an HP6890 gas chromatograph (GC) equipped with an HP 7673 auto-sampler, a micro electron-capture detector (µ-ECD). A 30 m × 0.32 mm capillary column coated with a 0.25 µm thick film of 5% phenyl methyl poly siloxane (HP-5) from Hewlett and Packard was used in combination with the following oven temperatures: Initial temperature 190 °C for 2 min, 20 °C/min up to 220 °C and held for 4 min, 20 °C/min up to 280 °C and held for 1 min. The nitrogen carrier gas had a 2 ml/min flow rate and split less injection of a 1 µL volume was carried out, injector and detector temperatures were 280 °C and 300 °C, respectively. The retention time was 7.45 min.
2.4.3 Determination of sulfoxaflor using high-performance liquid chromatography with diode-array detection
An Agilent 1260 series high-performance liquid chromatography (HPLC) equipped with an analytical column (150 mm × 4.6 mm id × 5 µm ODS) attached to a photodiode array detector. Flow rate of mobile phase (acetonitrile 90% + water 10% (2% formic acid)) was 1.5 ml/min with the injection volume of 20 µL. Detection wave length was set at 210 nm with retention time of 2.33 min.
2.5 Residue calculations
The residues were calculated with the following equation (Möllhoff, 1975): mg/kg = (Ps × B × V)/(Pst × G × C) × F; where: F (recovery factor) = 100/R, R: average of recovery, Ps: sample peak area, B: amount of standard injected (ng), V: final volumes of samples solution (mL), Pst: standard peak area, G: sample weight (g), C: amount of sample injected (µL). Residues half-life value (RL50) were calculated mathematically according to this equation: RL50 = Ln2/K = 0.6932/K (Moye et al., 1987).
2.6 Recovery studies
The recovery study was carried out on untreated fennel leaves by fortifying five replicates of the samples with tested pesticides standards at three levels ranging from 0.01 to 1 mg/kg (Table 2). Recovery percentages were calculated as follow: % Recovery = [(µg) found/(µg) added] × 100. Accuracies were calculated as the percentage between the found and the known concentrations and precisions were obtained as the relative standard deviations (%RSD), which are the ratio between standard deviations and average concentrations obtained (Table 2). n – Number of replicates, RSD – relative standard deviation.
Fortification levels mg/kgn = 5
Thiocyclam
Sulfoxaflor
mean recovery
RSD
mean recovery
RSD
0.01
79.26 ± 5.69
7.26
85.72 ± 3.59
5.43
0.1
82.14 ± 9.25
6.32
90.42 ± 5.82
3.99
1
83.59 ± 8.16
9.89
95.79 ± 4.70
4.92
2.7 Measurements of vegetative growth
Plant heights (cm), the numbers of total branches/plant, umbels numbers/plant, plant fresh weights (g), 1000 seeds weights, and fruit yields (g/plant or kg/6 × 7 m) were recorded.
2.8 Fruit protein content
Protein contents of fennel fruits were assessed as total nitrogen by Micro-Kjeldahl method, then N was multiplied by 6.25 (Tripathi et al., 1971).
2.9 Fruit essential oils percentage and oil yield
To determine the EO %, the extractions of the EOs were carried out by hydro- distillation method in a Clevenger apparatus. One-hundred g of fennel fruit were ground and hydro distillated for 3 h (Salem et al., 2020) and then the oil yield/plant was estimated.
2.10 Gas chromatography analysis of essential oils
The EOs were analyzed using DsCrom 6200 GC equipped a flame ionization detector (GC-FID) for separation of volatile oil components. The analysis condition was as follows; the chromatograph fitted with capillary column DP-WAX-122–7032 polysillphenylene-siloxane 30 × 0.25 mm ID × 0.25 μ film. Temperature program ramp was increased by a rate of 13 °C/min from 60 °C to 220 °C. Flow rate of nitrogen gas was set to 1 ml/min, hydrogen 30 ml/min and 330 ml/min for air. Detector and injector temperatures were 250 °C and 280 °C, respectively.
2.11 Statistical analysis
The variances homogeneity was subsequently tested by Levene’s test in order to analyse data by SPSS program (version 23.0). One-way ANOVA was conducted for all experiments. Means were compared with the Duncan test (α = 5%).
3 Results
3.1 Field evaluation of tested insecticides
In the first season, all tested insecticides caused high initial effect on A. craccivera adult where the reduction rate ranged from 91.3 to 97.7% except azadirachtin (a reduction ratio of 52.01%), which was significantly different from all other tested insecticides (Table 3). On 5th and 7th days after application, both of acetamiprid and azadirachtin were not significantly different and lower than other four tested insecticides in their impact on aphids. Means bearing the same letter(s) within each column are not significantly different (α = 5%) according to Duncan’s' multiple range test.
Insecticides treatments
Reductions (%) (Mean ± SD)
1st day
3rd day
5th day
7th day
2017/2018
2018/2019
2017/2018
2018/2019
2017/2018
2018/2019
2017/2018
2018/2019
Acetamiprid
95.70 ± 0.17a
89.98 ± 1.52a
91.76 ± 2.28 cd
95.02 ± 1.98b
89.41 ± 6.59b
94.32 ± 1.26b
85.61 ± 10.30b
92.22 ± 1.98b
Thiamethoxam
92.19 ± 1.58a
95.50 ± 1.69a
93.75 ± 2.48bc
97.75 ± 0.16a
96.32 ± 1.03a
98.58 ± 0.74a
97.82 ± 0.75a
99.11 ± 0.83a
Imidacloprid
91.31 ± 2.66a
95.45 ± 1.58a
94.73 ± 6.19abc
98.84 ± 0.51a
98.79 ± 0.14a
99.50 ± 0.33a
99.30 ± 0.48a
100.0 ± 0.0a
Azadirachtin
52.00 ± 10.34b
44.44 ± 12.88b
88.07 ± 1.78d
83.82 ± 2.36c
89.73 ± 2.86b
85.29 ± 5.05c
86.83 ± 6.69b
82.25 ± 3.75c
Thiocyclam
97.66 ± 0.52a
97.40 ± 0.77a
98.25 ± 1.26ab
98.56 ± 1.09a
99.06 ± 0.69a
99.66 ± 0.40a
99.44 ± 0.66a
100.0 ± 0.0a
Sulfoxaflor
97.47 ± 0.69a
98.11 ± 0.98a
98.83 ± 0.45a
98.43 ± 1.15a
99.86 ± 0.28a
100.0 ± 0.0a
100.0 ± 0.0a
100.0 ± 0.0a
F5,18
63.96
60.08
7.17
66.33
10.20
28.41
7.12
66.61
P values
<0.0001
<0.0001
<0.001
<0.0001
<0.0001
<0.0001
<0.001
<0.0001
During the second season (Table 3), the same trend approximately was achieved in the aphid reduction. This finding during the two successive seasons indicate that thiamethoxam, imidacloprid, thiocyclam and sulfoxaflor had higher effects on aphids and stable until 7 days post –treatment without significant differences among them. In addition, the reduction percent of A. craccivora by acetamipridon 7th day after application in the first season (85.61%) was approximately the same with that of azadirachtin (86.83%). Meanwhile, acetamiprid in the second season achieved significant higher reduction rate for aphids (92.22%) than that induced of by azadirachtin (82.25%).
3.2 Dissipation of thiocyclam and sulfoxaflor residues in fennel samples
The dissipation trends of thiocyclam and sulfoxaflor in fennel is shown in Table 4. Thiocyclam and sulfoxaflor were progressively decreased after the foliar application. The concentrations of thiocyclam and sulfoxaflor after 3 day of treatment were 2.65 and 2.17 mg/kg respectively, with less persistence of 20.76 and 24.89%, respectively. The residues amount was decreased to 1.95 and 1.55 mg/kg respectively, in fennel within the first 3 days after application. After this period, thiocyclam and sulfoxaflor residues were decreased to 0.55 and 0.21 mg/kg for thiocyclam and 0.54 and 0.04 mg/kg for sulfoxaflor at 3 and 7 days, respectively. Later foliar application contained no detectable amount of thiocyclam and sulfoxaflor (below the quantification limit 0.03 mg/kg) in fennel. The dissipation rat of the used insecticides in fennel exhibited a first order kinetics, and the half-life of thiocyclam and sulfoxaflor were calculated in fennel treated with recommended concentrations were 1.89 and 1.75 day, respectively (Table 4).Table 5 ND: not dectable; PHI: Pre harvest intervals; MRL: Maximum residue limits; RL50 Residual half-life; LOD: limit of detection, LOQ: limit of quantitation.
Intervals (days)
Thiocyclam
Sulfoxaflor
Residue (mg/kg)
Loss (%)
Persistence
Residue (mg/kg)
Loss (%)
Persistence
0
2.65 ± 1.77
0.00
100
2.17 ± 1.29
0.00
100
1
1.95 ± 2.15
26.41
73.59
1.55 ± 3.17
28.57
71.43
3
0.55 ± 1.06
79.24
20.76
0.54 ± 1.42
75.11
24.89
7
0.21 ± 3.04
92.07
7.93
0.04 ± 5.23
96.31
3.69
10
ND
ND
15
ND
ND
All seed samples
ND
ND
MRL
0.01
0.05
PHI
10 days
7 days
RL50
1.89 days
1.75 days
Insecticides treatments
Major essential oil constituents (%)
α-Pinene
Myrcene
Limonene
1,8-Cineole
γ-Terpinene
Fenchone
Methyl chavicol
Anethole
Control
1.19
0.90
12.22
2.68
0.19
0.24
79.90
0.54
Acetamiprid
0.11
1.3
0.4
14.59
4.62
0.3
77.59
0.09
Thiamethoxam
1.58
1.06
16.22
4.69
0.45
ND
74.66
1.13
Imidacloprid
0.91
0.37
17.30
6.32
0.55
0.65
73.00
0.42
Azadirachtin
1.73
1.13
11.36
2.54
0.13
0.96
68.10
10.06
Thiocyclam
0.74
0.78
14.04
2.32
ND
0.41
76.44
0.81
Sulfoxaflor
2.18
1.27
12.79
4.74
0.28
0.59
77.70
0.20
3.3 Plant vegetative growth parameters
Significant effects on plant growth characters (plant height, number of branches/plant, plant fresh weight (g), number of umbels/plant, and 1000 seeds weight (g)) were detected regarding the foliar spray of the insecticides. In both seasons, the data in comparison to control showed that the highest values from plant height (151.83 and 157.42 cm), and number of umbel/plant (77.63 and 96.25/plant) were attained from plants sprayed with thiocyclam (Fig. 1). The highest supreme values from number of branches/plant (9.00 and 9.40) and plant fresh weight (468.75 and 585.94 g) were obtained from plants treated with azadirachtin and 1000 seed weight (12.10 and 15.13 g) were achieved from plants treated with thiamethoxam during the 2 seasons (Fig. 1).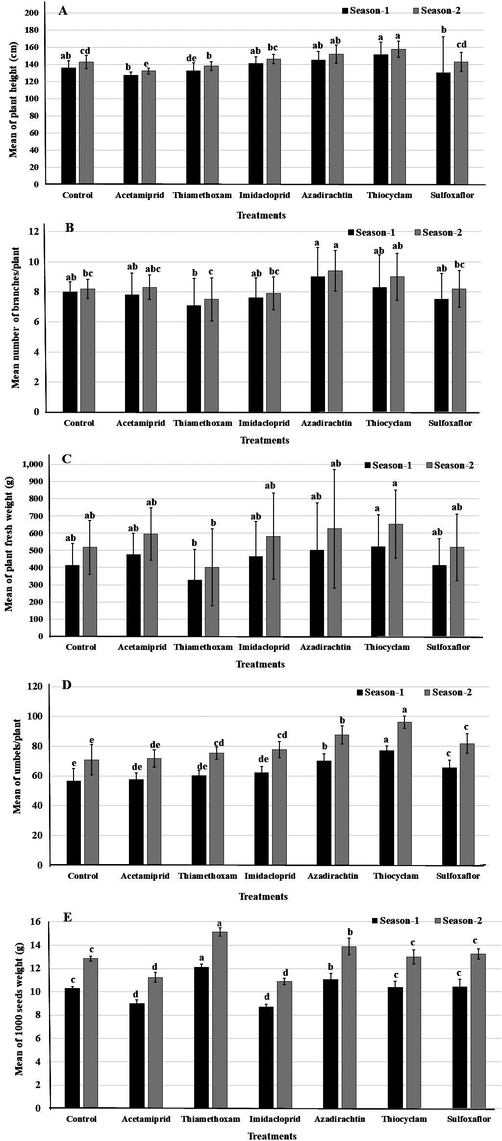
Vegetative growth parameters of fennel plant treated with foliar applications by six insecticides in two successive seasons. A- plant height, B- number of branches, C- plant fresh weight, D- umbels number, E- 1000 seeds weight. Different letters above bars indicate significantly different means according to Duncan test (α = 5%) within the same season.
3.4 Fruits yield
Concerning the effect of different insecticides application on fennel fruit yield, the data in Fig. 2, indicate that the foliar application with thiocyclam and azadirachtin increased significantly the fruit yield per fed, compared to the control (df = 6, F = 5.46, P ≤ 0.0001). In the first season, thiocyclam as foliar application gave the highest value from fruit yield (2504 kg/6 × 7 m) followed by azadirachtin (2281 kg/6 × 7 m) in the second season.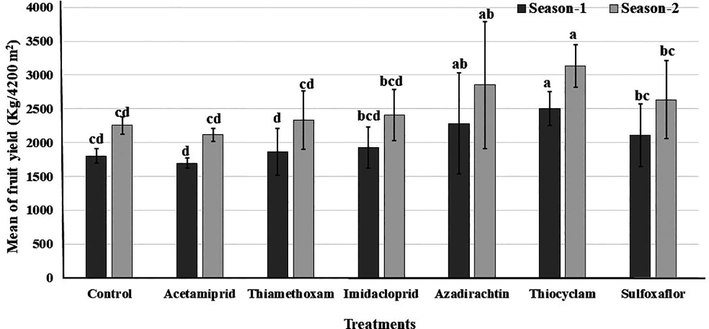
Fruit yield of fennel plants treated with foliar application by six insecticides in two successive seasons. Different letters above bars indicate significantly different means according to Duncan test (α = 5%) within the same season.
3.5 Fruit essential oil % and oil yield
The foliar application with thiamethoxam or azadirachtin had no significant effect on fruit EO % (Fig. 3a). They showed the highest percentages of EO compared to the control in the 1st season (3.38 and 3.30 %) for thiamethoxam and azadirachtin, respectively. In the 2nd season, a similar trend had been obtained.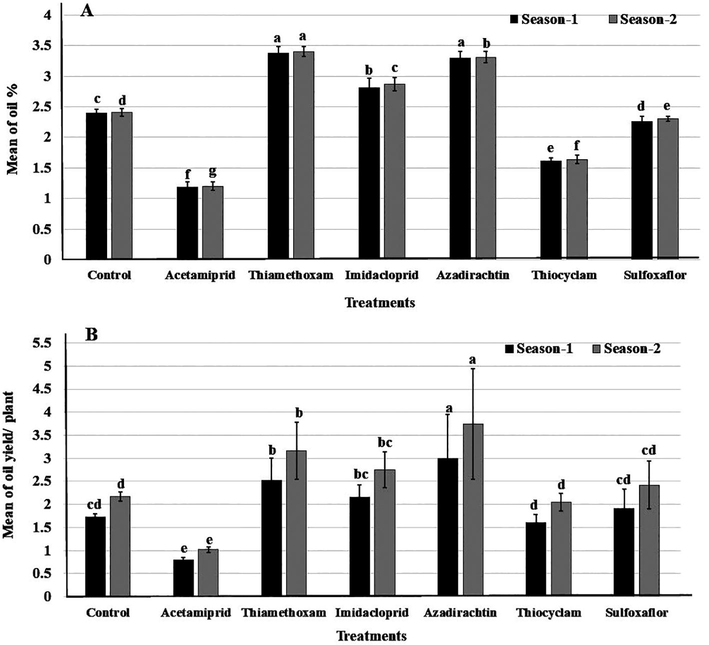
Fruit essential oil % (A) and oil yield (B) of fennel plants treated with foliar application by six insecticides in two successive seasons. Different letters above bars indicate significantly different means according to Duncan test (α = 5%) within the same season.
Considering the oil yield per plant, the results in Fig. 3b show the influence of the different insecticides on the oil yield (ml/plant) compared to the unsprayed plant (control) in both seasons. The highest value of oil yield was determined on plant treated with azadirachtin (2.99 and 3.77 ml/plant), followed by thiamethoxam (2.52 and 3.17 ml/plant) in both seasons, respectively.
3.6 Fruit protein content (%)
There was a significant effect of the foliar application with the different insecticides on the fruit protein (%) (Fig. 4). Furthermore, the thiocyclam treatment offered the highest percentage of fruit protein in the 1st season (16.22%) and in the 2nd season (16.39%). No significant differences were observed in protein content with treatments of acetamiprid, thiamethoxam or sulfoxaflor (15.66, 15.59 or 15.84 %, respectively) in the 1st season, and the similar trend was observed in the 2nd season.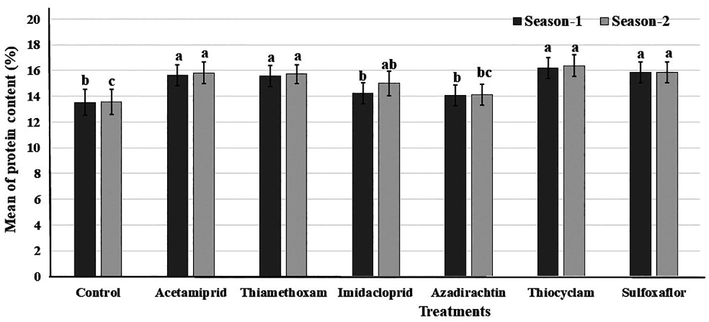
Fruit protein content (%) of fennel plants treated with foliar application by six insecticides in two successive seasons. Different letters above bars indicate significantly different means according to Duncan test (α = 5%) within the same season.
3.7 Chemical constituents of essential oil
The GC-FID analysis of the EO extracted from fennel fruits were taken from the 2nd season for treatments that have the highest growth, fruit and oil yield. Data in Table)5(illustrated the percentage of the major constituents in the fennel EOs. The major constituent was methyl chavicol (estragole) with percentage ranged from 68.10 to 79.90% followed by limonen (11.36 to 17.30%) then 1,8-cineole (2.32 to 6.32%) in all the treatments, while, the minor compounds were α-pinene (0.74–2.18%), myrcene (0.37–1.27%) and anethole (0.20–10.06%). The best treatment for decreasing the concentration of methyl chavicol (68.10%), with increasing anethole (10.06%) resulted from fennel plants treated with foliar application of azadirachtin. While, the untreated plant showed the highest value of methyl chavicol (79.90%) followed by the other treatments, which ranged from 73% to 77.70%.
4 Discussion
The efficiency and determining of insecticide residues in crops including the medicinal and aromatic plant like fennel is necessary to enhance the productivity of respective plant. In present study, the main chemical compounds in the Egyptian fennel seed EO were methyl chavicol (estragole), limonene, 1,8-cineole (eucalyptol) and anethole. The methyl chavicol was also the main compound in the fennel plants after treatments with Moringa oleifera leaf extracts and benzyladenine by 77.5% to 87.3% respectively (Cavaleiro et al., 1993). Furthermore, the EO from seeds of plants growing in Egypt showed the presence of estragole, fenchone, limonene and α-pinene with percentages of 69.78%, 13.19%, 12.43% and 2.44%, respectively, as main compounds (Cavaleiro et al., 1993). Fruit EO of F. vulgare Mill from Portugal showed the presence of methyl chavicol as main compound ranged from 79.3 to 82% (Miguel et al., 2010). Other studies showed the main compounds in the EO were (E)‐anethole (72.27%–74.18%), fenchone (11.32%–16.35%) and methyl chavicol (3.78%–5.29%) (Mimica-Dukić et al., 2003). The content of trans-anethole (81.63% and 87.85%) and other monoterpenes, β-myrcene, α-terpinene, α-pinene, and limonene were identified in the EO from Sweet fennel (Telci et al., 2009).
In this study, azadirachtin caused mortality within the first day after application, while after 3 and 5 days from the application, the mortality was highly increased by 88.08 and 89.73% respectively. These results were supported previously since neem oil has contains an effective compound against insect pest as antifeedant or as inset growth regulator that led gradually to the death of insects (Lee et al., 1991).. Importantly, sulfoxaflor is effective against various pest insect strains that are resistant to imidacloprid and other insecticides (Zhu et al., 2011). A few trials with synthetic nereistoxin insecticide was used in the control of aphids’ pests. Thiocyclam hydrogen oxalate could be used very effectively against the leaf miner Coelaenom enodera, in West Africa (Turner and Gillbanks, 2003).
Results indicated that all experiments achieved significant reductions in aphid population after 1,3,5, and 7 days compared to the control where imidacloprid, thiomethoxam and acetamiprid induced the highest efficiency against A. craccivora. Similar results were observed with imidacloprid which had a high effect in aphid control, where the trans– laminar transports of imidacloprid from the treated upper side of leaves to lower surface were excellent (Elbert et al., 1991). Also, imidacloprid was effective in aphid reductions on brinjal (auberggine) and in increasing the seedling total leaf chlorophyll over those of untreated plants (Jarande and Dethe, 1994). The neonicotinoid insecticides were highly effective against various aphid species and reduced the aphid population under field conditions (Abdu-Allah, 2012).
The dissipation of the pesticide residues in crop protection usually are depends on type of application, plant species, environmental condition, dosage, interval among applications, the treated surface and living state of the plant surface, in addition to harvest time (Khay et al., 2008).
Although many reports deal with neonicotinoids insecticidal properties, minimal details on the effect on plant growth are reported (Preetha and Stanley, 2012). The present results indicated that the neonicotinoids, were found to exert an effect on vegetative growth characters in the fennel. Acetamiprid and thiamethoxam induced a gradual increase in the total protein content of fruits. A significant influence of thiamethoxam and imidacloprid on the oil% was found in fennel fruits. In this context, neonicotinoids such as thiomethoxam has variable and potentially beneficial influences on plant strength, growth and stress response (Afifi et al., 2015).
Our results indicated that azadirachtin had a significant influence on all growth attributes, this is maybe due to that azadirachtin is the major constituent of neem oil extract, and has toxic effects on insects and antifeedant effect (Morgan, 2009). Moreover, neem is safe for workers, and can be used during the entire crop production periods (Boeke et al., 2004).
5 Conclusion
Results showed that using of thiocyclam, sulfoxaflor and azadirachtin gave highest control of aphid and increased the fruit yield of fennel plant. Thus, neonicotinoids group could be included as useful tactic in aphid management. Moreover, these insecticides could be used to compute pre-harvest residues in fennel at any point time and hence ensure safe application. Therefore, they could be used in the integrated pest management program of this insect pest. However, more investigations are needed to improve the understanding of the complex interactions between plants and these tested insecticides.
Funding
This publication was funded by Taif University Researchers Supporting Project number (TURSP-2020/92), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aphicidal activity of imidacloprid and primicarb compared with certain plant extracts on Brevicoryne brassicae L. and Aphis craccivora Koch. Assiut J. Agric. Sci.. 2012;43:104-114.
- [Google Scholar]
- Thiamethoxam as a seed treatment alters the physiological response of maize (Zea mays) seedlings to neighbouring weeds. Pest Manag. Sci.. 2015;71(4):505-514.
- [Google Scholar]
- A review of sulfoxaflor, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Entomol. Acarol. Res.. 2018;50:7836.
- [Google Scholar]
- The global status of insect resistance to neonicotinoid insecticides. Pesticide Biochem. Physiol.. 2015;121:78-87.
- [Google Scholar]
- Characterization of fennel fruits: types and quality (I) Life Sci. J.. 2012;9:686-691.
- [Google Scholar]
- Safety evaluation of neem (Azadirachta indica) derived pesticides. J Ethnopharmacol. 2004;94(1):25-41.
- [Google Scholar]
- Evaluation of pesticide residues in open field and greenhouse tomatoes from Colombia. Food Control. 2013;30(2):400-403.
- [Google Scholar]
- Contribution for the characterization of Portuguese fennel chemotypes. J. Essen. Oil Res.. 1993;5(2):223-225.
- [Google Scholar]
- Assessment of the attack of Hyadaphis foeniculi (Passerini) (Hemiptera: Aphididae) on biomass, seed and oil in fennel intercropped with cotton with colored fibers. Indu. Crop Prod.. 2015;77:511-515.
- [Google Scholar]
- Imidacloprid-a new systemic insecticide. Pflanzenschutz-Nachr Bayer. 1991;44:113-136.
- [Google Scholar]
- Hyadaphis foeniculi na cultura de erva-doce no Estado de Pernambuco. Pesqui Agropecu Bras. 2004;39(12):1265-1266.
- [Google Scholar]
- Can estragole in fennel seed decoctions really be considered a danger for human health? A fennel safety update. Evid.-Based Complement. Alternat. Med.. 2012;2012:1-10.
- [Google Scholar]
- IRAC, Insecticide Resistance Action Committee., 2019. IRAC Mode of Action Classification, v. 9.3, IRAC Mode of Action Working Group. file:///C:/Users/admin/Downloads/MoA_-brochure_Ed6_19July19.pdf.
- Effective control of brinjal sucking pests by imidacloprid. Plant Prot. Bull. Faridabed. 1994;46:43-44.
- [Google Scholar]
- Overview of the status and global strategy for neonicotinoids. J. Agric. Food. Chem.. 2011;59(7):2897-2908.
- [Google Scholar]
- Dissipation behavior of lufenuron, benzoyphenylurea insecticide, in/on Chines cabbage applied by foliar spraying under greenhouse condition. Bull. Environ. Contamin. Toxicol.. 2008;81:369-372.
- [Google Scholar]
- Lee, S.M., Klocte, A., Barnaby, M.A., Yamasak, R.B., Balandrina, M.F., 1991. Insecticide constituents of Azadirachta indica and Melia azedarach pages 293-304. In naturally occurring pest Bioregalators. P.A. Hedin (Editor). American Chemical Society Symposium Series, No. 449, Washington DC.
- Miguel, M.G., Cruz, C., Faleiro, L., Simões, M.T., Figueiredo, A.C., Barroso, J.G., Pedro, L.G., 2010. Foeniculum vulgare essential oils: chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Commun. 5, p.1934578X1000500231.
- Essential oil composition and antifungal activity of Foeniculum vulgare Mill. Obtained by different distillation conditions. Phytother. Res.. 2003;17(4):368-371.
- [Google Scholar]
- Residues of avermectin B1a in rotational crops and soils following soil treatment with (C14) avermectin B1a. J. Agric. Food. Chem.. 1987;35:859-864.
- [Google Scholar]
- Method for gas chromatography determination of residue tokuthion and its Oxon in plants and soil samples. Pflanzenschutz-Nachrichten Bayer. 1975;28:382-387.
- [Google Scholar]
- Extraction techniques for pesticide residues analysis in edible oils and role of sorbents in cleanup. Sep. Sci. Plus. 2020;3(3):51-62.
- [Google Scholar]
- Integrated management of Aphis craccivora in Cowpea using intercropping and entomopathogenic fungi under field conditions. J. Fungi.. 2020;6:60.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia. 2003;74(3):317-319.
- [Google Scholar]
- Influence of neonicotinoid insecticides on the plant growth attributes of cotton and okra. J. Plant Nutr.. 2012;35(8):1234-1245.
- [Google Scholar]
- Anti-termitic activity of three essential oils, chlorpyrifos, and a bioagent compound (Protecto) against Termite Microcerotermes eugnathus Silvestri (Isoptera: Termitidae) in Egypt. Insects. 2020;11:756.
- [CrossRef] [Google Scholar]
- Aphids on Trees and Shrubs. HYG-2031-90. Ohio State University Extension Fact sheet, Department of Horticulture and Crop Science. USA: Ohio State University; 2001.
- Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Indu. Crop Prod.. 2009;30(1):126-130.
- [Google Scholar]
- Oilpalm Cultivation and Management (Second Edition). Incorporated Society of Planters; 2003. p. :915.
- An Introduction to Insecticides, The Pesticide Book (6th Ed). Willoughby, Ohio: MeisterPro Information Resources; 2004.
- Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. Agric. Food. Chem.. 2011;59(7):2950-2957.
- [Google Scholar]







