Translate this page into:
Effect of tellurite on growth of extensively drug resistant (XDR) Mycobacterium tuberculosis and action on mycobacterial drug efflux pump
⁎Correspondence author at: Institute of Microbiology & Molecular Genetics University of the Punjab, New Campus, Lahore 54590, Pakistan. rehman.mmg@pu.edu.pk (Abdul Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study genotypic resistance of bacterial strain to first- and second-line anti-tuberculosis drugs was determined by Line probe assay. Toxic effect of tellurite on the growth of Mycobacterium tuberculosis (MTB) was determined by growing cells in different concentrations of tellurite (1 to 5 mM). Morphological effects of tellurite and uptake of metal in bacterial cells were confirmed by Scanning electron microscope (SEM) and energy dispersive X-ray (EDX) analysis. Change in fold expression of the efflux pump gene was measured by RT-PCR. Mycobacterial strain was characterized as XDR-TB based on the genotypic resistance to rifampicin and isoniazid, along with resistance to fluoroquinolones and second line injectable. XDR-TB showed black colonies in tellurite presence and growth was delayed (2–3 weeks) when compared with the control. The reduced cell size, metal accumulation and the characteristic tellurite peaks appeared in metal-treated cells. MTB showed MIC value of 1 mM and had high susceptibility for higher concentrations (2–5 mM). However, no significant metal inhibitory effect on the mmpL7 efflux system was determined. Tellurite shows significant growth reduction potential against XDR-TB strain. However, the exact mechanism of action needs to be elucidated with further research.
Keywords
Tellurite
XDR-TB
mmpL7 gene
Drug efflux pump
1 Introduction
Tellurium is not an essential biological micronutrient. It exists in nature in different states such as telluride, tellurite (TeO2-) and tellurate (Arenas et al., 2014). The toxicity of the tellurium is not well defined. However, tellurite (Te) oxyanions are highly toxic for most microorganisms with concentration as low as 1 µg/mL (Taylor, 1999; Chasteen et al., 2009). Te has long been used in ancient times against various infections prior to the use of antibiotics (Fleming, 1932; Taylor, 1999; Nguyen et al., 2019). It has been demonstrated that the Te exerts its toxic effects by oxidant nature and generation of reactive oxygen species (ROS) in various cellular compartments (Tremaroli et al., 2007; Presentato et al., 2019). With the exposure of cells to the metal(loid), cells display oxidative stress with increased ROS compounds like hydrogen peroxide, hydroxyl radical and superoxide anion (Pérez et al., 2007). The role of glutathione (GSH) is also revealed in tellurite toxicity. The reduced GSH pool is oxidized in the presence of tellurite and this process elicits the generation of superoxide radicals (Presentato et al., 2019). These ROS compounds can affect a number of macromolecules and metabolic pathways. According to the other mechanism of tellurite toxicity, Te induces DNA damage and translational arrest by depletion of cellular ATP and phosphorylation of histones and some translation initiation factors (Sandoval et al., 2010).
Despite the low abundance of Te in nature and its high toxicity, bacteria have developed tellurite resistance (TeR). The expanded use of tellurite in electronics, rubber, optics and metallurgy has posed a potential threat of increased environmental contamination (Sandoval et al., 2012) leading to the isolation of various TeR bacteria from environmental (Passet and Brisse, 2015) and clinical samples (Arenas et al., 2014).
The bacterial cells show black deposits inside the cells when grown in presence of Te due to the cytosolic accumulation of Te precipitates by the reduction of Te to the less toxic metal form (Te0) (Missen et al., 2020). The reduction can occur enzymatically (such as nitrate reductase, oxidase, catalase and dihydrolipoamide dehydrogenase) or non– enzymatically (Acuña et al., 2009). TeR determinants are found in bacterial chromosomes and plasmids. These include some genes for tellurite resistance such as kilA, telAB (klaABC), tehAB, terBCDE, terC and trgAB. Additionally, genes encoding for enzymes that are involved in oxidative stress response (katG, grxA, ahpCF, trxC gor), iron uptake regulator (fur), multidrug efflux pump (acrAB), metalloid efflux (arsABC), tellurite/selenite-induced transporter (gutS) are also involved in resistance to tellurite (Chasteen et al., 2009).
To date, very limited or no data is available for tellurite resistance in drug resistant Mycobacterium tuberculosis (MTB). There is interplay of several mechanisms which contribute to the emergence of drug resistance in tuberculosis. Broadly, intrinsic and extrinsic factors are involved in promoting resistance to MTB. The intrinsic factors are associated with resistance mechanisms of the microorganisms due to acquisition of genetic mutations, low permeability of the drug through cell envelope, modification of drug target enzymes, presence of drug efflux pumps, drug inactivation and the fitness cost (Swain et al., 2020; Allue Guardia et al., 2021). Efflux pumps (EP) are also known as transporters. The Mycobacterial EPs are classified into five superfamilies: i) ATP-binding cassette (ABC); ii) small multidrug resistance (SMR); iii) major facilitator superfamily (MFS); iv) multidrug and toxic-compound extrusion (MATE) and v) resistance nodulation division (RND). All these efflux pumps (EPs) together contribute 6–18% of all EPs found in any bacterial cell (da Silva et al., 2011).
The present study was designed to investigate the toxicity of tellurite against extensively drug resistant (XDR) tuberculosis strains. Additionally, the inhibitory effect of tellurite on drug efflux pump was also determined.
2 Material and methods
2.1 Isolation and characterization of the strains
The Mycobacterial strain was isolated from a clinical sputum sample. The sample was processed and characterized for genotypic resistance to first line and second line injectable drugs (SLIDs) by Line probe assay as previously described by Kabir et al. (2020,2021).
This study was approved by the Research Ethics and Biosafety Committee (No.D/650/MMG) of the Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan.
2.2 Effect of tellurite on MTB growth
The toxic effect of tellurite on the growth of MTB was determined by growth in the presence of tellurite and absence of tellurite (control). The five concentrations of tellurite (K2TeO3) (Sigma Aldrich) 1, 2, 3, 4, and 5 mM were used in the assay. The inoculum of the test strain was prepared to adjust the turbidity with 0.5 McFarland standards by scrapping 3–4 colonies of MTB from Löwenstein–Jensen (LJ) medium. The culture tubes were inoculated with 100 µl of prepared bacterial inoculum and tellurite whereas only organism suspension was inoculated in a control tube without tellurite.
2.3 Scanning electron microscope (SEM) and energy dispersive X-ray (EDX)
Mycobacterial cells with tellurite stress and without tellurite stress were further determined by SEM for comparison in their morphology (Khan et al., 2015). EDX was used to determine the metal and microbe interaction and to confirm the uptake of metal by the Mycobacterium. The samples were prepared for this analysis by taking 1 ml of the sample in a microcentrifuge tube. The tube was centrifuged at 14000 rpm for 5 min. The supernatant was discarded and the pellet was resuspended in 50 µl deionized double distilled water. A spot of the sample (30 µl) was dispensed into the straight piece of aluminium foil. The spot sample was allowed to air dry and fix on the foil. Samples were observed under SEM (Nova NanoSEM 450) equipped with the Oxford energy dispersive X-ray microanalysis system.
2.4 Extraction of RNA and cDNA synthesis
The RNA was extracted using 1 ml TRIzol by following the same protocol as described by Rio et al. (2010) with slight modification. Briefly, the colonies were scrapped from LJ culture and homogenized in prechilled pestle mortar with liquid nitrogen and allowed to thaw at room temperature. The homogenized culture was added with 1 ml of Trizol (Thermo Fisher Scientific, USA) and treated with chloroform and isopropanol in subsequent steps. The pellet was washed with 1 ml of 70 % ethanol and resuspended in 40 µl of RNase free water. Extracted RNA was quantified by Nanodrop plate (Skanit RE 4.1, Thermoscientific™) and was readily converted into cDNA using cDNA synthesis kit (Vivantis cDSK01-050™).
2.5 Real time PCR
Real time PCR (RT-PCR) was performed on Mic PCR (Bio Molecular System™) mmpL7 gene was used for relevant expression profiling while DNA Gyrase A was used as housekeeping gene. RT-PCR reaction mixture was prepared using Eva Green qPCR master mix (Solis Biodyne, Estonia). The sequence of the primers is given in Table S1. All samples were run in quadruples and relative expression was determined using double delta Ct method.
3 Results
3.1 Characterization of bacterial strain
The strain was characterized as XDR-TB by genotypic resistance to various first- and second-line anti-tuberculosis drugs (SLIDs). The first line drugs showed resistance conferring mutations for rifampicin (rpoB gene) and isoniazid (KatG gene) while SLIDs conferred resistance to fluoroquinolones (FQs) in gyrA gene and second line injectable (rrs gene). The mutations of these genes are given in Table S2.
3.2 Effect of tellurite on XDR-TB growth
A delay in the Mycobacterium growth containing tellurite was observed when the growth positivity was compared with control. This delay (2–3 weeks) was observed both in MGIT and LJ medium. The uptake and reduction of Te by bacterial cells was confirmed by the observation of black color of the colonies which were grown in the presence of tellurite whereas control showed creamy white colonies (Fig. 1). Mycobacterium showed growth only at the concentration of 1 mM and there was complete inhibition of growth at the concentration of 2 to 5 mM (Fig. 1).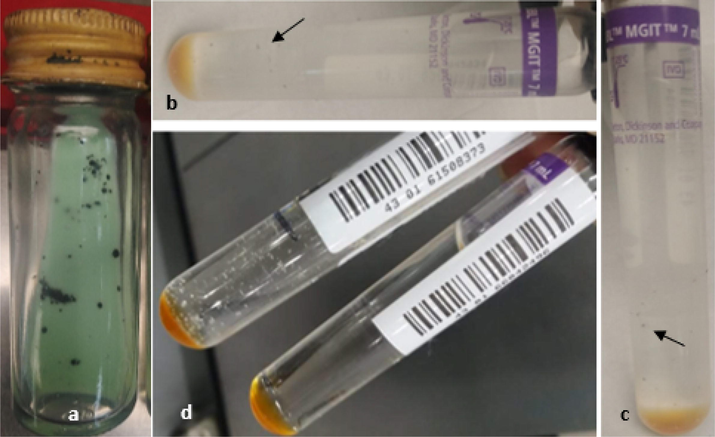
Effect of tellurite on bacterial growth (a) black color of the colonies showing conversion of K2TeO3 to black tellurium (Te0), (b & c) MGIT medium showing small black color colonies (indicated by arrows) in Te presence, (d) growth in control sample (without Te) (creamy white colonies) and MGIT control (inoculum free tube).
3.3 SEM and EDX analyses
The uptake of tellurite was confirmed by the EDX analysis. The characteristic peaks of Te appeared in tellurite treated cells (Fig. 2a) which confirmed the presence of these moieties in MTB. While no peak of Te appeared in the control (Fig. 2b). SEM analysis revealed a difference in the size of the cells and the accumulation of black metallic tellurium inside the cells in presence of tellurite (Fig. 2c, 2d).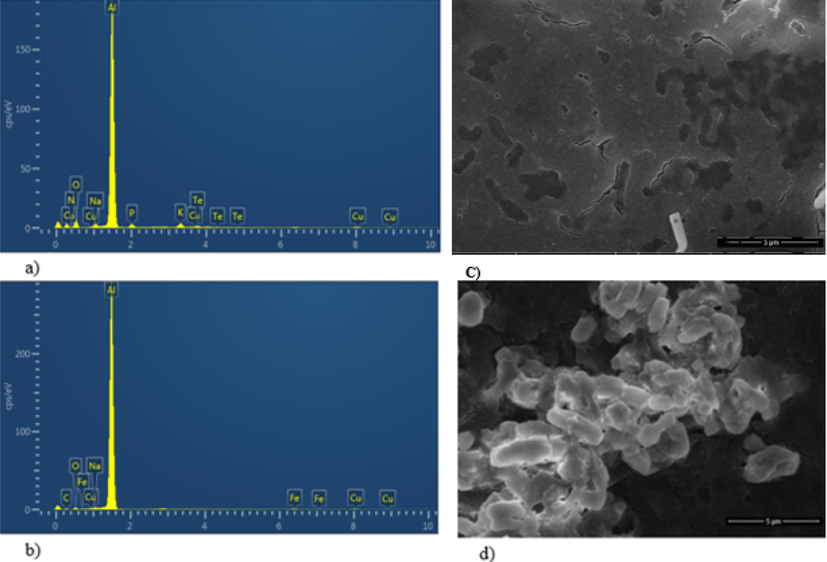
EDX analysis of the strain (a) in presence of tellurite (1 mM) confirming its uptake by Te peaks and (b) in absence of tellurite (c) SEM of the strain with tellurite showing black (Te0) inside the cells (d) SEM image of the control sample containing no tellurite.
3.4 Relative expression of drug efflux pump gene
The inhibitory effect of tellurite on the mmpL7 efflux system has been evaluated by relative expression of the genes in presence and absence of the Te oxyanions. The analysis of the expression revealed a negligible reduction in fold expression of the efflux pump gene in the presence of tellurite (Fig. 3).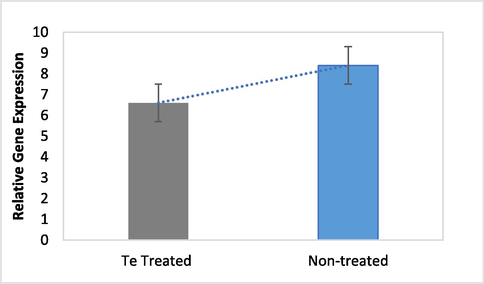
Relative gene expression of mmpL7 in the presence and absence of tellurite.
4 Discussion
The use of K2TeO3 as an antibacterial compound is reported in various studies. It has been used in the treatment of diseases such as leprosy, tuberculosis, syphilis and dermatitis (Ba et al., 2010; Akhtar and Rehman, 2017; Nguyen et al., 2019). A very low lethal concentration (4 µM) for prokaryotic cells such as E. coli is reported. Tellurite can also be toxic for the eukaryotic cells. However, its actual lethal concentration for eukaryotic cells is not well determined and the toxic concentration varies from 50 to 1600 µM according to different studies (Molina-Quiroz et al., 2012; Nguyen et al., 2019). Very limited data is available for the toxicity of tellurite against MTB. MTB acquires resistance by different mechanisms predominantly by developing genetic mutations. The drug efflux pump is also very important in maintaining a balance of the drug inside the cell to potentiate its effects. The active efflux of the drug can lead to the resistance of these drugs in bacteria. Though the idea to use tellurite against tuberculosis was an old one, the approach to use it against XDR-TB growth and inhibition of efflux pump was novel.
In the current study, the growth of bacterial strain in the presence of tellurite was associated with black colonies. The studies have associated the appearance of black colonies with the reduction of tellurium. The reduced metallic tellurium is insoluble which forms black precipitates around bacterial colonies (Coral et al., 2006; Nguyen et al., 2019). The reduction of tellurite is not strictly associated with tellurite resistance as some tellurite sensitive strains are also found to be associated with tellurite reducing activity (Arenas et al., 2014). The results obtained in the present study indicated that the presence of tellurite has significantly affected the growth rate of bacteria when compared with the control. The difference in the growth rate reflects the potential metal toxicity which has slowed down the growth of strains.
The metal can absorb into the cell due to their cationic nature and can change the morphological appearance of the cell. The results of the study have not found any morphological changes in cell shape but the cell size has been reduced in tellurite presence when observed under electron microscope. The morphological changes in bacterial cells were also observed in previous studies (Arenas et al., 2014; Nguyen et al., 2019). The uptake of tellurite by the XDR strain has been studied by EDX analysis which showed peaks of metal. The presence of tellurite peak indicates uptake of the metal. The results are comparable with the study where the authors investigated the cadmium resistance mechanism in E. coil and confirmed the cadmium uptake by obtaining peaks in EDX (Khan et al., 2015).
Bacteria have developed strategies to cope with the intracellular accumulation of heavy metals and ions (Khan et al., 2015). The one strategy to cope with the toxic concentrations of the drug is the presence of an efflux pump. Mycobacterial EPs provide resistance to different anti-TB drugs by extruding the drug molecules that enter into the cell (Pule et al., 2016). MmpL7 protein overexpression is involved with high resistance to one of the most potent first line anti-TB drug isoniazid in which no genetic mutations are found (Pasca et al., 2005). The drug efflux inhibitors are very useful in reducing efflux mediated resistance. Various studies have reported the activity of different type of inhibitors against drug efflux pump such as verapamil (de Souza et al., 2020), reserpine (Shaheen et al., 2019), thioridazine and chlorpromazine (Rodrigues et al., 2012). We tried to evaluate the effect of tellurite on the drug efflux pump. The two-fold difference in expression of the mmpL7 gene under metal stress was calculated. No considerable change in the expression of the gene was measured with and without tellurite stress.
5 Conclusion
In conclusion, Te was determined for growth reduction potential against XDR-TB at very low concentration of 1 mM. XDR-TB showed black colonies in tellurite presence and growth was delayed between 2 and 3 weeks as compared to the control. The reduced cell size, metal accumulation, and the characteristic tellurite peaks appeared in metal-treated cells. MTB showed MIC value of 1 mM and had high susceptibility for higher concentrations (2–5 mM). This tellurite concentration has not shown any considerable inhibitory effect on the mmpL7 efflux system. However, metal effects on other genes of the efflux system should also be evaluated. It is plausible that metal may use other mechanisms for inhibition of growth which should be investigated further.
Authors’ contributions
SK performed experiments, analyzed the data and wrote the manuscript. HE and KJ provided sources. SZH and MAR helped in experiments and data analysis. AR helped in research design and in manuscript editing. All authors contributed substantially to the interpretation of the results. All authors approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
No funding was obtained for the current study.
Ethical approval
The study was approved by the Research Ethics and Biosafety Committee (No.D/650/MMG) of the Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Expression of the yggE gene protects Escherichia coli from potassium tellurite-generated oxidative stress. Arch. Microbiol.. 2009;191(5):473-476.
- [Google Scholar]
- Tellurite reduction potential of bacteria isolated from industrial wastewater. Punjab Univ. J. Zool.. 2017;32:129-135.
- [Google Scholar]
- Evolution of drug-resistant Mycobacterium tuberculosis strains and their adaptation to the human lung environment. Front. Microbiol.. 2021;12:137.
- [Google Scholar]
- Isolation, identification and characterization of highly tellurite-resistant, tellurite-reducing bacteria from Antarctica. Polar Sci.. 2014;8(1):40-52.
- [Google Scholar]
- Tellurium: an element with great biological potency and potential. Org. Biomol. Chem.. 2010;8(19):4203-4216.
- [Google Scholar]
- Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol. Rev.. 2009;33(4):820-832.
- [Google Scholar]
- A preliminary study on tellurite resistance in Pseudomonas spp. isolated from hospital sewage. Pol. J. Environ. Stud.. 2006;15(3):517-520.
- [Google Scholar]
- Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol.. 2011;63(1):1-9.
- [Google Scholar]
- Isoniazid and verapamil modulatory activity and efflux pump gene expression in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis.. 2020;24(6):591-596.
- [Google Scholar]
- On the specific antibacterial properties of penicillin and potassium tellurite incorporating a method of demonstrating some bacterial antagonisms. J. Pathol. Bacteriol.. 1932;35:831-842.
- [Google Scholar]
- Fluoroquinolone resistance and mutational profile of gyrA in pulmonary MDR tuberculosis patients. BMC Pulm. Med.. 2020;20:1-6.
- [Google Scholar]
- Variations in rifampicin and isoniazid resistance associated genetic mutations among drug naïve and recurrence cases of pulmonary tuberculosis. Int. J. Infect. Dis.. 2021;103:56-61.
- [Google Scholar]
- Cadmium resistance mechanism in Escherichia coli P4 and its potential use to bioremediate environmental cadmium. Appl. Microbiol. Biotechnol.. 2015;99(24):10745-10757.
- [Google Scholar]
- Love is in the Earth: A review of tellurium (bio) geochemistry in surface environments. Earth-Sci. Rev.. 2020;204:103150
- [Google Scholar]
- Enhancing the antibiotic antibacterial effect by sub lethal tellurite concentrations: tellurite and cefotaxime act synergistically in Escherichia coli. PloS One. 2012;7(4):e35452.
- [Google Scholar]
- Microbial tellurite reduction and production of elemental tellurium nanoparticles by novel bacteria isolated from wastewater. J. Indust. Engineer. Chem.. 2019;78:246-256.
- [Google Scholar]
- mmpL7 gene of Mycobacterium tuberculosis is responsible for isoniazid efflux in Mycobacterium smegmatis. Antimicrob. agents Chemother.. 2005;49(11):4775-4777.
- [Google Scholar]
- Association of tellurite resistance with hypervirulent clonal groups of Klebsiella pneumoniae. J. Clin. Microbiol.. 2015;53(4):1380-1382.
- [Google Scholar]
- Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One. 2007;2(2):e211.
- [Google Scholar]
- Tellurite-dependent blackening of bacteria emerges from the dark ages. Environ. Chem.. 2019;16(4):266-288.
- [Google Scholar]
- Efflux pump inhibitors: targeting mycobacterial efflux systems to enhance TB therapy. J. Antimicrob. Chemother.. 2016;71(1):17-26.
- [Google Scholar]
- Purification of RNA using TRIzol (TRI reagent) Cold Spring Harbor Protocols. 2010;5:1-4.
- [Google Scholar]
- Contribution of efflux activity to isoniazid resistance in the Mycobacterium tuberculosis complex. Infect. Genet. Evol.. 2012;12(4):695-700.
- [Google Scholar]
- Tellurite-induced oxidative stress leads to cell death of murine hepatocarcinoma cells. Biometals. 2010;23(4):623-632.
- [Google Scholar]
- A comparative study of tellurite toxicity in normal and cancer cells. Mol. Cell Toxicol.. 2012;8(4):327-334.
- [Google Scholar]
- Reserpine is the new addition into the repertoire of AcrB efflux pump inhibitors. Mol. Biol.. 2019;53(4):596-605.
- [Google Scholar]
- Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerg. Microbes Infect.. 2020;9(1):1651-1663.
- [Google Scholar]
- Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch. Microbiol.. 2007;187(2):127-135.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102629.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







