Translate this page into:
Effective removal of biofilm formation in Acinetobacter baumannii using chitosan nanoparticles loaded plant essential oils
⁎Corresponding author: State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory of Plant Resources and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China. liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study focused on anti-biofilm property of chitosan loaded essential oils, which were obtained and evaluated against biofilm forming Acinetobacter baumannii. The results of agar well punctured method demonstrated its effeciency at 150 µg/mL concentration. The complete biofilm arrangement was demolished at 150 µg/mL concentration as suggested by quantitative invitro inhibition assay measurement. The biofilm metabolic cells lost their virulence, which was confirmed by color changes of XTT assay. Furthermore, the biofilm metabolic product inactivation was proved by congo red agar assay with the evidence of complete exopolysaccharide arrest. The interior and exterior portions of the chitosan loaded essential oils were completely damaged, as suggested by confocal laser scanning electron microscope and scanning electron microscope observations. Hence, all the invitro inhibition assays, and morphological examination confirmed that the biofilm forming Acinetobacter baumannii lost its virulence factors and its bacterial growth was eradicated efficiently.
Keywords
Chitosan
Essential oils
Biofilm inhibition
Metabolic activity degradation
Exopolysaccharide cleavage
Confocal laser scanning electron microscope
1 Introduction
Worldwide, the most threatening bacteria is Gram negative, opportunistic nature of the Acinetobacter baumannii (Eze et al., 2021). It is a top threatening bacteria in healthcare delivery system and comes under highly critical list by World Health Organization (Al-Shamiri et al., 2021). In mostly caused nosocomial and urinary tract regions. In this virulence factors are the main factors for high occurrence of drug tolerance and produced the biofilm. Acinetobacter baumannii is resistant to almost all the existing classes of antibiotics, particularly beta lactum, tetracyclines, trimethoporin-sulfamethoxazole, fluoroquinolones (Elbehiry et al., 2021). The accumulation of biofilm forming Acinetobacter baumannii is 1000 times more resistant than normal pathogens and it completely developed the exopolysaccharide mediated slime layer (Gautam et al., 2021). This kind of consortium often helped to bacteria to prevent the other environmental stress, antibiotics and drugs. It can be spread easily in some related environments particularly, hospital atmosphere, catheter, foley catheter and cerebrospinal fluid (Mea et al., 2021).
Among the various constituents of plant materials, essential oils are heightened for biomedical application because of the more biological properties (Prakash et al., 2015; Khajenoori et al., 2009). The plant Morinda citrifolia is a tropical plant which is available mostly in South India, Australia and many parts of Southeast Asia (Purkayastha et al., 2012). It also simply called as “noni”, an important medicinal plant that proved as excellent phytochemical derivatives and various biomedical properties including anti-microbial, anti-viral, larvicidal and insecticide (Zhang et al., 2008). In industry, the products of Morinda citrifolia are an inevitable source to use in various processes. Till-date, the complete chemical properties, bioactive compounds, hormones and other biomedical properties are not driven clearly (Alves Veloso et al., 2020; Zhang et al., 2018). Till-date, the researchers are reported that the own metabolism of Morinda citrifolia was used for synthesis of essential oils, which is a complex molecules, arranged a clear organic structure. Previously, more researchers are reported that the M. citrifolia involved in the various biomedical properties, in particular essential oils shown excessive nature (Osorio et al., 2021; Silva et al., 2017). Previously, the chemical composition of M. citrifolia was purified as juice format in industry for eradicate the control of pathogenic microorganisms (Piaru et al., 2012; Jahurul et al., 2021).
Few studies of the essential oils with increased activity against phytopathogens, and more reports with biomedical properties including anti-cancer (Rajivgandhi et al., 2020), anti-oxidant (Suktham et al., 2021), anti-microbial (Sharmeen et al., 2020) and anti-viral properties. For these advantages, the current research was focused on South Indian medicinal plant of Morinda citrifolia essential oils for evaluated against biofilm producing bacteria. To improve the essential oils efficiency, the essential oils were loaded on natural polymer of chitosan, and then evaluated the biomedical activity against biofilm forming Acinetobacter baumannii to detect the essential oils efficiency.
Chitosan is a natural polymer which approved by Food and Drug Administration for use in industry, biopharmaceutical and biomedical process (Granata et al., 2021; Kanimozhi et al., 2018a,b). It highly covered by high units of glucosamine and connected with β (1–4) glycosidic bond. It is available more content in crustacean shells and somewhat in fungal cell wall (Pereira dos Santos et al., 2019; Zhang et al., 2020). In addition, the polymer nanoparticles have received a lot of attention for their use in biological applications. This is due to their exceptional electronic, thermal and optical properties along with their biocompatibility (Kanimozhi et al., 2018a,b; Nalini et al., 2019; Siddhardha et al., 2020). The chitosan structure was arranged with enormous amount of polysachharides (Jamil et al., 2016). Compared with other carrier molecules, chitosan is acted as a excellent reservoir to deliver the drugs and antibiotics. It has highly non-toxic nature, increased adhesive property, flexible biocompatibility, biodegradability and various biological properties such as anti-oxidant, anti-cancer, anti-microbial, larvicidal (Bilal et al., 2020; Zhang et al., 2020; Keykhosravy et al., 2020). Recent years, all the fields including soil and agricultural, food and food additives, industrial process, biopharmaceutical and biomedical properties (Mondéjar-López et al., 2022; Kalagatur et al., 2018). In addition, the incorporation of plant extract, secondary metabolites, essential oils, drugs and other existing antibiotics are delivered in target points and also increasing efficiency level (Ebrahimzadeh et al., 2021; Rajivgandhi et al., 2021; Barzegar et al., 2021). Previously, more researchers are reported with chitosan loaded essential oils have improved biomedical properties and chitosan as an excellent carrier molecule (Kavaz et al., 2019; Kanimozhi et al., 2019; Kayalvizhi et al., 2022). The targeting factors are also very clear and effective against any pathogens using chitosan loaded essential oils (Ashrafi et al., 2019). Therefore, the current study was highly focused on chitosan nanoparticles loaded essential oils which was obtained from School of Life Sciences, Sun Yat-Sen University, China for evaluated against biofilm producing A. baumannii.
2 Materials and methods
2.1 Collection of nanomaterial and pathogens
Initially, the chitosan loaded essential oils nanomaterial was obtained from Department of Ecology and Evolution, Sun Yat-Sen University, Gunagzhou, China for removal of biofilm producing gram negative bacteria. In addition, all the chemicals of this work were purchased from Suresh Scientific @ Co, Tiruchirappalli, Tamil Nadu, India who is the local supplier for Hi-Media, Mumbai, India. All the media and solutions, reagents were purchased from Ponmani @ Co, Tiruchirappalli, Tamil Nadu, India who is the local supplier for Merck, Mumbai, India.
2.2 Agar well puncture method for eradication of biofilm
The initial confirmation effect of essential oils loaded chitosan nanoparticle was evaluated against biofilm forming bacteria using agar well punctured method (Ma et al., 2020). The 48 h mate formed culture was swabbed on already prepared nutrient agar plates after solidification of 30 min. Then, the chitosan loaded essential oils were added various concentration 25–150 μg/mL into the wells and gently shake for equally filled in all places. Next, all the plates were maintained in 37 °C setup incubator 1 day. After one day, the result was compared based on the positive and internal controls result and noted in mm of diameter.
2.3 Quantitative detection of biofilm eradication by 24-well polystyrene method
In adherent cells or attached cells of the biofilm forming Acinetobacter baumannii culture was grown in 24-well polystyrene plate with the presence of tryptic soy broth. Consecutively, 25–200 μg/mL concentrations of chitosan loaded plant essential oils with diluted safely or gently by shaking method. This experiment was completely followed by Maruthupandy et al. (2020). Briefly, 24-well polystyrene plate was used to attached the biofilm cells on the wall was very effective for biofilm producing Acinetobacter baumannii. Based on the above statement, the Acinetobacter baumannii was used in this study to detect the eradication of biofilm formation or not using microtitre plate reader. After one day, the plates were shown highly turbidity with no any culture deposition or high deposition with no turbidity was seen on naked eye. In this experiment, the turbidity based detection of biofilm degradation was indicated that the chitosan loaded essential oils treated the sample. So, the turbidity range, inhibition concentration range and mate differentiation were quantitatively analyzed with the help of 4% crystal violet. Here, stained by 4% crystal violet solution was used for absorption of crushed biofilm places and discorded the crystal violet after 10 min. After treatment, first washed by double distilled water and second washed by PBS. Finally, 2 mL of ethanol solution was added into the wells for degradation of crystal violet solution after consecutive universal wash. Finally read the solution with microtitre plate for attached cells density and then calculated based on the control well and treated wells.
2.4 Metabolic activity inactivation of chitosan loaded essential oils
In live and dead conditions of biofilm forming Acinetobacter baumannii was monitored after treatment with chitosan loaded essential oils followed by the previously evidenced protocol of Govindan et al. (2020). After treatment in 24-well plate, the sample was transferred to 15 mL falcon tube and centrifuged by 2500 rpm for 15 min and then separated supernatant and pellet in falcon tube. After the solution of 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)–2H-tetrazolium-5- carboxanilide (XTT) was added each two ml in control and test samples. In lag phase, log (exponential phase), the biofilm producing bacteria was produced the different virulence factors vigorously. In the treatment of chitosan nanoparticles loaded essential oils, all the virulence factors were produced irregular role means of inactivation. Then, after 1 h time duration, the menadione acetone solution was added into the XTT plus chitosan nanoparticles treated biofilm pathogen containing mixture sample. Also added in untreated control samples also. Both the treated and untreated samples tubes were maintained 1 h and allowed to check the color production formed or not. The color changes were also confirmed by transferring color varied tubes by naked eye. Next, the color changes of the treated and absence of color in the untreated tubes were calculated using UV-spectrometer at 550 nm O.D of wavelength. Last, the O.D values were noted and calculated based on the formula for control and treated results.
2.5 Validation of XTT assay using agar plates assay
All the concentration of the XTT assay was evidently confirmed by agar streaking method using muller hinton agar and congo red agar assays. In both the plates, the control, half and complete inhibition concentrations were streaked and stored at 37 °C for 12 h. After, the color variations were indicated the nanomaterial efficiency (Jamal et al., 2021).
2.6 Internal biofilm factors modification
Increased efficiency of chitosan loaded essential oils against inside of the bacterial parts was evidently proved by fluorescence microscopic analysis using fluorescence dyes of AO. In this view, the bacterial culture was inoculated into the 24-well plate holding cysteine lactose electrolyte deficient broth and soak glass cover slip for adhere the biofilm on glass cover slip. Taken out the cover slip after 24 h, then initially washed by distilled water and followed by PBS. Back side of the cover slip was clearly washed until the adherent or attached cells removal and use front side of the cover slip. After successful washing, the slide was taken out and dried 10 min and followed by addition of AO stain to bind into the crushed parts of the intracellular bacterial body. Then, viewed by confocal laser scanning electron microscope at 40x magnification lens for clear image of the cells. The entire protocol was modified from previous protocol of Reichhardt et al. (2019).
2.7 External structure modification
The external structure and cell wall of the biofilm forming Acinetobacter baumannii by the influence of chitosan loaded plant essential oils. Initially, the mate formed Acinetobacter baumanni culture was grown in test tube with minimum inhibition concentration of chitosan loaded nanoparticles and allowed to grown together at 37 °C for 1 day. Then, taken out from tube and centrifuged at 5000 rpm 10 min for obtained pellet. Then, the pellet was carefully taken after PBS washing and made smear on cover slip. Then, 4% formaldehyde solution was gradually added on the smear and allowed to fix the sample with 4 h time duration. Then washed the fixation using prepared PBS and vacuum filtered by 0.1 mm polycarbonate membrane filter. Continuously done the dehydration process of filtered sample by 10–100% of ethanol (Namasivayam and Allen Roy, 2013). After successive time interval, the sample was dried properly and coated using gold palladium metal and directly viewed by scanning electron microscopy. The complete process of chitosan loaded essential oils against biofilm producing bacteria (Fig. 1).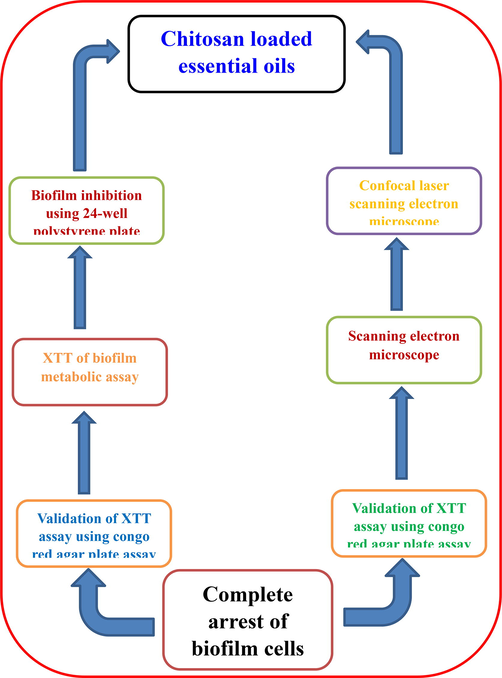
Complete process of chitosan loaded essential oils against biofilm producing bacteria.
3 Result
3.1 Effect of chitosan loaded essential oils
In this study, the chitosan loaded essential oils were obtained from Department of Microbiology, Sun Yat-Sen University, China. The synthesis and characterization was already reported in our previously published article (Rajivgandhi et al., 2020). All the spectroscopic result of FT-IR, XRD and morphological evidences of SEM and TEM results were confirmed the preparation of essential oils successfully loaded into the chitosan molecules. In addition, the synthesized nanomaterial was performed against A549 lung cancer cells. In result, it was inhibited the A549 lung cancer cells very effectively and this evidences were proved by various invitro experiments, notably cell cycle arrest using flow cytometer analysis.
3.2 Agar well punctured method
Different zones of the chitosan nanoparticles loaded essential oils wells were conveyed that the biofilm bacteria was more sensitive to nanoparticles. Also, the result was suggested that the obtained chitosan nanoparticles loaded essential oils was not only effective against cancer cells, it also very efficient against biofilm producing bacteria (Rahman et al., 2017). This result was also interpreted with previously report, the selected chitosan nanoparticles loaded essential oils was concentration dependent inhibitor. In this agar well punctured method, the 20 mm zones against 75 μg/mL concentration (Fig. 2a), 26 mm zones against 100 μg/mL concentration (Fig. 2b) and 30 mm zones against 150 μg/mL concentration (Fig. 2c) were screened. Previously more researchers were reported that the Morinda citrifolia essential oils were very effective against biofilm bacteria due to the presence of rich bioactive compounds (Marisa Halim et al., 2017; Kovendan et al., 2014; Abou Assi et al., 2017; Sharmeen Jugreet and Fawzi Mahomoodally, 2021). The similar statement was reported by Pongnaravane et al. (2006), and chitosan loaded plant essential oils have improved bioactivity than other plant derivatives (Rajivgandhi et al., 2021). The inactivation of biofilm production due to the alter the virulence factors by plant essential oils loaded chitosan material as strong nanomaerials in biomedical activities.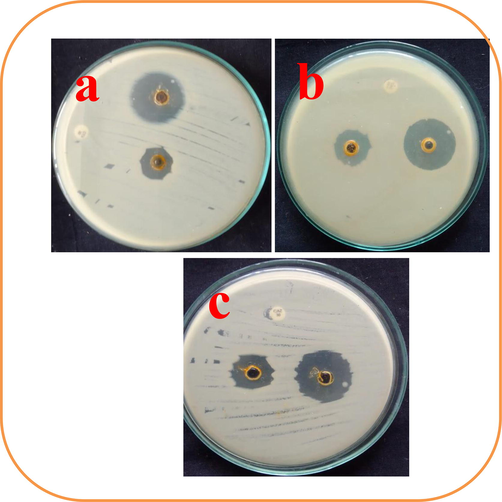
Agar well punctured method for detection of chitosan loaded essential oils against biofilm forming Acinetobacter Baumannii at concentrations of 75 μg/mL (a), 100 μg/mL (b) and 150 μg/mL (c).
3.3 Quantitative detection of biofilm eradication by 24-well polystyrene method
The primary biofilm eradication with increasing concentration process was evidently proved by quantitative confirmation using O.D value of microtitre plate results. When seen on the treated culture of the wells were not shown any mate formation compared to control. All the wells were shown clear turbidity and non-attached cells were shown clearly because of the chitosan nanoparticle loaded essential oils influence. In increasing concentration, the turbidity level was high and bacterial growth was low which is confirmed by microtitre plate reading result. As same as, the concentration of 150 μg/mL was indicated 96% of inhibition against Acinetobacter baumannii culture wells. In 75 μg/mL concentration, the inhibition percentage range was 54% and 34% of inhibition in 50 μg/mL concentration. Among the calculated values, the lowest concentration of 150 μg/mL with high turbidity based inhibition was fixed to further study (Fig. 3a, b). All the result was evidently supported the agar well punctured method and also similar to the statement of Rahman et al. (2017) with increasing concentration of the nanomaterial inhibited the biofilm growth highly. Usually, the chitosan loaded essential oils were inhibited the bacterial growth completely due to the invasion drug efficiency (Ma et al., 2020a,b). In this result was suggested that the essential oils efficiency was heightened by chitosan and it deactivate the bacterial virulence factors (Bala Subramaniyan et al., 2021). Bacterial virulence was altered, bacteria lost their internal communication system and production of internal regulating factors failure (Luna et al., 2022). Therefore, the result of chitosan loaded essential oils were clearly similar to previously reported material in the process of anti-bacterial activity (Rahman et al., 2017). I addition, the obtained chitosan nanoparticles loaded essential oils have increased efficiency against cancer cells and bacterial cells very effectively.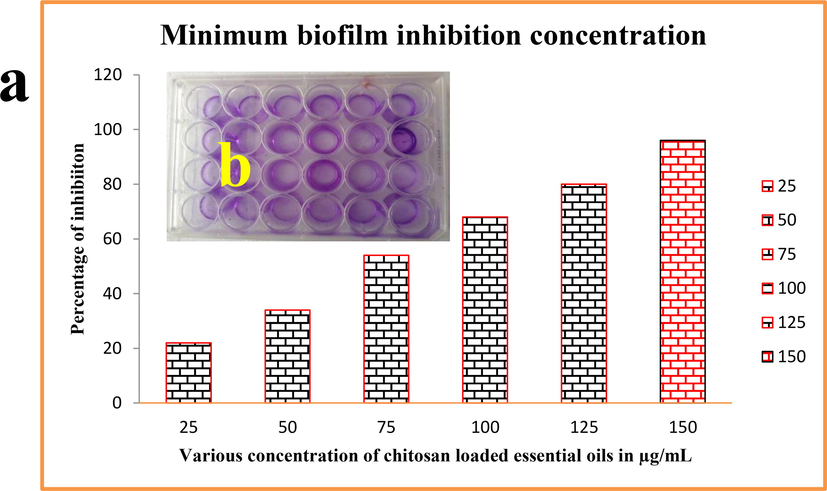
Quantitative measurement of biofilm inhibition concentration against Acinetobacter Baumannii using chitosan loaded essential oils.
3.4 Live/dead activity of biofilm producing Acinetobacter baumannii
Result of minimum biofilm inhibition concentration experiment was used to help the metabolic activity of biofilm forming Acinetobacter baumannii. In this experiment, the same 25–150 μg/mL concentration was used to analyze the live condition of bacteria and their biofilm formation. Evidently, after addition of menadione acetone solution, the color was changed after incubation and confirmed the deactivation ability against virulence factors. As same as, the result was more similar to minimum biofilm inhibition concentration experiment and shown effectively inhibited the bacteria in increased concentration. In 75 μg/mL concentration, almost all the bacteria was enter to decline phase due to the continuous arrest of internal formazan production. Also, the inhibition percentage was 60%, it was very high compared with previous reports of biofilm inhibition by plant essential oils alone (Zhang et al., 2018). In 150 μg/mL. In addition, the dose-dependent inhibition of chitosan loaded essential oils were suggested that the inhibitions were depends on the nature of the chitosan. The inhibition percentage was 99%, and absence of bacterial activity was observed (Fig. 4). Chitosan may influence the essential oils efficiency and delivered the essential oils in proper manner. This statement was agreed by Reichhardt and Parsek (2019), and chitosan is an excellent biopolymer which used as a carrier to various molecules particularly for essential oils. Similar evidences has been observed by Bala Subramaniyan et al. (2021) and reported that the chitosan nanoparticle loaded essential oils have high efficiency in decrease the pathogenicity in bacteria. Therefore, the XTT assay result was suggested that the obtained chitosan nanoparticle mediated essential oils have virulence factors mediated biofilm eradication an it can be confirmed by streaking on congo red agar assay.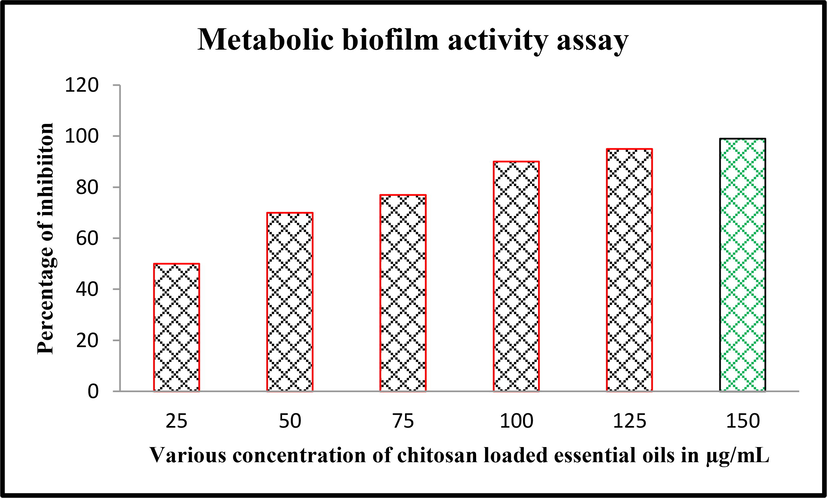
Confirmation of metabolic activity inhibition measurement against Acinetobacter Baumannii using various concentration of chitosan loaded essential oils.
3.5 Validation of metabolic activity assay
In congo red agar plates, the chitosan nanoparticle loaded essential oils treated XTT result was streaked for detection of virulence factors inactivated or not. As same as, the congo red agar plate result was agreed to XTT result due to the color changes. In 150 μg/mL concentration, the bacterial growth was completely arrested (Fig. 5b). When seen on the untreated control tube, the clear growth was obtained (Fig. 5c). Both the results were further rechecked in microtitre plate with effective biofilm inhibition (Fig. 5a). Further, the biofilm formation was arrested or not by XTT using chitosan nanoparticle loaded essential oils were restreaked on the congo red agar plates and confirmed that the obtained nanomaterial were very effective against biofilm formation of Acinetobacter baumannii. Based on the color differences, control wells with XTT solution was shown bright black color appearance whereas no nay color formation of 150 μg/mL and slightly pink color formation in 75 μg/mL were observed. Based on the color variations, the black color formation of control indicates, the exopolysachharide, amino acid, proteins were continuously produced to Acinetobacter baumannii and able to produce strong biofilm. Contrary result of no any color formation in the 150 μg/mL concentration plate was clearly indicated that the bacteria Acinetobacter baumanni lost their pathogenicity and also antigenicity. The plate was shown with no any color and no any bacterial growth was observed, this evident was conveyed the important information that the Acinetobacter bamannii was completely lost their structure. Surprisingly, the 75 μg/mL concentration treated Acinetobacter baumanni was shown pink color in congo red agar plate. This evident was proved that the bacteria lost their pathogenicity and remain hold their antigenicity. Therefore, all the three results were clearly suggested that the chitosan nanoparticle loaded essential oils not only contributed in biofilm inhibition in Acinetobacter baumanni, it also destroy the bacteria also.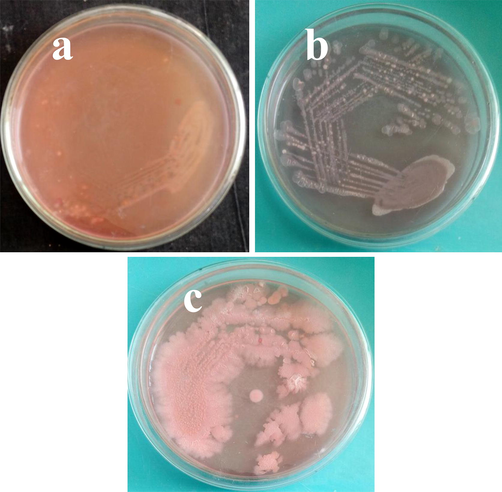
Validation of biofilm metabolic activity using muller hinton agar plate for growth observation (a, b) and biofilm growth of control (c), pathogenicity of biofilm inhibition (d) and complete biofilm bacterial inhibition (e) by congo red agar plate assay.
3.6 Internal biofilm factors modification
The internal structure of the biofilm arrangement was demolished by chitosan loaded essential oils were clearly seen in the picture of Fig. 6b. In result, the original rod shape structure of tightly closed chain formation was observed for control with developed internal materials (Fig. 6a). After treatment, the internal virulence factors, protein, amino acids, exopolysachharides, other responsible genes were stopped due to the complete essential oils delivery of chitosan was observed (Fig. 6b). After addition of AO dye, the damaged membrane was easily shown in fluorescence microscope with the help of glittering the damaged rod shaped colonies. After treatment with nanomaterial, the internal membrane layers were shown with confluent, and loosely arranged colonies were observed. Whereas, the dye was not shown with high intensity level in untreated cells (Fig. 6a). All the arrangement of the biofilm was demolished and undergone to decline phase due to the depletion of virulence factors effect was conveyed that the chitosan loaded essential oils were very effective against biofilm forming Acinetobacter baumannii. Also, the 3D image of treated bacterial cells was clearly shown in Fig. 6c.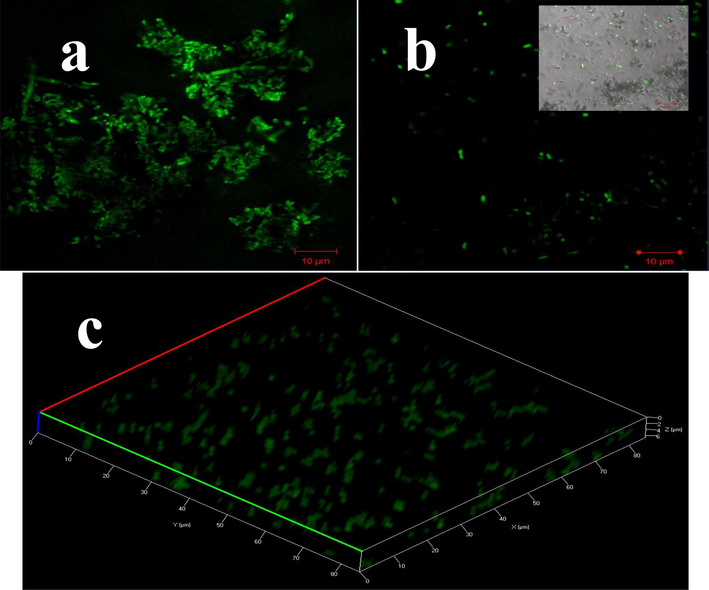
Internal damages of biofilm forming cell and their parts of the Acinetobacter baumannii by confocal laser scanning electron microscope. The untreated control sample shown clear shape (a), treated sample shown damaged shape and arrangement (b) and 3D image of damaged cells (c).
3.7 External morphological modification
Fig. 7b of chitosan loaded essential oils treated Acinetobacter baumanni was shown irregular shape, smooth surface with collapsed nucleolus. Contrary, the strong formation of punch of colonies with rod shaped colonies was observed in control image (Fig. 7a). Both the results were interpreted each other, and suggested that the original structure, size and shape of the bacterial morphology was affected clearly due to the effect of chitosan loaded essential oils and confirmed by SEM result of Fig. 7b. The intensity was low and exopolysachharide production was stopped and hank like structure was absence. Also, the increased membrane polarization and fluid discharge was started on the periplasmic space and cell sheath. In bacteria, the polarization was very high and permeability was very easy in the treated cells. Entirely contrast result of control cells image was suggested that the present result was very useful and confirmation for external damages (Muthuchamy et al., 2020).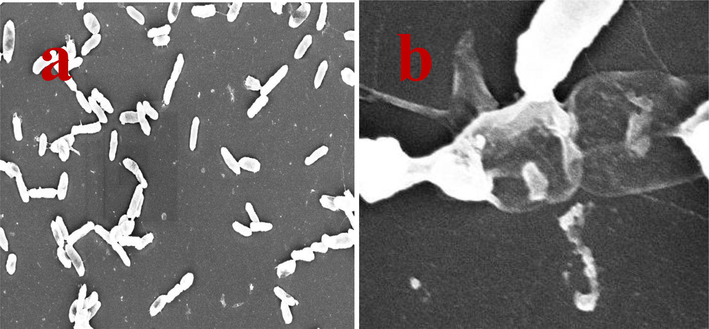
External cell wall and surrounding layers cleavage by scanning electron microscope and result of clear size and rod shape morphology (a) and unclear rod shape and surface layers damaged (b) cells of Acinetobacter baumannii.
4 Conclusion
Based on the observed result, the chitosan was more efficient carrier to deliver the essential oils against biofilm producing bacteria. The essential oils efficiency was more compared with without loading chitosan and proved by quantitative biofilm inhibition and metabolic activity assay. Both the assays were more supported to agar well punctured result and proved that the chitosan loaded essential oils were inhibited the biofilm at concentrated dependent manner. The black, pink and no color formation of control, half treatment and complete treatment of the congo red agar plates were evidently proved that the Acinetobacter bamannii lost their virulence factors and more sensitive to tested nanomaterial. Both the intracellular and extracellular surface structures were also supported to the chitosan loaded nanomaterial not only inhibits the cancer cells, it also highly effective against biofilm forming Acinetobacter baumannii.
Acknowledgements
All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Numbers: 41950410573) and Postdoctoral Science Foundation of China (Project Approval Number: 2019M663213) for financial support for this work. Wen-Jun Li was also supported by Introduction project of high-level talents in Xinjiang Uygur Autonomous Region. The authors extend their appreciation to researchers supporting project number (RSP-2021/119), king Saud University, Riyadh, Saudi Arabia for funding this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arabian J. Chem.. 2017;10:691-770.
- [Google Scholar]
- Phenotypic and genotypic characteristics of Acinetobacter baumannii enrolled in the relationship among antibiotic resistance, biofilm formation and motility. Microb. Pathog.. 2021;155:104922
- [Google Scholar]
- Chemical composition and bioactivity of essential oil from Morinda citrifolia L. fruit. J. Med. Plants Res.. 2020;14:208-214.
- [Google Scholar]
- Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym.. 2019;212:142-149.
- [Google Scholar]
- V. Artocarpus integrifolia seed lectin enhances membrane damage, oxidative stress and biofilm inhibition activity of silver nanoparticles against Staphylococcus aureus. Colloids Surf. A: Physicochem. Eng. Aspects. 2021;624:126842.
- [Google Scholar]
- Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm.. 2021;597:120288
- [Google Scholar]
- Development and characterization of essential oils incorporated chitosan-based cues with antibacterial and antifungal potentialities. J. Radiat. Res. Appl. Sci.. 2020;13:174-179.
- [Google Scholar]
- Essential oils-loaded electrospun chitosan-poly(vinyl alcohol) nonwovens laminated on chitosan film as bilayer bioactive edible films. LWT. 2021;144:111217.
- [Google Scholar]
- Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J. Biol. Sci.. 2021;28:1158-1166.
- [Google Scholar]
- Antibiotic resistance and biofilm formation of Acinetobacter baumannii isolated from high-risk effluent water in tertiary hospitals in South Africa. J. Global Antimicrob. Resistance. 2021;27:82-90.
- [Google Scholar]
- Attenuation of Acinetobacter baumannii virulence by inhibition of polyphosphate kinase 1 with repurposed drugs. Microbiol. Res.. 2021;242:126627
- [Google Scholar]
- Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumonia. Colloidal Surf. A. 2020;600:124830
- [Google Scholar]
- Oregano and thyme essential oils encapsulated in chitosan nanoparticles as effective antimicrobial agents against foodborne pathogens. Molecules. 2021;26:4055.
- [Google Scholar]
- A review on functional and nutritional properties of noni fruit seed (Morinda citrifolia L.) and its oil. Food Biosci.. 2021;41:101000.
- [Google Scholar]
- Anti-biofilm activity of LC-MS based Solanum nigrum essential oils against multi-drug resistant biofilm forming P. mirabilis. Saudi J. Biol. Sci.. 2021;28:302-309.
- [Google Scholar]
- Encapsulation of cardamom essential oil in chitosan nano-composites: in-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol.. 2016;7:1580.
- [Google Scholar]
- Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol.. 2018;9(610):1-13.
- [Google Scholar]
- In vitro cytocompatibility of chitosan/PVA/methylcellulose – Nanocellulose nanocomposites scaffolds using L929 fibroblast cells. Appl. Surf. Sci.. 2018;449:574-583.
- [Google Scholar]
- Salt Leaching Synthesis, Characterization and In Vitro Cytocompatibility of Chitosan/Poly(vinyl alcohol)/Methylcellulose – ZnO Nanocomposites Scaffolds Using L929 Fibroblast Cells. J. Nanosci. Nanotechnol.. 2019;19(8):4447-4457.
- [Google Scholar]
- Kanimozhi, K, Basha, S. Khaleel, Kumari, V. Sugantha, Kaviyarasu, K, Development of Biomimetic Hybrid Porous Scaffold of Chitosan/Polyvinyl Alcohol/Carboxymethyl Cellulose by Freeze-Dried and Salt Leached Technique, Journal of Nanoscience and Nanotechnology, 18, 7, 2018, 4916-4922.
- Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol.. 2019;123:837-845.
- [Google Scholar]
- Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads – A kinetic and thermodynamic study. Environ. Res.. 2022;203:111814.
- [Google Scholar]
- Chitosan-loaded nanoemulsion containing Zataria Multiflora Boiss and Bunium persicum Boiss essential oils as edible coatings: its impact on microbial quality of turkey meat and fate of inoculated. Int. J. Biol. Macromol.. 2020;150:904-913.
- [Google Scholar]
- Proposed models for subcritical water extraction of essential oils. Chin. J. Chem. Eng.. 2009;17:359-365.
- [Google Scholar]
- Mosquitocidal properties of Morinda citrifolia L. (Noni) (Family: Rubiaceae) leaf extract and Metarhizium anisopliae against malaria vector, Anopheles stephensi Liston. (Diptera: Culicidae) Asian Pac. J. Tropical Dis.. 2014;4:S173-S180.
- [Google Scholar]
- High antibacterial performance of hydrophobic chitosan-based nanoparticles loaded with Carvacrol. Colloids Surf., B. 2022;209:112191
- [Google Scholar]
- MatthiasLeonhardeBeritSchneider-SticklereYulongTane, Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym.. 2020;241:116254
- [Google Scholar]
- Citral-loaded chitosan/carboxymethyl cellulose copolymer hydrogel microspheres with improved antimicrobial effects for plant protection. Int. J. Biol. Macromol.. 2020;164:986-993.
- [Google Scholar]
- The hot-water extract of leaves of noni, Morinda citrifolia, promotes the immunocompetence of giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol.. 2017;64:457-468.
- [Google Scholar]
- An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Microbiol. Res.. 2021;247:126722.
- [Google Scholar]
- Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr. Polym.. 2022;277:118815.
- [Google Scholar]
- Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumonia. Carbohydr. Polym.. 2020;230:115646
- [Google Scholar]
- Development and characterization of alginate / chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Delivery Sci. Technol.. 2019;52:65-72.
- [Google Scholar]
- Anti biofilm effect of medicinal plant extracts against clinical isolate of biofilm of Escherichia coli. Int. J. Pharm. Pharm. Sci.. 2013;5(2) ISSN-0975-491
- [Google Scholar]
- Essential oil of Noni, Morinda citrifolia L., fruits controls the rice stem-rot disease without detrimentally affect beneficial fungi and ladybeetles. Ind. Crops Prod.. 2021;170:113728.
- [Google Scholar]
- Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials. 2019;12:2223.
- [Google Scholar]
- Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Tropical Med.. 2012;5:294-298.
- [Google Scholar]
- Extraction of anthraquinones from roots of Morinda citrifolia by pressurized hot water: antioxidant activity of extracts. J. Supercrit. Fluids. 2006;37:390-396.
- [Google Scholar]
- Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities – potentials and challenges. Food Control. 2015;47:381-391.
- [Google Scholar]
- Evaluation of antimicrobial and phytochemical screening of Fennel, Juniper and Kalonji essential oils against multi drug resistant clinical isolates. Asian Pac. J. Tropical Biomed.. 2012;2:S1625-S1629.
- [Google Scholar]
- Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J. Biol. Sci.. 2017;24:324-330.
- [Google Scholar]
- Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol.. 2020;164:4010-4021.
- [Google Scholar]
- Physiochemical characterization and anti-carbapenemase activity of chitosan nanoparticles loaded Aegle marmelos essential oil against K. pneumoniae through DNA fragmentation assay. Surf. Interfaces. 2021;23:100932.
- [Google Scholar]
- Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol.. 2019;10:677.
- [CrossRef] [Google Scholar]
- Reprint of: Essential oils from 9 exotic and endemic medicinal plants from Mauritius shows in vitro antibacterial and antibiotic potentiating activities. S. Afr. J. Bot.. 2021;140:478-485.
- [Google Scholar]
- Chemical variability, pharmacological potential, multivariate and molecular docking analyses of essential oils obtained, from four medicinal plants. Ind. Crops Prod.. 2020;150:112394.
- [Google Scholar]
- Chrysin-loaded chitosan nanoparticles potentiates antibiofilm activity against Staphylococcus aureus. Pathogens. 2020;9(115):1-11.
- [Google Scholar]
- The Efficiency of Noni (Morinda citrifolia L.) Essential Oil on the Control of Leaf Spot Caused by Exserohilum turcicum in Maize Culture. Medicines. 2017;4:60.
- [Google Scholar]
- Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia L (Rubiaceae): Errata and review of technological development and prospects. Sep. Purif. Technol.. 2021;117844
- [Google Scholar]
- Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumonia, Saudi. J. Biol. Sci.. 2020;27:3449-3455.
- [Google Scholar]
- Chemistry and Antimicrobial Activity of the Essential Oils from Ripe and Unripe fruits of the Fijian Morinda citrifolia (noni/kura) Rubiaceae. J. Essential Oil Bearing Plants. 2008;11
- [Google Scholar]
- Morinda officinalis How. – A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol.. 2018;213:230-255.
- [Google Scholar]







