Translate this page into:
Efficacy and safety of Amomum villosum extracts in obese adults: A randomized, double-blind, placebo-controlled trial
⁎Corresponding author at: Ilwonbio Co., Ltd, & Department of Physiology, College of Korean Medicine, Wonkwang University, 460 Iksandaero, Iksan, Jeonbuk 54538, South Korea. desson@wku.ac.kr (Kang-Beom Kwon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
This study aimed to assess how well Amomum villosum water extract (AVE) assisted overweight to moderately obese people in losing weight.
Methods
Eighty participants were chosen at random for the AVE group or the placebo group for this experiment. Subjects were given two tablets of the test substance after their two consistent meals daily for 12 weeks. At the starting and completion of the experiment, measurements of body mass index (BMI), body weight, percent body fat, body fat mass, visceral adipose tissue (VAT), lean body mass, subcutaneous adipose tissue (SAT), percent VAT (%), and percent SAT (%) were taken. Before and after the study, blood samples were obtained for safety laboratory parameters.
Results
After taking the products for 12 weeks, the AVE group lost considerably more weight than the placebo group (-2.04 ± 3.04 kg vs −0.30 ± 2.88 kg, respectively; P < 0.014). Additionally, the AVE group showed reduction in body fat mass of −1393.63 ± 2268.80 g as compared to the placebo group's reduction of 51.67 ± 2111.05 g (P < 0.006). In comparison to the placebo group, VAT and SAT weight significantly lowered in the AVE group. There were no negative effects associated with AVE consumption. Before and after AVE administration, 31 of 38 participants (81.6 %) lost weight. Similarly, 26 of 38 members (68.4 %) lost weight and body fat mass before and after AVE administration.
Conclusions
These results suggest that AVE might be a reliable and secure weight regulation. WKUIOMH-IRB-2020–01 is the registration number for this trial.
Keywords
Amomum villosum
Body weight
Obesity
Visceral adipose tissue
Randomized controlled trial
1 Introduction
Obesity is the most common contributor to the probability of numerous metabolic diseases and chronic illnesses like diabetes, heart disease, and some types of cancer (Guo et al., 2018). Frequency of overweight and obesity has reached crisis levels globally over the past 50 years (Yanovski, 2018; NCD Risk Factor Collaboration, 2017; NCD Risk Factor Collaboration, 2016). The most comprehensive data on changes in obesity prevalence around the world over the past 40 years has been supplied by researchers from the Noncommunicable diseases (NCDs) Risk Factor Collaboration (NCD Risk Factor Collaboration, 2017).
Obesity rates grew throughout each country between 1975 and 2016 according to the current study that provides patterns in BMI for all countries worldwide depending on the measurement of body mass and heights data from 128.9 million individuals including children, adolescents, and adults (NCD Risk Factor Collaboration, 2017). Obesity is defined by an increase in body mass and fat tissue as a result of chronically consuming more calories than one expends (Spiegelman and Flier, 2001). As a result of the energy deficit, adipose cells store too many calories followed by triglyceride buildup that causes fat cell enlargement, and abnormal fat cell differentiation causes hyperplasia of fat cells (Hara-Chikuma et al., 2005).
The production of adipocytokines, free fatty acids, and inflammatory mediators is sparked by the hypertrophy, hyperplasia, and associated intracellular malfunction of adipocytes, which results in overall ill health and the beginnings of obesity (De Ferranti and Mozaffarian, 2008). Medicinal plants based medication is a popular management method nowadays, although the majority of synthetic medications have negative outcomes (Schroll et al., 2016). As an example, orlistat works to treat adiposity through blocking activity of lipase and decreasing the long-chain triglycerides absorption. But there is a large list of adverse effects associated with orlistat, including fecal incontinence, oily spotting, diarrhea, and far more significant adverse effects such acute hepatotoxicity (Rucker et al., 2007).
A nutritional supplement is a material designed to complement one or more nutritional elements, such as protein, vitamins, and minerals (Ahmad et al., 2020). The use of natural supplements has become increasingly widespread worldwide. Weight-loss products are one of the key contributors and the estimated value of the 123 billion USD global market for dietary supplements in 2015 (Sun et al., 2016). In the prevention and treatment of various diseases, the bioactive elements of dietary and medicinal plants drawn increased awareness due to their lack of toxicity or low toxicity (Xu et al., 2020).
Additionally, various plants, including white bean, Yerba Mate, garcinia, and green tea have been produced as natural dietary supplements for weight loss (Jayawardena et al., 2020). Various researches have revealed that numerous medicinal plants, including lingonberries, ginger can combat obesity (Walid et al., 2018). Additionally, several plant-based bioactive substances with anti-obesity properties include capsaicin, dioscin, kaempferol, anthocyanin, and quercetin have been reported previously (Poudel et al., 2014). Furthermore, the major weight-loss strategies encompass suppressing appetite, lowering carbohydrate and lipid absorption, inhibiting adipogenesis and lipid synthesis, controlling lipid metabolic activity, increasing energy expenditure, controlling intestinal microbiota, and reducing inflammation caused by obesity (Walid et al., 2018; Poudel et al., 2014).
AVE is an herbal extract prepared from A. villosum Lour. (AV). According to a prior study, AV showed substantial pharmacological effects on ulcer, inflammation, and diarrhea (Huang et al., 2014). Previous studies have also demonstrated that AV extract (AVE) therapy had a substantial impact on postprandial glycemia and insulin secretion in healthy subjects (Kim et al., 2020) and high fat diet (HFD) and high cholesterol diet (HCD)-induced obesity in mice models (Kim et al., 2021, Kim et al., 2022). Additionally, AVE significantly reduced a-glucosidase activity (Kim et al., 2022).
In the current investigation, we conducted a 12-week, double-blind, randomized, placebo-controlled trial to look at the efficacy of AVE on reducing adiposity in obese people. In order to assess AVE efficiency, we have applied dual-energy X-ray absorptiometry (DEXA) to analyze total and regional body fats, anthropometric measurements, and metabolic markers.
2 Materials and methods
2.1 Investigation materials
The identification of A. villosum Lour. (AV) was made using a voucher specimen that was placed at the Department of Herbology at the Wonkwang University Korean Medical School by G. Lee, a specialist in the field. Amomum villosum, which had been finely ground, was steeped in boiling water for four hours before being centrifuged. The resulting supernatant was then concentrated under decreased pressure and permitted to thoroughly dry. In the end, a water extract from Amomum villosum was collected and given the acronym AVE and employed in additional studies. Composition of test (AVE) and placebo products were illustrated in Table 1.
Composition of materials
Test Product (%)
(AVE)Placebo Product (%)
(Placebo)
AVE
50.0
–
Dextrin
–
50.0
Lactose
12.0
12.0
Cellulose, Crystalline
32.4
32.4
Hydroxypropyl Cellulose
2.4
2.4
Glycerine Ester of Fatty acid
0.54
0.54
Titanium Dioxide
0.1
0.1
Cacao Color
0.5
0.5
Powder of Gardenia Yellow
0.06
0.06
Silicon Dioxide
1.0
1.0
Magnesium Stearate
1.0
1.0
Total
100
100
2.2 Subjects
Eighty (80) female and male participants between the ages of 20 and 75 were took part in this study. Other requirements for participation included maintaining a routine exercise throughout the clinical trial. Aside from those characteristics, prior to enlistment, maintain a constant and steady body weight for three months. Gestation period or midwifery, within 6 months earlier to the program, drug abuse, endocrine disorders, uncontrolled diabetes mellitus type 2, diabetes mellitus type 1, hypersensitivity reactions to the study’s test supplements, hypothyroidism or hyperthyroidism, hepatic disease, Cushing syndrome, renal disease, malignant tumor, high blood glucose, digestive disease or any additional sickness disturbing the outcomes of the clinical trial, involvement in additional experimental trial were the main exclusion criteria.
All participants freely provided written informed consent before the study. The institutional review board (IRB) at Wonkwang University Korean Medicine Hospital (Iksan, Korea) approved the clinical trial (IRB No. WKUIOMH-IRB-2020-01) which followed the principles set forth in the Helsinki Declaration (Williams, 2008).
2.3 Treatments
Before being randomly assigned to the 12-week treatment phase, subjects had a 2-week run-in phase to evaluate their amenability with the study's conditions and the suggested eating regimen. According to the randomization code provided by a separate statistical analyst, randomly chosen groups of individuals received either AVE treatment or placebo treatment. Both researchers and participants were unaware of the participants' group assignment. Each subject received a single-sealed, opaque packet from the clinical research unit that held the treatment code and was only to be unsealed in an urgent situation. Thus, products allocation was hidden, and masking was successfully accomplished throughout the trial since no closed package was broken knowingly or unknowingly, or was damaged with. Each active tablet contains 250 mg AVE and 250 mg excipients. Each placebo tablet contains 500 mg of excipient and is identical in look, size, weight, and color. Individuals were told to take two tablets each day, one tablet at a time, after lunch and dinner for 12 weeks. The researcher distributed test materials every six weeks, and compliance was evaluated by numbering the tablets that were retrieved and simultaneously checking the subjects' diaries. A 3-day eating history was used to track the individuals' dietary patterns throughout two weekdays and one weekend. They were also asked to complete a Global physical activity questionnaire (GPAQ) in order to track their physical patterns over the course of a typical week.
2.4 Measurement of abdominal fat area
DEXA (Lunar Prodigy Advance; GE, Wisconsin, USA) scan was utilized to examine body compositions such as percentage body fat, lean body mass, body fat mass, subcutaneous and visceral fat weight with percentage measures.
2.5 Measurement of anthropometric and circulatory parameters
Every test involved measuring the subject's body weight, height, body fat percentage, hip and waist circumferences, systolic and diastolic blood pressures, and pulse rate. While the subject was standing, the subject's waist was measured to the nearest 0.1 cm between the iliac crest and the lowest rib. The circumference of the hip was measured to the closest 0.1 cm. Only when doing a screening test were height measurements taken. Height and weight were used to calculate the BMI values. This equation was used to determine the BMI: BMI = Kg (Weight)/m2 (Height).
2.6 Hematological examination, blood biochemistry, and urinalysis
At Wonkwang University Korean Medicine Hospital, routine laboratory procedures were used to analyze complete blood count (CBC) including WBC, RBC, hemoglobin, and hematocrit. Blood biochemistry including total protein, albumin, total bilirubin, alkaline phosphatase (ALP), creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LD), Gamma-glutamyl transpeptidase (GGT), glucose, blood urea nitrogen (BUN), creatinine, adiponectin and leptin. Urinalysis including pH and specific gravity. Vital sign readings (body temperature, heart rate, and blood pressure), and adverse event monitoring were all done as part of safety evaluations.
2.7 Statistical analysis
The baseline characteristics of the two groups were compared using independent t-tests, chi-square tests, and fisher's exact tests. CBC, blood biochemistry, urinalysis, and anthropometric characteristics were compared between the two groups using an independent t-test. Results were shown as the mean ± SD. If P < 0.05, the result was regarded as significant. Applying the SAS software program, version 9.3 for all statistical analyses (SAS Institute, Cary, NC, USA).
3 Results
3.1 Baseline characteristics
This clinical investigation was conducted from June 2020 to January 2021. Eighty (80) subjects were randomized into two different (active and placebo) groups. Due to withdrawal of consent (n = 1) and adverse event (n = 1), there were two individuals in the placebo group who were dropped from the study. Two individuals from each group (two from the active group and two from the placebo group) Four people, two from each group were excluded during the efficacy analysis as a result of protocol breaks. In terms of body height, gender distribution, age, weight, BMI, hip to waist ratio, body fat mass, diastolic blood pressure (DBP), systolic blood pressure (SBP), thyroid stimulating hormone (TSH), pulse, alcohol intake, and smoking, there was no statistically significant difference between the study groups. Fig. 1 shows a flowchart of the study population. Table 2 lists the baseline characteristics of the two different study groups. In each group, there were more female subjects (n = 34) than male (n = 6) ones, with a mean subject age of 37.20 ± 8.94 years. Values are presented as mean ± SD or number (%). TSH, thyroid stimulating hormone; SBP, systolic blood pressure; DBP, diastolic blood pressure.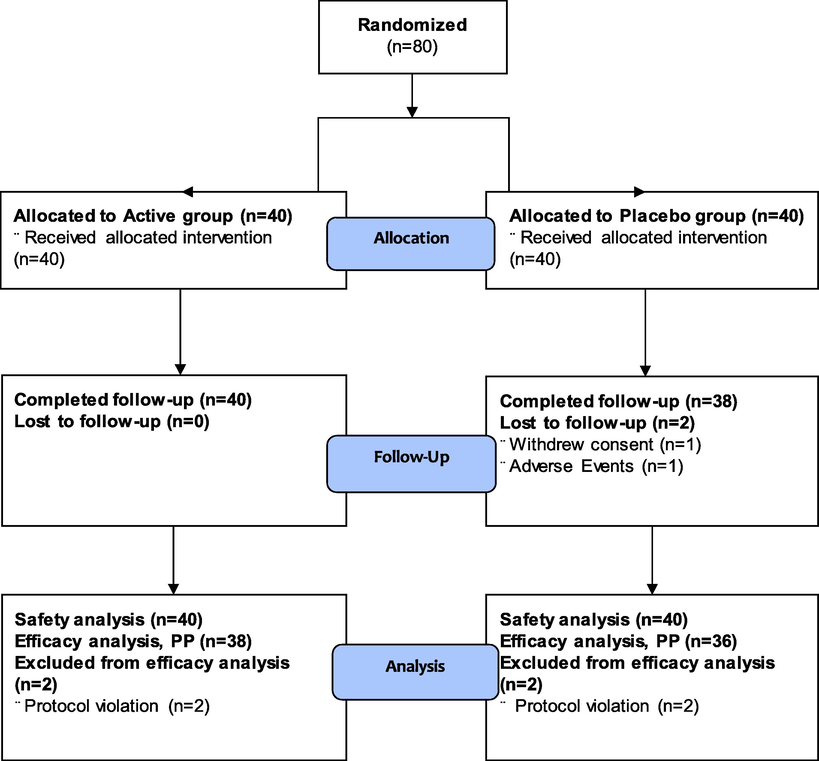
Clinical trial flowchart.
Parameters
AVE (n = 40)
Placebo (n = 40)
Total (n = 80)
p-value1)
Sex (M/F)
6/34
6/34
12/68
1.0002)
Age (years)
36.98 ± 9.08
37.43 ± 8.91
37.20 ± 8.94
0.824
Height (cm)
163.78 ± 6.58
162.85 ± 6.95
163.31 ± 6.74
0.824
Weight (kg)
69.90 ± 9.70
70.57 ± 13.04
70.23 ± 11.43
0.797
BMI (kg/m2)
26.03 ± 3.05
26.52 ± 3.92
26.27 ± 3.50
0.533
Waist circumference (cm)
89.70 ± 7.57
89.40 ± 10.05
89.55 ± 8.84
0.881
Hip circumference (cm)
100.39 ± 4.89
99.95 ± 7.69
100.17 ± 6.41
0.758
Waist to hip ratio
0.89 ± 0.05
0.89 ± 0.06
0.89 ± 0.06
0.923
Percent body fat (%)
35.37 ± 6.57
35.88 ± 5.72
35.63 ± 6.12
0.713
TSH (μIU/mL)
1.98 ± 1.06
2.17 ± 1.23
2.08 ± 1.15
0.468
SBP (mmHg)
125.73 ± 10.69
124.45 ± 13.82
125.09 ± 12.29
0.646
DBP (mmHg)
74.95 ± 9.16
76.88 ± 6.89
75.91 ± 8.11
0.291
Pulse (beats/min)
80.00 ± 10.80
83.53 ± 10.11
81.76 ± 10.54
0.136
Alcohol (n, %)
25 (62.50)
20 (50.00)
45 (56.25)
0.2602)
Alcohol (units/week)
5.50 ± 3.96
5.39 ± 4.38
5.45 ± 4.10
0.933
Smoking (n, %)
4 (10.00)
5 (12.50)
9 (11.25)
1.0003)
3.2 Anthropometric measures
Table 3 displays the variations in anthropometric measurements before and after AVE treatment. The study groups all started out with similar body weights. However, in the AVE treated group compared to the placebo group, body mass substantially decreased from baseline to week 12 (-2.04 ± 3.04 vs −0.30 ± 2.88 kg; P < 0.014). At the end of the study, the mean BMI of the AVE-treated group was considerably lesser compared to the placebo group (-0.76 ± 1.16 vs −0.10 ± 1.10 kg/m2, P < 0.014). Compared to the placebo group, the AVE treated group showed decline of body fat mass (-1393.63 ± 2268.80 vs 51.67 ± 2111.05 g, P < 0.006), and percent body fat (-1.05 ± 2.21 vs 0.12 ± 2.08 %, P < 0.022) from baseline to week 12. Lean body mass is also reduced by AVE treatment compared to the placebo group, but the values are statistically insignificant (-643.16 ± 1512.97 vs −354.44 ± 1666.82 g, P < 0.437). Furthermore, AVE showed significant reductions in visceral adipose tissue (VAT) of −41.39 ± 111.75 vs 21.17 ± 66.60 g (P < 0.005), and subcutaneous adipose tissue (SAT) of −19.13 ± 35.40 vs 6.67 ± 24.85 g (P < 0.001) from baseline to week 12. Percent of VAT (-1.13 ± 2.97 vs 0.17 ± 2.08 %, P < 0.032) and SAT (-0.58 ± 0.89 vs 0.03 ± 0.51 %, P < 0.001) values were also considerably reduced in the AVE treated group compared to placebo from baseline to week 12. Variation of obesity-related hormones in serum at week 0 and 12 are shown in Table 4. Serum adiponectin level (123.54 ± 459.75 vs −127.76 ± 524.67 ng/mL, P < 0.031) significantly increased and serum leptin level (-658.40 ± 2130.66 vs 217.82 ± 1472.25 pg/mL, P < 0.043) significantly decreased in AVE treated group compared to placebo from baseline to week 12. Values are presented as mean ± SD. Values are presented as mean ± SD.
Parameters
AVE (n = 38)
Placebo (n = 36)
p-value1)
0 week
12 week
Change value
0 week
12 week
Change value
Weight
(kg)69.15 ± 9.00
67.11 ± 8.51
−2.04 ± 3.04
71.73 ± 12.99
71.42 ± 13.07
−0.30 ± 2.88
0.014*
BMI
(kg/m2)25.82 ± 2.90
25.06 ± 2.70
−0.76 ± 1.16
26.82 ± 3.99
26.72 ± 4.12
−0.10 ± 1.10
0.014*
Body Fat Mass
(g)22208.82 ± 5623.17
20815.18 ± 5476.83
−1393.63 ± 2268.80
23902.56 ± 7251.02
23954.22 ± 7477.65
51.67 ± 2111.05
0.006**
Percent Body Fat (%)
32.06 ± 6.33
31.01 ± 6.90
−1.05 ± 2.21
32.98 ± 6.46
33.10 ± 6.54
0.12 ± 2.08
0.022*
Lean Body Mass (g)
46938.61 ± 7611.99
46295.45 ± 7823.84
−643.16 ± 1512.97
47822.50 ± 8710.55
47468.06 ± 8354.81
−354.44 ± 1666.82
0.437
VAT (g)
653.37 ± 250.08
611.97 ± 233.76
−41.39 ± 111.75
752.72 ± 287.74
773.89 ± 298.77
21.17 ± 66.60
0.005**
SAT (g)
435.97 ± 50.35
416.84 ± 50.85
−19.13 ± 35.40
454.14 ± 69.44
460.81 ± 72.52
6.67 ± 24.85
0.001**
Percent VAT (%)
24.58 ± 6.80
23.45 ± 7.00
−1.13 ± 2.97
26.61 ± 6.03
26.78 ± 6.02
0.17 ± 2.08
0.032*
Percent SAT
(%)16.89 ± 0.76
16.32 ± 1.07
−0.58 ± 0.89
16.61 ± 1.02
16.64 ± 1.10
0.03 ± 0.51
0.001**
Parameters
AVE (n = 38)
Placebo (n = 36)
p-value1)
0 week
12 week
Change value
0 week
12 week
Change value
Adiponectin (ng/mL)
6169.56 ± 3822.25
6293.10 ± 3873.15
123.54 ± 459.75
4806.36 ± 2932.54
4678.61 ± 3117.31
−127.76 ± 524.67
0.031*
Leptin
(pg/mL)12430.03 ± 6727.17
11771.64 ± 6882.24
−658.40 ± 2130.66
12933.24 ± 6045.42
13151.06 ± 6503.48
217.82 ± 1472.25
0.043*
3.3 Biochemical variables in blood
Table 5 displays various hematological tests and blood biochemical characteristics. Hematology (WBC, RBC, hematocrit, platelets), blood biochemistry (total proteins, albumin, total bilirubin, ALP, CK, AST, ALT, LD, GGT, glucose, BUN, creatinine) and urinalysis (pH, specific gravity) were conducted to assess the safety of AVE. The two groups did not significantly differ on any of the characteristics. Values are presented as mean ± SD.
Parameters
AVE (n = 40)
Placebo (n = 40)
p-value1)
0 week
12 week
Change value
0 week
12 week
Change value
CBC
WBC (✕103/μl)
6.03 ± 1.32
5.79 ± 1.29
−0.24 ± 1.12
6.31 ± 1.81
6.39 ± 1.84
0.08 ± 1.14
0.216
RBC (✕103/μl)
4.44 ± 0.40
4.45 ± 0.40
0.01 ± 0.22
4.52 ± 0.47
4.50 ± 0.49
−0.03 ± 0.22
0.520
Hemoglobin (g/dL)
13.36 ± 1.36
13.58 ± 1.43
0.22 ± 0.66
13.40 ± 1.44
13.42 ± 1.55
0.02 ± 0.64
0.167
Hematocrit (%)
39.63 ± 3.74
39.31 ± 3.85
−0.32 ± 1.85
39.88 ± 3.98
39.23 ± 4.08
−0.65 ± 2.07
0.454
Platelets count (✕103/μl)
274.13 ± 58.56
275.48 ± 51.35
1.35 ± 29.02
285.30 ± 65.52
283.18 ± 59.16
−2.13 ± 31.24
0.608
Blood biochemistry
Total proteins (g/dL)
6.99 ± 0.33
7.00 ± 0.27
0.01 ± 0.34
7.01 ± 0.31
6.97 ± 0.36
−0.04 ± 0.29
0.462
Albumin (g/dL)
4.26 ± 0.23
4.20 ± 0.20
−0.06 ± 0.17
4.24 ± 0.20
4.19 ± 0.22
−0.05 ± 0.21
0.908
Total bilirubin (mg/dL)
0.67 ± 0.22
0.70 ± 0.28
0.03 ± 0.20
0.69 ± 0.32
0.61 ± 0.21
−0.08 ± 0.33
0.101
ALP (IU/L)
169.90 ± 37.87
173.53 ± 38.80
3.63 ± 23.30
174.38 ± 40.80
178.25 ± 48.34
3.88 ± 19.65
0.959
CK (U/L)
115.53 ± 98.69
99.38 ± 48.35
−16.15 ± 80.85
143.45 ± 358.55
101.50 ± 101.81
−41.95 ± 366.86
0.666
AST (IU/L)
20.63 ± 6.58
21.90 ± 6.72
1.28 ± 8.04
20.58 ± 8.47
20.98 ± 6.90
0.40 ± 7.38
0.613
ALT (IU/L)
17.88 ± 9.91
17.68 ± 9.05
−0.20 ± 9.33
18.88 ± 12.23
18.98 ± 10.71
0.10 ± 6.63
0.869
LD (U/L)
168.68 ± 28.85
183.38 ± 30.08
14.70 ± 21.92
166.63 ± 37.98
184.05 ± 31.64
17.43 ± 36.21
0.685
GGT (IU/L)
22.10 ± 11.74
21.78 ± 11.28
−0.33 ± 6.01
23.38 ± 16.84
22.70 ± 14.32
−0.68 ± 8.05
0.826
Glucose (mg/dL)
85.93 ± 8.41
81.38 ± 8.43
−4.55 ± 7.65
84.98 ± 8.41
78.45 ± 8.16
−6.53 ± 11.06
0.356
BUN (mg/dL)
11.05 ± 3.00
11.57 ± 2.96
0.52 ± 3.57
12.54 ± 4.19
11.90 ± 2.93
−0.64 ± 3.18
0.130
Creatinine (mg/dL)
0.83 ± 0.16
0.83 ± 0.16
−0.01 ± 0.07
0.86 ± 0.13
0.83 ± 0.13
−0.03 ± 0.06
0.241
Urinalysis
pH
6.18 ± 1.05
5.79 ± 0.85
−0.39 ± 1.13
5.75 ± 0.95
5.83 ± 0.91
0.08 ± 1.01
0.057
Specific gravity
1.02 ± 0.01
1.02 ± 0.01
0.00 ± 0.01
1.02 ± 0.01
1.02 ± 0.00
0.00 ± 0.01
0.584
4 Discussion
In this research article, we examined the impact of AVE supplementation on obese adults. The risk of metabolic diseases rises with obesity. Numerous studies on medications and foods with special functions that can prevent obesity are being done as a result of the fast spreading worldwide obesity epidemic.
It is challenging methodologically to compare AVE to other fat-consuming weight-loss medications like orlistat, chitosan, or Litramine®. After receiving chitosan therapy for twelve weeks, Ho et al. noticed no significant alterations in body mass from base point (Ho et al., 2001). After twelve weeks of consumption, a medication with the patented fiber combination Litramine® resulted in a 3.8 kg weight reduction (Grube et al., 2013). According to a research by Anderson et al., orlistat (a gastric and pancreatic lipase inhibitor) influenced a 3.05 kg body weight reduction in obese participants after sixteen weeks treatment at 180 mg per each day (Anderson et al., 2006), whereas it contributed a body mass decline of 8.3 kg after twelve weeks in overweight subjects at a high dosage of 360 mg per each day. According to our research, AVE considerably reduced, percent body fat, body fat mass, BMI, VAT in addition to SAT. The probability of non - alcoholic fatty liver disease, diabetes, and coronary heart disease may all be reduced by treatment with AVE if body fat mass, percent body fat, VAT and SAT, an indicator of abdominal fat content, decreases (Flint et al., 2010; Clemente et al., 2016; Gautier et al., 2010). According to the current study, 31 of 38 individuals (81.6 %) lost weight before and after AVE treatment. The current clinical investigation also shows that 26 of 38 individuals (68.4 %) lost weight and body fat mass before and after AVE treatment.
Leptin and adiponectin are two adipokines that are produced by adipose tissue and are involved in the control of the energy balance and have an impact on cellular metabolic processes. Leptin regulates appetite and keeps the body's energy levels balanced. Its levels rise in cases of obesity (Klok et al., 2007). Unlike leptin, adiponectin levels drop in cases of obesity. Adiponectin mediates anti-inflammatory, anti-atherosclerotic, and anti-hyperglycemic activities (Matsubara et al., 2002). A widespread shift in the levels of many adipokines in the bloodstream is associated with the disproportional formation of white adipose tissue in overweight and obesity. The increased risk of a number of associated diseases in obese persons is assumed to be caused by adipokine dysregulation and adipokine dysfunction, or at the very least, it is one of the major contributors to this risk. For example, higher levels of proinflammatory adipokines, such as leptin, and decline of anti-inflammatory adipokines, for example adiponectin, in obese people generate a chronic condition of low-grade inflammatory response that endorses the insulin resistance development, coronary heart disease, high blood pressure, and type-2 diabetes (Hotamisligil, 2017). In addition, because adiponectin also functions as an insulin-sensitizing hormone in the liver and muscles, reduced adiponectin level in obese people substantially increase peripheral insulin resistance (Saltiel and Olefsky, 2017). On the contrary, elevated levels of leptin in the blood cause hypothalamic leptin resistance, which suppresses signals related to anorexia and energy expenditure and worsens obesity (Waterson and Horvath, 2015).
In the current study, AVE significantly decreased serum leptin level and considerably increased serum adiponectin level suggested that its potential effects on augmentation of insulin sensitivity and anti-obesity nature. Our findings stand in line with previous results (Ghanbari et al., 2022; Kim et al., 2018). AVE therapy decreased hepatic triglyceride level and dramatically raised adiponectin expression in adipocytes in the preliminary investigation in mice fed a high-fat diet (Kim et al., 2021). Additionally, SREBP1, FAS, CCAAT/enhancer binding protein (C/EBP)-α, PPAR-γ, and tumor necrosis factor (TNF)-α mRNA expression were all considerably downregulated by AVE therapy, which further significantly reduced adipogenesis (Kim et al., 2021). In a similar manner, rats fed a high-cholesterol diet and given AVE treatment for four weeks exhibited significantly lower levels of blood TG, TC, LDL-C, and hepatic TG as well as lower liver, epididymal fat, and body weights. The AVE therapy also significantly increased the levels of mRNA expression for the HMG-CoA reductase (HMGCR), the sterol regulatory element-binding protein 2 (SREBP2), and the LDL receptor (LDLR) (Kim et al., 2022).
In the previous human investigation, AVE supplementation showed a substantial decrease in postprandial blood glucose and postprandial blood insulin level compared to the placebo group (Kim et al., 2020). The VAT and SAT of obese persons in this study were considerably reduced with AVE supplementation. Fat is divided into visceral and subcutaneous compartments in humans. In the relationship between obesity, metabolism, and health, many studies have demonstrated that the regional distribution of body fat is a more significant factor than the degree of generalized obesity (Leizorovicz et al., 1994), demonstrating that VAT and SAT were more strongly associated with a poor metabolic risk profile. Decreases in VAT and SAT may therefore have a favorable impact on metabolic risk factors (Aronsson et al., 2010; Wernsdorfer et al., 2009).
In conclusion, the current investigation showed that AVE supplementation had an antiobese impact by lowering the VAT and SAT in obesity people. The results of this investigation are consistent with those of earlier animal experiments. The findings, along with those of earlier research, point to the possibility that AVE may reduce adiposity in obese people.
Acknowledgement
This present study was supported by Wonkwang University, South Korea in 2020.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Extraction and UHPLC-DAD detection of undeclared substances in market-available dietary supplements and slimming products in Eastern region, Saudi Arabia: An application of principal component analysis. Biomed. Chromatogr.. 2020;34(1):e4698.
- [Google Scholar]
- Low-dose orlistat effects on body weight of mildly to moderately overweight individuals: a 16 week, double-blind, placebo-controlled trial. Ann. Pharmacother.. 2006;40(10):1717-1723.
- [CrossRef] [Google Scholar]
- Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4) PloS one. 2010;5(9):e13087.
- [Google Scholar]
- Waist circumference as a marker for screening nonalcoholic fatty liver disease in obese adolescents. Rev. Paul. Pediatr.. 2016;34(1):47-55.
- [CrossRef] [Google Scholar]
- The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem.. 2008;54(6):945-955.
- [CrossRef] [Google Scholar]
- Flint, A. J., Rexrode, K. M., Hu, F. B., Glynn, R. J., Caspard, H., Manson, J. E., Willett, W. C., Rimm, E. B., 2010. Body mass index, waist circumference, and risk of coronary heart disease: a prospective study among men and women. Obes. Res. Clin. Pract. 4(3), e171–e181. https://doi.org/10.1016/j.orcp.2010.01.001.
- Gautier, A., Roussel, R., Ducluzeau, P. H., Lange, C., Vol, S., Balkau, B., Bonnet, F., DESIR Study Group., 2010. Increases in waist circumference and weight as predictors of type 2 diabetes in individuals with impaired fasting glucose: influence of baseline BMI: data from the DESIR study. Diabetes care 33(8), 1850–1852. https://doi.org/10.2337/dc10-0368.
- Artemisia annua L. extracts improved insulin resistance via changing adiponectin, leptin and resistin production in hfd/stz diabetic mice. J. Pharmacopuncture. 2022;25(2):130-137.
- [CrossRef] [Google Scholar]
- A natural fiber complex reduces body weight in the overweight and obese: a double-blind, randomized, placebo-controlled study. Obesity (Silver Spring, Md.). 2013;21(1):58-64.
- [CrossRef] [Google Scholar]
- The relationship between lipid phytochemicals, obesity and its related chronic diseases. Food funct.. 2018;9(12):6048-6062.
- [CrossRef] [Google Scholar]
- Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem.. 2005;280(16):15493-15496.
- [CrossRef] [Google Scholar]
- In the absence of dietary surveillance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singapore Med. J.. 2001;42(1):6-10.
- [Google Scholar]
- Foundations of Immunometabolism and Implications for metabolic health and disease. Immunity. 2017;47(3):406-420.
- [CrossRef] [Google Scholar]
- SNP typing for germplasm identification of Amomum villosum Lour. Based on DNA barcoding markers. PloS One. 2014;9(12):e114940.
- [Google Scholar]
- Availability and composition of weight-loss supplements in Sri Lanka. Nutr. Diet.. 2020;77(2):247-252.
- [CrossRef] [Google Scholar]
- Amomum villosum Lour. fruit extract ameliorates high-fat dietinduced body mass gain and adipogenic pathways in C57BL/6 mice. J. King Saud Univ. Sci.. 2021;33:101473
- [Google Scholar]
- Inhibitory effect of Amomum villosum water extracts on α-glucosidase activity. Physiol. Mol. Plant Pathol.. 2022;117:101779
- [CrossRef] [Google Scholar]
- Amomum villosum Lour. Fruit extract mitigates hyperlipidemia through SREBP-2/LDLR/HMGCR signaling in high-cholesterol diet-fed mice. J. King Saud Univ. Sci.. 2022;34:102230
- [Google Scholar]
- Acute effects of Amomum villosum Lour. fruit extract on postprandial glycemia and insulin secretion: A single-blind, placebo-controlled, crossover study in healthy subjects. Saudi. J. Biol. Sci.. 2020;27(11):2968-2971.
- [CrossRef] [Google Scholar]
- Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: a randomized, double-blind, placebo-controlled trial. J. Med. Food. 2018;21(5):454-461.
- [CrossRef] [Google Scholar]
- The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev.. 2007;8(1):21-34.
- [CrossRef] [Google Scholar]
- Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: a meta-analysis. BMJ. 1994;309(6950):299-304.
- [CrossRef] [Google Scholar]
- Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol.. 2002;147(2):173-180.
- [CrossRef] [Google Scholar]
- Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int. J. Mol. Med.. 2014;34(5):1401-1408.
- [CrossRef] [Google Scholar]
- NCD Risk Factor Collaboration (NCD- RisC)., 2016. Trends in adult body- mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population- based measurement studies with 19.2 million participants. Lancet 387, 1377–1396.
- NCD Risk Factor Collaboration (NCD- RisC)., 2017. Worldwide trends in body- mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population- based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642.
- Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ (Clinical research ed.). 2007;335(7631):1194-1199.
- [CrossRef] [Google Scholar]
- Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest.. 2017;127(1):1-4.
- [CrossRef] [Google Scholar]
- Assessment of adverse events in protocols, clinical study reports, and published papers of trials of orlistat: A document analysis. PLoS Med.. 2016;13(8):e1002101.
- [Google Scholar]
- Obesity and the regulation of energy balance. Cell. 2001;104(4):531-543.
- [CrossRef] [Google Scholar]
- Natural Dietary and Herbal Products in Anti-Obesity Treatment. Molecules. 2016;21(10):1351.
- [CrossRef] [Google Scholar]
- Beneficial effects of Aloe vera gel on lipid profile, lipase activities and oxidant/antioxidant status in obese rats. J. Funct. Foods. 2018;48:525-532.
- [CrossRef] [Google Scholar]
- Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab.. 2015;22(6):962-970.
- [CrossRef] [Google Scholar]
- Activity of Eurycoma longifolia root extract against Plasmodium falciparum in vitro. Wien Klin. Wochenschr.. 2009;121(Suppl. 3):23-26.
- [CrossRef] [Google Scholar]
- The Declaration of Helsinki and public health. Bull World Health Organ.. 2008;86(8):650-652. PMID: 18797627; PMCID: PMC2649471
- [CrossRef] [Google Scholar]
- Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr.. 2020;60(10):1693-1705.
- [CrossRef] [Google Scholar]
- Obesity: Trends in underweight and obesity - scale of the problem. Nat. Rev. Endocrinol.. 2018;14(1):5-6.
- [CrossRef] [Google Scholar]







