Translate this page into:
Effect of β-sitosterol on PEL and PSL of Pseudomonas aeruginosa
⁎Corresponding author at: Zagazig University Hospitals, Infection Control Unit, Zagazig 44519, Egypt. mfabuzaid@zu.edu.eg (Marwa Fady),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pseudomonas aeruginosa is one of the major reasons of nosocomial infections and well known of its high morbidity and mortality in hospitals. Additionally, it is well known of its intrinsic resistance to most of antibiotics. P. aeruginosa virulence factors have been investigated well in recent years and it has been classified according to their secreted factors for attachment into alginate, PEL, PSL. In this study we investigated reduction effect of β- sitosterol which is a natural compound found in most of plants, on biofilm formed by different biofilm-associated mutant strains and wild types of P. aeruginosa. The results revealed that β- sitosteral has antibacterial and showing reduction in biofilm formation on P. aeruginosa. Also, β- sitosterol significantly affects swarming and swimming of most strains.
Keywords
Pseudomonas aeruginosa
PEL
PSL
Biofilm
Data availability
Data will be made available on request.
1 Introduction
Pseudomonas aeruginosa has been considered as one of the most virulent pathogen in hospitals related to percentage of mortality, near-ubiquitous presence, widely intrinsic antibiotic resistance and ability to form biofilm on any indwelling medical devices (Ma et al.2020), it represents a great bluster in nosocomial infections especially in immunocompromised patients (Liao et al.2022).Fig 1.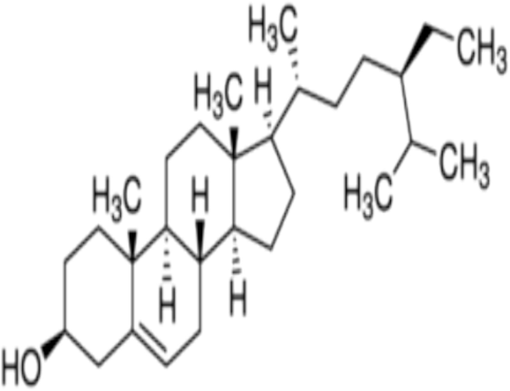
Structure of B-sitosterol (National Center for Biotechnology Information, 2024).
Bacterial pathogenesis depends on best demonstration of virulence factors. According to virulence factors of P. aeruginosa they can be classified into three classes the first one is bacterial structures including pili which facilitates attachment to host, biofilm formation (Burrows, 2012), flagella which responsible for bacterial adhesion and biofilm formation (Sampedro et al. 2015), lipopolysaccharide (LPS) is a crucial surface structural component. It also reduces the endotoxicity of Lipid A in LPS, facilitates tissue damage, adhesion, and host receptor recognition (Park, et al., 2022), while second class includes exopolysaccharides like alginate, PSL, and PEL which may aid in the development of biofilms and prevent the removal of bacteria (Ozer, E. et al. 2021).Third class comprise cell to cell interaction Quorum sensing which regulates production of virulence factors and biofilm which enables bacteria to resist antimicrobial agents. Biofilm combined bacteria in extracellular polysaccharide that adapt bacteria to harsh environmental conditions and increase resistance against antimicrobial agents (Thi et al., 2020). The extracellular materials forming biofilm matrix shield microbes from antimicrobials and the host immune system (Fleming et al., 2022).
Some strains of P. aeruginosa rely more on PEL like PA14 its mutant strain failed to attach to surface since it can’t produce PEL.Also, strains rely on PSL like PAO1 its mutant strain couldn't attach to surface due to failure to produce Psl (Colvin et al., 2012). The presence of PSL and PEL inside biofilm of PAO1 is different than PA14, since PSL is present on the margin of grown biofilm and PEL is centralized on the base of grown biofilm this in case of PAO1 and the vice versa occurs in PA14 (Jennings et al., 2015).
Bioprospecting for antimicrobial and antibiofilm agents have been widely investigated from natural sources in recent years to combat resistance of bacteria.β-sitosterol which is isolated from cell membranes of some plants and was previously demonstrated to have antibacterial properties (Ododo et al., 2016). In our study we investigate the effect of β- sitosterol on P. aeruginosa biofilms.
2 Materials and methods
β-sitosterol was purchased from Sigma Aldrich (St. Louis, MO, USA).
2.1 Bacterial strains and cultivation requirements
PAO1 wild type strain served as the parental strain for the genetic mutant used in this study, ΔwspF mutant has elevated intracellular c-di-GMP, which results in over-expression of both PEL and PSL, Δpel mutants produce no PEL, Δpsl mutants produce no PSL, and Δpel Δpsl mutants produce neither PEL nor PSL. PA14 was used as an alternative wild type strain. All bacterial strains were cultured in lysogeny broth (LB) and any additional supplements are described below.
2.2 Disc diffusion assay
Antibacterial effect of β-sitosterol was investigated by using disc diffusion method according to CLSI (2015). Briefly, a concentrated solution of β-sitosterol was made by dissolving 10 mg/ml in ethyl acetate. Sterile filter paper discs were cut into 6 mm diameter then dipped in for 30 min, let dry then placed on Muller Hinton agar with different strains of P. aeruginosa, incubated for 24 h. at 37 °C and the diameters of the zones of inhibition were measured this is repeated three times.
2.3 Determination of MIC and MBC
The MIC and MBC were determined by using broth microdilution method in 96 wells plates. A 100 µl of 3.2 mg/ml of β-sitosterol was serially diluted, then 100 µl of p. aeruginosa were added into each well and incubated for 24 h. at 37 °C. MIC was determined and recorded as the minimum concentration that prevented the growth of bacteria, this is repeated three times.
For MBC, we took 10 µl from the wells at and above MIC, spread on agar plates technique. The MBC was determined as the concentration at which no bacterial colonies formed on plates after 24 h incubation at 37 °C this is repeated three time.
2.4 Antibiofilm activity
To determine the effects of β-sitosterol on pre-formed biofilm, 100 µl cultures of P.aeruginosa strains were inoculated into 96 wells plates overnight in LB after adjusting turbidity to 0.5 McFarland standard. After 24 h the broth was discarded and the wells were washed with distilled water three times to remove unattached bacteria. 100 µl of 32 mg/ml of β-sitosterol was serially diluted in 96 wells with one well left without addition of β-sitosterol to act as poitive control and one well with 100 µl ethyl acetate to act as negative control, the wells were overnight incubated at 37 °C then biofilm reduction were detected qualitatively by observing turbidity in 96 microtiter plates and quantitatively by calorimetry assay using crystal violet staining. Biofilms were also evaluated using Scanning Electron Microscopy (SEM). PAO1 Δpsl biofilm (24 h) was scanned before and after treatment with 100 µl of 800 µg/ml β-sitosterol for 24 h.
2.5 Motility assays
The effects of β-sitosterol on motility of P. aeruginosa were tested using modified protocols from (Ha et al., 2014) for swimming motility and (Packiavathy et al., 2014) for swarming motility. Briefly, overnight culture of P. aeruginosa with (100 µl) β-sitosterol (400 µg), 2 µl were inoculated on the media of swimming (0.3 % agar) and swarming (0.6 % agar). Plates were incubated at 37 °C for 24 h and the diameter of the migration zones from the inoculation points were measured.
2.6 Tissue culture cytotoxicity test of β-sitosterol
To determine the cytotoxicity of β-sitosterol against mammalian cells, Madin Darby canine kidney (MDCK) cells were used as a model for mammalian epithelial cells MDCK tissue culture cells were grown to ∼ 90 % confluency in a 24 well sterile Corning plate. Then, we added 100 µl of β-sitosterol, 100 µl of PAO1 was used as positive cytotoxicity control. The cells were observed at 24, 48 and 72 h under a microscope to determine when the epithelial culture has gone below 75 % confluence with tight junctions between the cells broken down. We also used lactate dehydrogenase (LDH) release assay to supplement our microscopy data. Cell death was measured by assessing plasma membrane damage. If the cell is damaged it releases a product LDH which can be measured using a colorimetric assay with a plate reader.
2.7 Statistical analysis
Three separate bacterial cultures were used in MIC, MBC antibiofilm and cytotoxicity tests. The one-way ANOVA test, with Dunnett's and Tukey's adjustments, has been used to establish statistical significance. P values less than 0.05 are regarded as statistically significant.
3 Result
3.1 Antibacterial effect
β-sitosterol shows antibacterial and antibiofilm properties towards different strains of P. aeruginosa as shown in Fig. 2 which revealed that β sitosterol exhibited wide range of antibacterial effect against P. aeruginosa strains where the most antibacterial effect was recorded with Δ Pel Psl, Δ Psl and PA 14 the inhibition zone measured between (16.51 ± 0.51 mm to 24.13 ± 0.51 mm), while in case of PAO1, Δ wspf, Δ Pel the range of zone inhibition was (12.62 ± 0.45 mm to 18.58 ± 0.45 mm).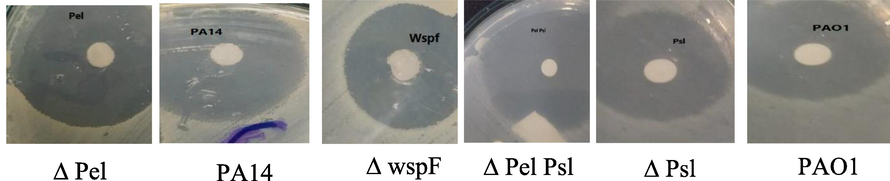
Antibacterial effect of β-sitosterol was measured by using disc diffusion method.
3.2 MIC and MBC and antibiofilm activity
The susceptibilities of various wild and mutant strains of P. aeruginosa were contrast in Table 1. For planktonic bacteria the MBC was 4–8 fold more than MIC. The results revealed that β- sitosterol has antibacterial and antibiofilm against wild and mutant strains of P. aeruginosa. Calorimetry assay showed that β- sitosterol reduced biofilms of wild type PAO1at concentrations 400, 800, 1600, 3200 µg/ml by 50.27± 0.23 %, 72. 37 ± 0.26 %, 85.26 ± 0.31 % and 98.15 ± 0.29 % respectively, while for PA14 the percentage of biofilms reduction with concentrations 200, 400, 800 and 1600 µg was 48.24 ± 0.17 %, 75.97 ± 0.26 %, 87.06 ± 0.26 %and 96.30 ± 0.26 %respectively. Biofilms of ΔwspF was reduced by reduction 50.08 ± 0.29 %, 69.40 ± 0.34 %, 83.89 ± 0.25 % and 85.50 ± 0.27 % with concentrations 400, 800, 1600 and 3200 µg/ml respectively.
Strain
Vegetative cells
MIC(µg/ml)
MBC(µg/ml)
MBC/MIC ratio
PA14
25
200
8
PAO1
50
200
4
ΔPel
25
100
4
ΔPsl
50
200
4
ΔwspF
100
800
8
Δ Pel Psl
12.5
50
2
The anti-biofilm effect of β-sitosterol on ΔPel with concentrations 200, 400 and 800 µg was 51.17 ± 0.28 %, 82.42 ± 0.27 % and 99.8 ± 0.22 % respectively while on ΔPsl concentrations were 200, 400 and 800 µg/ml and reduction percentage of biofilm formation was 44.34 ± 0.23 %, 76.97 ± 0.22 and 90.40 ± 0.25 % respectively. Evaluation of anti-biofilm in case of ΔPel Psl with 100, 200, 400 µg was 42.85 ± 0.26 %, 74.60 ± 0.31 %and 99.68 ± 0.33 % respectively. These results are shown in Figs. 3, 4 and 5.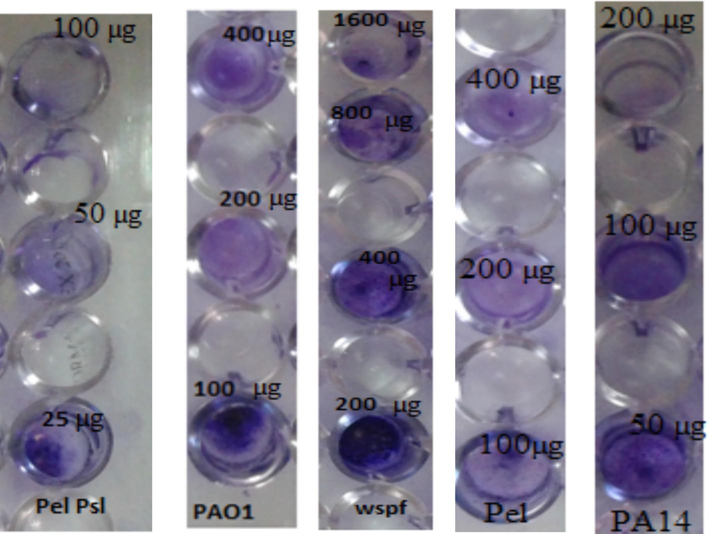
Showing reduction of biofilm with different mutant isolates by staining with crystal violet.
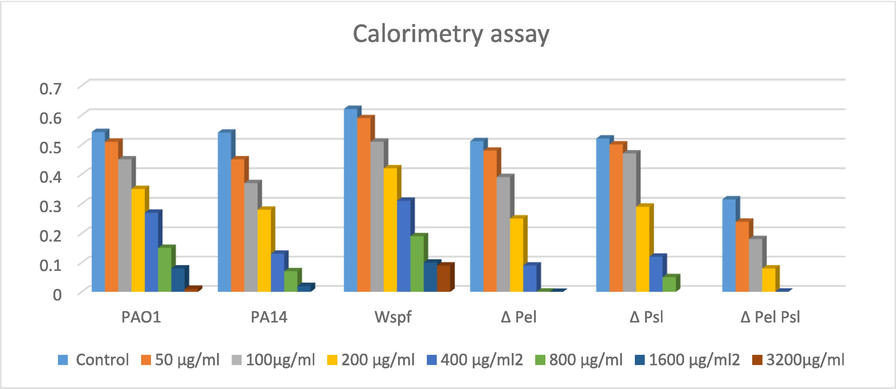
Calorimetry assay showing reduction effect of β-sitosterol on biofilm formed by PAO1, PA14, Δwspf, Δ Pel, ΔPsl, and Δ Pel Psl.
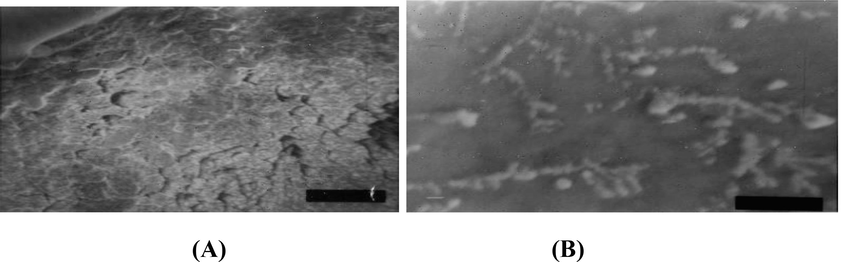
Scanning Electron Microscope (A) Biofilm formation of PAO1 Δpsl before treatment with β-sitosterol (B) Biofilm of PAO1 Δpsl after treatment with β-sitosterol showing disruption of biofilm.
3.3 β-sitosterol inhibits motility
The effect of β-sitosterol(400 µg) on swimming and swarming was shown in Fig. 6 (A, B).Fig. 6A showing effect of β-sitosterol on swimming of PA14 it was 44.4 ± 0.21 % reduction and in case of PAO1 was 42.5 ± 0.22 % reduction. The swarming reduction of PA14 it was 43.30 ± 0.16 % reduction. Additionally, reduction was 44.40 ± 0.16 % with PAO1.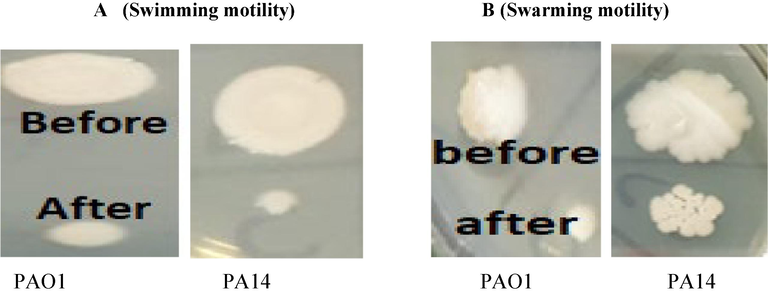
(A) Effects of β-sitosterol on swimming motility of different wild and mutant strains of P. aeruginosa, figure showing swimming before and after treatment.(B) Effect of β-sitosterol on swarming motility of different wild and mutant strains of P. aeruginosa, figure showing swarming before and after treatment.
3.4 Cytotoxicity of β-sitosterol
Fig. 7 shows that β-sitosterol has low cytoxicity onto MDCK epithelial cells after exposure for 24, 48 and72 h. Also, Fig. 8: A, B revealed that epithelial cells still alive under microscope even after incubation with β-sitosterol for 24 h.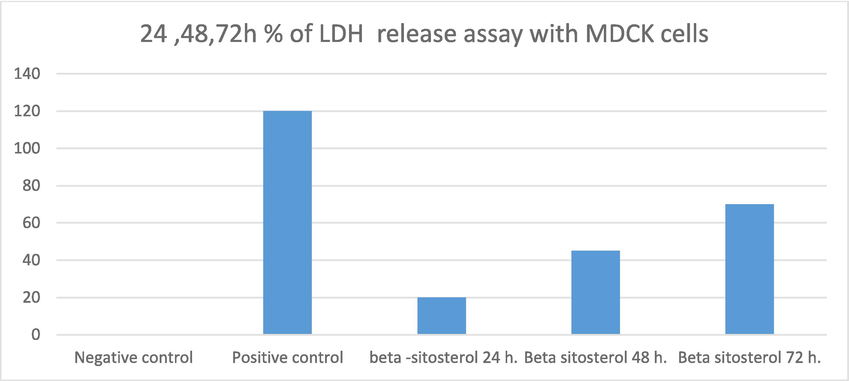
Showed the % of LDH release with β-sitosterol, control and positive control after 24, 48, 72 h incubation with MDCK cells.
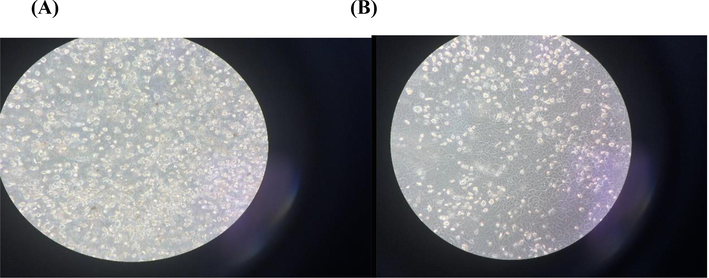
(A) showed MDCK under microscope at 0-time control condition, fig (B) showed 72 h. incubation with β-sitosterol.
4 Discussion
P. aeruginosa is a common opportunistic Gram negative pathogen found in various ecological and medical environments that has capabilities to form biofilms on many types of surfaces including indwelling medical devices. Moreover, it has intrinsic resistance against most classes of antibiotics, which is a major reason why it is one of the most widely studied organisms. P.aeruginosa can produce three distinct extracellular biofilm polysaccharides: PSL, PEL and alginate.β-sitosterol is found extensively in plants and has demonstrated to have antibacterial properties. (Anokwah et al., 2021; Anwar et al. 2022) and anti- Quorum sensing (Rasamiravaka et al., 2017). In this research we focus on investigating β-sitosterol as a potential antibiofilm compound.
To assess the antibacterial effect of β-sitosterol, several assays were performed against P. aeruginosa: disc diffusion assays, and determination of MIC and MBC which revealed that β-sitosterol has antibacterial properties against P. aeruginosa this is in convenience with (Anwar et al. 2022) who reported that β-sitosterol extracted from Kalanchoe tomentosa exhibited antibacterial effect against S. aureus and K. pneumonia. Also, (Subramaniam et al., 2014) reported that β-sitosterol-D-glucopyranoside exhibited antibacterial activity against E. faecalis, S. dysenteriae, and P. aeruginosa. A recent study (Abo-Elghiet et al. 2023) isolated β-sitosterol from Pulicaria crispa as one of the most abundant compounds and revealed antibacterial effect against S.aureus and P. aeruginosa.
The MIC of β-sitosterol ranged from 12.5 to 100 µg/ml with different planktonic strains of P. aeruginosa while MBC recorded 50 to 200 µg/ml. A study reported that MIC of β-sitosterol was 125 µg/ml with P. aeruginosa (Anokwah et al. 2021) Also, (Akbar et al., 2021) Showed that MIC of β-sitosterol with p. aeruginosa was 700 µg/ml. Also, A report (Anwar et al., 2022) showed that MIC of β-sitosterol against K. pneumonia is 31.25 µg/ml.
P. aeruginosa biofilm represents a scaffolding network under which sessile bacterial communities are differ completely in resistance to antimicrobials than planktonic (Limoli et al., 2015). Antibiotic resistant pathogens are an increasing problems in hospitals all over the world. To eradicate and combat p. aeruginosa biofilms, doctors usually use combination of two antimicrobial agents but this way has many side effects and adverse effects moreover it may produce multidrug resistance (Chung et al., 2023). Consequently, we are in an urgent need for new natural antimicrobial compounds that can combat biofilm infections, including p. aeruginosa.
PSL consists of D-glucose, D mannose and L-rhamnose with neutral charge(Byrd et al., 2009) which plays an important role in starting cell adhesion and cell to cell communications (Jones et al., 2017). In addition, PSL plays an important role in resistance of sessile form to antimicrobial agents (Billings et al., 2013) In contrast, PEL consists of N-acetyl-d-glucosamine and N-acetyl-d-galactosamine with positive charge which contributes in initiation, integrity of biofilm and repulsion of positively charged antimicrobial agents (Jennings et al., 2015).
Antibiofilm effect of β-sitosterol was assessed calorimetry using crystal violet assay which showed reduction effect with most mutated strains and wild types. This is in agreement with (Rasamiravaka et al., 2017) who reported that β-sitosterol at 200 µM has reduced of extracellular polysaccharides of PAO1 after 24 h by 42 ± 3 % and has diminished production of the pelA gene by 32 ± 7 % while biofilm formation was prohibited by 44 ± 3 %.
The motility of P. aeruginosa is accompanied by flagella and type IV pili (T4P) (Gellatly and Hancock, 2013) where flagella plays an important role of swimming in liquid media while T4P participates effectively of twitching on solid media and when both of them combine together participate with swarming effect on semi-solid (Nirody et al., 2017).
PSL plays a role in swimming (Wang et al. 2013), since β-sitosterol has a reduction effect on swimming so it will affect PSL too.
Swarming and swimming motility play an important role in bacterial colonization and primary attachment of vegetative bacteria to sessile form(Conrad et al., 2011).Our results revealed that β-sitosterol inhibited a swimming motility and swarming motilityof P. aeruginosa.
β-sitosterol has an effect on quorum sensing (QS) since (Rasamiravaka et al. 2017) reported that β-sitosterol diminished expression of lasB and rhlA by 25 %, 32 % respectively. Also, they reported 44 % inhibition for both pyocyanin production and biofilm formation while extracellular polysaccharide in PAO1was diminished by 42 % and reduced expression of Pel A by 32 %.
Our results about cytotoxicity of β-sitosterol revealed that epithelial cells keep alive after 24, 48, 72 h. Our results are in line with a recent study showed that β-sitosterol exhibited no hepatotoxicity, did not inhibit cytochrome P450 2D6 and did not show mutagenic in the chemical Ames mutagenicity test; and LD 50 for rat oral LD50 was 1.57 g/kg (Akbar et al., 2021).
In animal model, a group of mice were treated with 5 mg /kg β-sitosterol 24 h before infection with p. aeruginosa where they recovered faster and there was a decrease in number of bacteria isolated from mice airways (Rossi et al., 2023).
Many investigations have been carried out recently to know mode of action of β-sitosterol, one of them interpreted mode of action of β-sitosterol by interfering with pneumolysin hence protects mice from death of pneumonia caused by Streptococcus pneumonia (Li. et al., 2015), while another interpretation of mode of action of β-sitosterol with Salmonella typhimurium by elevating secretion of antimicrobial peptides (Ding et al., 2010). Also, Protein ligand docking for β-sitosterol revealed that antibacterial action is due to prevention of enzyme dihydrofolate reductase (Ravi et al., 2020) which leads to interruption of synthesis of thymidylate and folate dependent formyltransferases (Rao and Tapale 2013).
A recent study (Fan et al., 2023) showed that β-sitosterol affects production of LPS which represents a pioneer in virulence factors of all gram negative bacteria. Additionally, lipid A in LPS is endotoxic, which allows for tissue injury and attachment (Park et al., 2022). They recommended β-sitosterol as a new natural alternative for antibiotics.
A viable strategy is to target matrix exopolysaccharides unique to P. aeruginosa biofilms because these exopolysaccharides are important for community architecture, cell aggregation, and substrate adhesion—factors that enhance P. aeruginosa tolerance to antimicrobials and host defenses and confer survival benefits (Malhotra et al. 2019).
Since β-sitosterol contain hydroxyl group terminal which may form a hydrogen bond with hydroxyl group of N-acetylglucosamine found in Pel gene and hydroxyl group of d-mannose found in PSL and this may interfere with their role in biofilm formation.
This suggestion must be investigated in future as it will be a valuable finding since positive charge of PEL enables it to bind to eDNA consequently increased resistance to aminoglycoside (Jennings et al.2021).
5 Conclusion
P. aeruginosa secretes three factors associated with initiation of infection and biofilm formation PEL, PSL and alginate. Our results showed that β-sitosterol has both antibacterial and antibiofilm effect on P. aeruginosa. Also, affects swarming and swimming of P.aeruginosa. Our findings suggests a potential novel compound to combat P. aeruginosa infections.
CRediT authorship contribution statement
Marwa Fady: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Data curation, Conceptualization. Yasuhiko Irie: Supervision, Writing – review & editing. Reem M. Aljowaie: Writing – review & editing, Resources. Saeedah Musaed Almutairi: Writing – review & editing, Resources.
Acknowledgments
The authors extend their appreciation to the Researchers supporting project number (RSP2024R418), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical profile, antibacterial, antibiofilm, and antiviral activities of pulicaria crispa most potent fraction: an in vitro and in silico study. Molecules. 2023;28:4184.
- [CrossRef] [Google Scholar]
- Evaluation of the antioxidant, antimicrobial, and anticancer activities of dicliptera bupleuroides isolated compounds using in vitro and in silico studies. Molecules. 2021;26:7196.
- [CrossRef] [Google Scholar]
- Bioactive constituents with antibacterial, resistance modulation, anti-biofilm formation and efflux pump inhibition properties from Aidia genipiflora stem bark. Clin. Phytosci.. 2021;7(28)
- [CrossRef] [Google Scholar]
- Antimicrobial activity of β-Sitosterol Isolated from kalanchoe tomentosa leaves against staphylococcus aureus and klebsiella pneumonia pak. J. Biol. Sci.. 2022;25:602-607.
- [Google Scholar]
- The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog.. 2013;9:e1003526.
- [Google Scholar]
- Pseudomonas aeruginosa twitching motility: type IV Pili in action. Annu. Rev. Microbiol.. 2012;66:493-520.
- [CrossRef] [Google Scholar]
- Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol.. 2009;73:622-638.
- [Google Scholar]
- How Three self-secreted biofilm exopolysaccharides of pseudomonas aeruginosa, Psl, Pel, and alginate, Can each be exploited for antibiotic adjuvant effects in cystic fibrosislung infectionInt. J. Mol. Sci.. 2023;24:8709.
- [CrossRef] [Google Scholar]
- CLSI, “Performance Standards for Antimicrobial Susceptibility Testing,” Twenty-Fifth Informational Supplement. CLSI Document M100-S25, 2015.
- The Pel and Psl polysaccharides provide pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol.. 2012;14(8):1913-1928.
- [CrossRef] [Google Scholar]
- Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys J. 2011;100:1608-1616.
- [CrossRef] [Google Scholar]
- β-Sitosterol improves experimental colitis in mice with a target against pathogenic bacteria. J. Cell Biochem.. 2010;120(4):5687-5694.
- [Google Scholar]
- β-sitosterol suppresses lipopolysaccharide-induced inflammation and lipogenesis disorder in bovine mammary epithelial cells. Int. J. Mol. Sci.. 2023;2023(24):14644.
- [CrossRef] [Google Scholar]
- Contribution of Pseudomonas aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front. Cell Infect. Microbiol.. 2022;12:835754
- [CrossRef] [Google Scholar]
- Pseudomonas aeruginosa: newinsights into pathogenesis and host defenses. Pathog Dis. 2013;67:159-173.
- [CrossRef] [Google Scholar]
- Plate-based assay for swimming motility in pseudomonas aeruginosa. Pseudomonas Methods Protoc.. 2014;1149:59-65.
- [Google Scholar]
- Pel is a Cationic exopolysaccharide that cross-links extracellular DNA in the pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci.. 2015;112:11353.
- [CrossRef] [Google Scholar]
- Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep.. 2021;34(8):108782
- [CrossRef] [Google Scholar]
- Psl produced by mucoid pseudomonas aeruginosa contributes to the establishment of biofilms and immune evasion. mBio. 2017;8:e00864-e00917.
- [Google Scholar]
- β-sitosterol inter- acts with pneumolysin to prevent Streptococcus pneumoniae infection. Sci. Rep.. 2015;5:17668-17677.
- [Google Scholar]
- Virulence factors of pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance front. Cell. Infect. Microbiol. 06 July 2022
- [CrossRef] [Google Scholar]
- Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr.. 2015;3:223-247.
- [Google Scholar]
- Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci.. 2020;7:1901872. [CrossRef] [PubMed]
- [Google Scholar]
- Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface. Clin. Microbiol. Rev.. 2019;32:e00138-e00218.
- [Google Scholar]
- National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 222284, Beta-Sitosterol. Retrieved June 13, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Beta-Sitosterol.
- The biophysicist’s guide to the bacterial flagellar motor. Adv. Phys. X. 2017;2:324-343.
- [CrossRef] [Google Scholar]
- Structure elucidation of βsitosterol with antibacterial activity from the root bark of Malva parviflora. Springerplus.. 2016;5(1)
- [CrossRef] [Google Scholar]
- An inside look at a biofilm: Pseudomonas aeruginosa flagella biotracking. Sci. Adv.. 2021;7:1-15.
- [Google Scholar]
- (2014) Inhibition of biofilm development of uropathogens by curcumin−ananti-quorum sensing agent from Curcuma longa. Food Chem.. 2014;148:453-460.
- [Google Scholar]
- Benzyl isothiocyanate attenuates inflammasome activation in Pseudomonas aeruginosa LPS-stimulated THP-1 cells and exerts regulation through the MAPKs/NF-kappaB pathway. Int. J. Mol. Sci.. 2022;23:1-10.
- [Google Scholar]
- A study on dihdrofolate reductase and its inhibitors: a review. Int. J. Pharm. Sci. Res.. 2013;4(7):2535-2547.
- [Google Scholar]
- Rasamiravaka T., Ngezahayo J., Pottier L., Ribeiro S., Souard F. , Hari L., Stevigny , El Jaziri M.and Duez P. Terpenoids from Platostoma rotundifolium (Briq.) A. J. Paton Alter the Expression of Quorum Sensing-Related Virulence Factors and the Formation of Biofilm in Pseudomonas aeruginosa PAO1. Int. J. Mol. Sci. 2017, 18, 1270.
- Ravi L., Girish S.,Harshini M.,Sreenivas B.(2020): β-Sitosterol: an antibacterial agent in aquaculturemanagement of vibrio infections. J. Pure Appl. Microbiol. | 14(4):2699-2714 10.22207/JPAM.14.4.48.
- β-sitosterol ameliorates inflammation and Pseudomonas aeruginosa lung infection in a mouse model. J. Cystic Fibrosis. 2022;22(2023):156-160.
- [CrossRef] [Google Scholar]
- Synergistic antibacterial action of sitosterol-D-glucopyranoside isolated from desmostachya bipinnata leaves with antibiotics against common human pathogens. Rev. Bras Farma. 2014;24:44-50.
- [Google Scholar]
- A spider web strategy of type IV Pili-mediated migration to build a fibre-like Psl polysaccharide matrix in pseudomonas aeruginosa biofilms. Environ. Microbiol.. 2013;15:2238-2253.
- [Google Scholar]







