Translate this page into:
Effect of endophytic entomopathogenic fungi on soybean Glycine max (L.) Merr. growth and yield
⁎Corresponding author. leticiarusso@conicet.gov.ar (M.L. Russo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The soybean is a crop of economic importance and has a great number of potential pests which cause significant economic losses. The entomopathogenic fungi Beauveria bassiana, Metarhizium anisopliae and Metarhizium robertsii are important biological control agents, which can live as endophytes within plants and causes no apparent damage to the host. The aims of this study were to assess whether the entomopathogenic fungi B. bassiana, M. manisopliae and M. robertsii are able to colonize soybean plants as endophytes by using different inoculation techniques; and assess if these fungi produce any effect on the growth and yield of soybean plants under field conditions. We demonstrate the effectiveness of three inoculation methods (foliar spray, seed immersion and root immersion) to establish fungal entomopathogens as endophytes. Percentage of recovery for the different fungal strains was higher after 7 days of inoculation, through the organ that was in direct contact with the fungus during the inoculation. B. bassiana LPSc 1098 inoculated by leaf aspersion was the most successful strain. It was also demonstrated for the first time that inoculation with B. bassiana promoted the growth and increased the yield of soybean plants under filed conditions, with no adverse effects observed in the inoculated plants.
Keywords
Glycine max
Endophytes
Beauveria bassiana
Metarhizium sp.
1 Introduction
The soybean Glycine max (L.) Merril is native to China and belongs to the family Fabaceae. Due to its oils and proteins, which are widely used in the production of food for animals and humans, soybean is currently cultivated worldwide. Soybean is affected from plant emergence to grain maturity by a great diversity of pest arthropods, which usually limit the growth and yield of this crop. The main management strategy of these pests is focused on the use of pesticides however, they also have direct effects on humans and the environment. Entomopathogenic fungi including Beauveria bassiana (Bals-Criv.) Vuill. (Hypocreales: Cordicypitaceae), Metarhizium anisopliae (Metschn.) Sorokin and Metarhizium robertsii Bisch., Rehner & Humber (Hypocreales: Clavicipitaceae) are used as biocontrol agents worldwide (Vega et al., 2012). Most research on these fungi has focused on the development of inundative methods, however, their endophytic behavior indicates that the ecology of these microorganisms is far beyond the fungus-insect interaction. Endophytic fungi can live inside plants and, in general, do not cause visible damage to the host (Gurulingappa et al., 2010). Several species of entomopathogenic fungi occur naturally in different plant species (Vega, 2008). Others have been artificially introduced into plants by different inoculation techniques, such as foliar spray, stem injection, root and seed immersion, and soil drenching (Akello and Sikora, 2012; Gurulingappa et al., 2010; Jaber and Enkerli, 2016; Castillo Lopez and Sword, 2015; Parsa et al., 2013; Quesada Moraga et al., 2014a; Russo et al., 2015), being able to colonize the plants either locally (Wearn et al., 2012; Yan et al., 2015) or systemically (Gurulingappa et al., 2010; Quesada Moraga et al., 2006; Russo et al., 2015). Recently, many studies have demonstrated that some species are able to play a wider role in nature than previously thought, for example, as promoters of plant growth through the increasing of root length, dry and wet weight, foliar area, seed germination, plant height, yield and even the nutritional status (Akello et al., 2008a,b; Castillo Lopez and Sword, 2015; Greenfield et al., 2016; Griffin et al., 2005; Kabaluk and Ericsson, 2007; Liao et al., 2014; Ownley et al., 2004, 2008; Qayyum et al., 2015; Sánchez Rodríguez et al., 2015; Sasan and Bidochka, 2012; Vega, 2008; Vega et al., 2009).
The aims of this study were to (1) assess whether the entomopathogenic fungi B. bassiana, M. anisopliae and M. robertsii are able to colonize soybean plants as endophytes either locally or systematically by using different inoculation techniques; and (2) use the most efficient inoculation technique and the most frequent fungal isolate recovered from inoculated plants under laboratory conditions, to assess if these fungi produce any effect on the growth and yield of the soybean plants under field conditions.
2 Material and methods
2.1 Experiment-I
2.1.1 Fungal strains and inoculum preparation
Sixteen strains were used in the experiments including fourteen of B. bassiana, one of M. anisopliae and one of M. robertsii. All strains of entomopathogenic fungi were obtained from the fungal collection of “Instituto Spegazzini” (LPSc), La Plata, Argentina and preserved by freezedrying (lyophilization) technique. To confirm the identity of all strains, previously characterized on the basis of morphological characters according to taxonomic keys of Humber (2012), molecular techniques were used. DNA was extracted from fungal cultures developed on potato dextrose liquid medium after 7 days incubation at 25 °C in darkness using the DNA easy Plant Mini kit (Qiagen, Hilden, Germany). Amplification of the internal transcribed spacers (ITS) was carried out using the universal primers ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990). Polymerase-chain reactions (PCRs) were carried, amplicon sizes were checked by electrophoresis and purified PCR products were sent to Sequencing Service – CERELA (Tucumán, Argentina) for sequencing in both directions. The sequences obtained were edited using the program BioEdit version 7.0.9.0 (Hall, 1999) and submitted to the National Center for Biotechnology Information (NCBI) GenBank database for gene annotation. Sequences are available under the accession numbers: Beauveria bassiana MG712618 (LPSc 1060), MG712619 (LPSc 1061), MG712620 (LPSc 1062), MG712624 (LPSc 1063), MG712621 (LPSc 1066), MG712622 (LPSc 1080), MG712623 (LPSc 1081), MG712625 (LPSc 1083), MG712626 (LPSc 1086) and MG712627 (LPSc 1156). While M. anisopliae LPSc 907, M. robertsii LPSc 963, B. bassiana LPSc 902, 1067, 1082 and 1098 were previously determined with GeneBank accession numbers KT163258, KJ772494, KT952326, KF500409, KJ7722495 and KT163259 respectively.
To obtain the conidial suspensions, different isolates of each species were cultivated onto potato dextrose agar (PDA) (Britania®) and incubated at 25 °C in darkness. After 15 days, conidia were harvested by scrapping them off the Petri dishes and transferred to 10 ml of 0.01% (v/v) Tween 80 (polyoxyethylene sorbitan monolaurate) (Merck®). The suspension was filtered and homogenized by shaking for 10 min. Conidial concentration was determined by using a Neubauer chamber and adjusted to 1 × 108 conidia/ml (Gurulingappa et al., 2010). The conidial viability of each isolate was evaluated according to Greenfield et al. (2016), and in all cases, the mean conidial viability was >95%.
2.1.2 Soybean plants substrates
Seeds of the variety DM3810 (Don Mario, Argentina) were used for all experiments. The seeds were surface-sterilized (Posada et al., 2007) and were transferred to plastic pots (330 cm3) containing a mixture of equal parts of earth-perlite-vermiculite (1: 1: 1), used as planting substrate. The substrate was tindalized in an autoclave for 45 min at 121 °C. Sterilization was performed three times with 24-h intervals between each process (Quesada Moraga et al., 2014b). Plants were produced and maintained under greenhouse conditions (25 ± 2 °C, 12:12 LD photoperiod) until used.
2.1.3 Inoculation methods and determination of endophytic colonization
Three methods of inoculation were tested: leaf aspersion, root immersion and seed immersion, according to Russo et al. (2015).
Plant colonization by different fungal isolates was evaluated after 7, 14, 21, and 28 days of inoculation. Each plant was separated into roots, shoot and leaves, and surface-sterilized by successive immersions in 70% ethanol for 2 min, sodium hypochlorite (55 g Cl/L of commercial bleach) for 2 min and finally rinsed twice with sterile distilled water. To determine the efficiency of the surface sterilization method, surface-sterilized stem pieces were plated onto solid PDA (Reddy et al., 2009). The absence of fungal or bacterial growth was considered indicative of a successful sterilization technique.
Each plant organ was cut with a sterile scalpel into pieces of 1 cm2. Six pieces of each plant organ were placed onto 20 ml of PDA medium with 2 ml of antibiotics (5g streptomycin and 0.25 g chloramphenicol/200 ml) (Vega et al., 2008). All dishes were maintained at 25 °C in a growth chamber and examined after 10 days of incubation. A total of 1920 plants and 34,560 plant pieces were examined (120 plants and 2160 plant pieces for each fungal strain inoculated). Data were expressed as the frequency of colonization (FC) = (number of plant pieces colonized/total number of plant pieces examined) × 100 (Petrini and Fisher, 1986).
2.2 Experiment-II
2.2.1 Effect of fungal inoculation on plant growth and yield
To determine if the entomopathogenic fungus has any effect as endophyte on the soybean growth and yield, we selected leaf aspersion as inoculation technique because it proved to be the most efficient, and B. bassiana strain LPSc 1098 because it was the most frequently recovered from the inoculated plants under laboratory conditions. Plants were obtained according to the methodology described in Experiment I. After two weeks, all plants were inoculated and maintained in a greenhouse for 7 days before transferring them (according to the sowing time of the crop) to a 10 × 10 m field plot, in a randomized way and forming six rows of 10 plants each. Plants transferred to the field were previously checked for endophytic colonization according to Russo et al. (2015). The fungal effect on plant growth and yield was determined after five months, when plants completed their annual cycle. The following parameters were analyzed: total plant height, number of branches per plant, number of pods per branch and per plant, pod weight per branch and per plant, number of seeds per pod, per branch and per plant, seed weight per branch and per plant, yield (Diestéfano and Gadbán, 2010), and germinative capacity of seeds, according to the International Association of Seed Analysis (ISTA, 2007), following the protocol (modified) of Luna and Iannone (2013).
This experiment was performed in Alberti city, Buenos Aires province, Argentina (35° 1′ 53″ S-60° 16′ 49″ W). The mean annual rainfall was 1000 mm and the mean temperature was 16 °C (www.trigoklein.com.ar/estacion-meteorológica). Soybean is one of the main crops in this area, arriving at physiological maturity in optimal conditions. In this experiment, we used 30 inoculated plants and 30 controls. Controls were inoculated with a 0.01% (v/v) Tween 80 (Merck®) solution without the addition of fungal inoculum.
2.3 Statistical analyses
Inoculation techniques (Experiment-I), plant organs, time and frequency of colonization of the different strains were compared with a three-way Analysis of Variance (ANOVA) and the mean differences compared by Tukey’s test (p < 0.05) using InfoStat (2004).
To stabilize the variance, percent values were transformed to arcsine. Differences between the germinative capacity, the yield, and each growth parameter analyzed of plants inoculated with B. bassiana and non-inoculated (controls) (Experiment-II) were compared with a t-test.
3 Results
3.1 Experiment-I. Recovery of entomopathogenic fungi as endophytes
No growth of entomopathogenic fungi was observed in the non-inoculated controls (data not shown). All techniques of inoculation introduced successfully the strains of B. bassiana into soybean plants while the seed immersion technique was unsuccessful for introducing M. anisopliae and M. robertsii (Table 1). ANOVA results of the comparison between inoculation techniques, organs and time for each fungal strain are shown in Table 2.
Strain
Days
Leaf aspersion
Seed immersion
Root immersion
Root
Stem
Leaf
Root
Stem
Leaf
Root
Stem
Leaf
7
45 ± 5.6 defhi
90 ± 5.8 ij
100 ± 0 J
51.3 ± 8.8 efghi
36.7 ± 7.2 bcdefg
18.7 ± 11.3 abcd
40 ± 10.6 cdefgh
43.3 ± 11.2 cdefgh
40 ± 10.6 cdfgh
B. bassiana LPSc 1098
14
43.3 ± 5.1 defghi
66.7 ± 7 ghi
100 ± 0 J
16.7 ± 7.5 abcde
20 ± 8.2 abcde
23.3 ± 10.9 abcdef
40 ± 10.6 cdefgh
46.7 ± 6 defgh
5 ± 2 ab
21
35 ± 4.6 cdefghi
73.3 ± 3.7 hij
83.3 ± 7.9 ij
13.3 ± 7.4 abcd
11.7 ± 7.9 abc
15 ± 7.6 abcd
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
31. 7 ± 7.2 abcdefg
70 ± 5 ghi
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
31.6 ± 8 abcd
66.7 ± 4 bcd
80 ± 6 d
38.3 ± 4 abcd
40 ± 6 abcd
43.3 ± 6 abcd
33.3 ± 4 abcd
40 ± 2 abcd
33.3 ± 2 abcd
B. bassiana LPSc 1067
14
25 ± 7 abcd
25 ± 3 abcd
76.7 ± 4 cd
38.3 ± 4 abcd
33.3 ± 5 abcd
23.3 ± 5.5 abcd
25 ± 8.3 abcd
18.3 ± 9.4 abc
35 ± 5 abcd
21
16.6 ± 7 abc
25 ± 2 abcd
50 ± 2 abcd
20 ± 0 abc
23.3 ± 4 abcd
23.3 ± 1.2 abcd
0 ± 0 a
23.3 ± 4.5 abcd
15 ± 7 abc
28
16.6 ± 2 abc
25 ± 4 abcd
31.7 ± 6 abcd
8.3 ± 0 ab
15 ± 4 abc
10 ± 6.7 ab
0 ± 0 a
0 ± 0 a
10 ± 1 ab
7
36.6 ± 4 cde
83.3 ± 6 fg
88.3 ± 5 g
33.3 ± 0.5 cde
3.3 ± 1 ab
0 ± 0 a
30 ± 1 bcde
30 ± 7 bcde
26.7 ± 4 abcde
B. bassiana LPSC 1086
14
18. 3 ± 7 abcd
46.6 ± 5 de
58.3 ± 5.6 efg
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
36.7 ± 2 cde
13.3 ± 4.6 abc
21
0 ± 0 a
0 ± 0 a
16.6 ± 7.4 abcd
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
66.6 ± 4.4 fghi
86.7 ± 6 J
81.7 ± 6 ij
61.3 ± 7.3 efghij
31.7 ± 7 abcdef
13.7 ± 6 abcd
71.7 ± 7 ghij
36.7 ± 7 abcdef
40 ± 6 abcdef
B. bassiana LPSc 1080
14
40 ± 8 abcdef
66.7 ± 2.6 ghij
78.3 ± 6 hij
45.3 ± 3.3 abcdef
36 ± 6 abcdefg
23.7 ± 4.8 abcdef
16.7 ± 5 abcde
36.7 ± 3 abcdef
35 ± 6 abcdef
21
6.7 ± 2 abc
53.3 ± 1.3 defgh
48.3 ± 2.9 cdefgh
0 ± 0 a
0 ± 0 a
0 ± 0 a
6.7 ± 1 ab
16.7 ± 3 cdefgh
16.7 ± 2 abcde
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
20 ± 7.7 abcdef
60 ± 3 fgh
71.7 ± 6 h
71.7 ± 5 gh
58.3 ± 7.1 fgh
0 ± 0 a
40 ± 8 bcdefg
46.7 ± 8 cdefgh
40 ± 9.6 bcdefgh
B. bassiana LPSc 1063
14
11.7 ± 3.6 abcd
36.7 ± 4 abcdefg
53.3 ± 6 efgh
13.3 ± 5.9 abcd
25 ± 6.5 abcef
0 ± 0 a
15 ± 6.3 abcde
35 ± 7 abcdefg
13.3 ± 4.3 abcd
21
8.3 ± 2.2 abc
30 ± 5 abc
15 ± 2 abcd
10 ± 2 ab
10 ± 3 abc
0 ± 0 a
3.3 ± 1 ab
8.3 ± 3.7 ab
0 ± 0 a
28
3.3 ± 1.1 ab
5 ± 2.5 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
43.3 ± 8.6c
46.7 ± 6.9cd
83.3 ± 4.9f
45 ± 7 cd
31.7 ± 3.8c
0 ± 0 a
45.7 ± 8.2cd
45.3 ± 8.7cd
33.3 ± 1c
B. bassiana LPSc 1066
14
25 ± 4.4 bc
46.7 ± 6.9cd
71.7 ± 3.5 def
31.7 ± 4.6c
25 ± 3.7 bc
0 ± 0 a
43.3 ± 5 cd
30 ± 6.1c
0 ± 0 a
21
0 ± 0 a
28.3 ± 4.3c
48.3 ± 3.8 cde
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
10 ± 5 ab
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
10 ± 3.5 ab
83.3 ± 7.8 fg
86.7 ± 4.8 fg
5 ± 3.5 a
13.3 ± 3.3 ab
26.7 ± 3 abcd
68.3 ± 2.9 defg
40.7 ± 5.1 bcd
40.3 ± 1 bcd
B. bassiana LPSc 1156
14
13.3 ± 5.9 ab
60 ± 2.2 cdefg
81.7 ± 4 fg
23.3 ± 6 abc
16.7 ± 7 ab
28.3 ± 6.1 abcd
26.7 ± 2 abc
33.3 ± 2.8 abcd
33.3 ± 3.33 abcd
21
0 ± 0 a
35 ± 5.5 abcd
48.3 ± 6.5 bcde
0 ± 0 a
0 ± 0 a
0 ± 0 a
18.3 ± 8.4 ab
21.7 ± 7.4 abc
20 ± 6.9 abc
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
40 ± 7.5 cdefg
63.3 ± 8.5 gh
73.3 ± 9 h
56.7 ± 8.3 efg
31.7 ± 6.3 cdefg
0 ± 0 a
41.7 ± 8 cdefg
28.3 ± 6 bcde
16.7 ± 5.5 abc
B. bassiana LPSc 1060
14
13.3 ± 2.2 abcd
40 ± 8.3 cdefg
46.7 ± 7.7 efgh
43.3 ± 7.5 defg
23.3 ± 3.6 bcdef
0 ± 0 a
35 ± 8 cdefg
16.7 ± 4.6 abcde
0 ± 0 a
21
0 ± 0 a
21.7 ± 5.5 bcde
18.3 ± 5.2 abcde
18.3 ± 2 abcd
0 ± 0 a
0 ± 0 a
6.7 ± 2 ab
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
61.7 ± 5 fg
66.7 ± 4 gh
95 ± 3.5h
50 ± 0 defg
25 ± 0 abcdef
0 ± 0 a
66.3 ± 9.3 gh
55.3 ± 7.7 defg
55 ± 5 defg
B. bassiana LPSc 1061
14
48.3 ± 9 defg
46.7 ± 8 cdefg
50 ± 6.9 defg
0 ± 0 a
0 ± 0 a
0 ± 0 a
35 ± 8.4 bcdefg
26.7 ± 5.6 abcdef
23.3 ± 5.6 abcde
21
18.3 ± 7.2 abcd
11.7 ± 6 abc
13.3 ± 3 abc
0 ± 0 a
0 ± 0 a
0 ± 0 a
6.7 ± 2.7 ab
3.3 ± 1.2 ab
3.3 ± 1 ab
28
0 ± 0 a
1.7 ± 0 a
3.3 ± 1 ab
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
20 ± 5.4 abcdef
20 ± 5.4 abcdef
61.7 ± 5 g
18.3 ± 6 abcde
21.7 ± 3.4 abcdef
21.7 ± 2 abcdef
40 ± 3 bcdefg
43.3 ± 6 bcdefg
60 ± 4 fg
B. bassiana LPSc 1062
14
0 ± 0 a
20 ± 5.4 abcdef
51.7 ± 2 efg
16.7 ± 3 abcd
11.7 ± 2 abc
11.7 ± 2 abcd
31.7 ± 4 bcdefg
46.7 ± 5.9 defg
15 ± 2 abcd
21
0 ± 0 a
8.3 ± 2 a
25 ± 3.7 bcdefg
13.3 ± 3 abcd
11.7 ± 2 abc
10 ± 1 ab
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
30 ± 2 bcde
45 ± 4 defg
78.3 ± 6.1h
30 ± 0 cdef
16.6 ± 0 bcde
0 ± 0 a
68.3 ± 5.2 gh
51.7 ± 5.2 efg
48.3 ± 5 efg
B. bassiana LPSc 1082
14
13.3 ± 3 abc
35 ± 7 cdef
53.3 ± 5.9 fgh
0 ± 0 a
0 ± 0 a
0 ± 0 a
33.3 ± 3 cdef
21.7 ± 2.2 bcde
0 ± 0 a
21
0 ± 0 a
18.3 ± 3.8 abcd
18.3 ± 3.2 abc
0 ± 0 a
0 ± 0 a
0 ± 0 a
5 ± 1 ab
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
46.7 ± 4.1 de
73.3 ± 2.7 ef
73.3 ± 2.7 ef
76.7 ± 7.1f
48.3 ± 3 de
0 ± 0 a
20 ± 0 bc
6.6 ± 0 ab
0 ± 0 a
B. bassiana LPSc 1083
14
36.7 ± 3.3cd
56.7 ± 5.6 def
71.7 ± 2.5 ef
40 ± 4 cd
18.3 ± 7.6 bc
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
21
20 ± 2.1 bc
46.7 ± 4.1 de
50 ± 3.5 de
6.7 ± 1.7 ab
6.7 ± 2 ab
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
6.7 ± 2 ab
6.7 ± 1.7 ab
6.7 ± 1.7 ab
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
0 ± 0 a
51.7 ± 7 fg
76.7 ± 5.6h
30 ± 0 cdef
20 ± 0 bc
0 ± 0 a
53.3 ± 4.1 fg
53.3 ± 4.1 fg
30 ± 3.1 cde
B. bassiana LPSc 1081
14
0 ± 0 a
41.7 ± 6.2 efg
65 ± 6.3 gh
0 ± 0 a
0 ± 0 a
0 ± 0 a
53.3 ± 4.1 fg
43.3 ± 2.7 efg
20 ± 5.4 bc
21
0 ± 0 a
28.3 ± 3.5 cdef
36.7 ± 2.1 def
0 ± 0 a
0 ± 0 a
0 ± 0 a
16.7 ± 0.1cd
5 ± 1 a
6.7 ± 2.2 ab
28
0 ± 0 a
0 ± 0 a
3.3 ± 2.2 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
31.7 ± 6 abcd
65 ± 5 bcd
75 ± 5.6 d
38.3 ± 7 abcd
46.7 ± 6 abcd
43.3 ± 5.6 abcd
55 ± 3 bcd
40.3 ± 5 abcd
40 ± 2 abcd
B. bassiana LPSc 902
14
25 ± 4 abcd
25 ± 2 abcd
66.7 ± 5.6cd
38.3 ± 2.2 abcd
33.3 ± 2 abc
23.3 ± 3.3 abc
40 ± 1 abcd
36.7 ± 3.9 abcd
25 ± 3.4 abc
21
16.7 ± 3 abc
25 ± 2 abcd
25 ± 5.4 abcd
0 ± 0 a
11.7 ± 3.8 ab
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
16.7 ± 3 abc
16.7 ± 1 abc
23.3 ± 3 abcd
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
16.7 ± 0.1b
41.7 ± 6.2c
56.7 ± 7.2 d
0 ± 0 a
0 ± 0 a
0 ± 0 a
66.6 ± 0.4 d
33.3 ± 0.2c
16.7 ± 0.1b
M. anisopliae LPSc 907
14
0 ± 0 a
26.7 ± 3.6 bc
41.7 ± 5.1c
0 ± 0 a
0 ± 0 a
0 ± 0 a
33.3 ± 0.2c
16.7 ± 0.1b
0 ± 0 a
21
0 ± 0 a
0 ± 0 a
18.3 ± 3.8b
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
7
26.7 ± 3.6 bc
38.3 ± 6.4cd
48.3 ± 4.3 de
0 ± 0 a
0 ± 0 a
0 ± 0 a
66.6 ± 0.4 e
16.7 ± 0.1b
0 ± 0 a
M. robertsii LPSc 963
14
16.6 ± 1 a
20 ± 3.3b
26.7 ± 5 bc
0 ± 0 a
0 ± 0 a
0 ± 0 a
66.6 ± 0.4 e
16.7 ± 0.1b
0 ± 0 a
21
0 ± 0 a
0 ± 0 a
23.3 ± 3.6 bc
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
28
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
0 ± 0 a
Technique
Organ
Time
Technique * organ
Technique * time
Organ * time
Technique * organ * time
F
df
p
F
df
p
F
df
p
F
df
p
F
df
p
F
df
p
F
df
p
B.bassiana
LPSc 1098201.52
2
0.0001
28.13
2
<0.0001
81.4
3
<0.0001
26.5
4
<0.0001
5.43
6
<0.0001
2
6
0.0646
3.17
12
0.0003
B.bassiana
LPSc 10679.26
2
0.0001
7.12
2
0.0009
16.18
3
<0.0001
3.23
4
0.0128
0.11
6
0.9949
0.77
6
0.5920
0.88
12
0.5656
B.bassiana
LPSc 108661.67
2
<0.0001
8.1
2
0.0004
107.8
3
<0.0001
15.51
4
<0.0001
16.48
6
<0.0001
3.41
6
0.0028
5.02
12
<0.0001
B.bassiana
LPSc 108035.41
2
<0.0001
20.84
2
<0.0001
93.9
3
<0.0001
2.8
4
0.0260
11.15
6
<0.0001
5.97
6
<0.0001
4
12
<0.0001
B.bassiana
LPSc 10639.43
2
0.0001
8.47
2
0.0003
70.23
3
<0.0001
12.03
4
<0.0001
1.14
6
0.3408
0.78
6
0.5826
5.1
12
<0.0001
B.bassiana
LPSc 1066110.75
2
<0.0001
7.32
2
0.0008
339.78
3
<0.0001
81.88
4
<0.0001
21.99
6
<0.0001
17.27
6
<0.0001
8.77
12
<0..0001
B.bassiana
LPSc 115646.82
2
<0.0001
30.65
2
<0.0001
106.47
3
<0.0001
15.43
4
<0.0001
12.66
6
<0.0001
5.68
6
<0.0001
2.36
12
0.0065
B.bassiana
LPSc 106034.01
2
<0.0001
12.85
2
<0.0001
136.9
3
<0.0001
34.18
4
<0.0001
6.02
6
<0.0001
3.54
6
0.0021
5.87
12
<0.0001
B.bassiana
LPSc 1061117.8
2
<0.0001
0.48
2
0.6223
102.76
3
<0.0001
2.58
4
0.0371
26.68
6
<0.0001
0.5
6
0.8071
1.18
12
0.2984
B.bassiana
LPSc 10623.06
2
0.0482
8.66
2
0.0002
69.51
3
<0.0001
11.52
4
<0.0001
4.47
6
0.0002
1.74
6
0.1101
3.52
12
0.0001
B.bassiana
LPSc 1082129.51
2
<0.0001
2.52
2
0.0819
135.86
3
<0.0001
26.16
4
<0.0001
37.91
6
<0.0001
2.06
6
0.0572
5.21
12
<0.0001
B.bassiana
LPSc 1083461.75
2
<0.0001
10.45
2
<0.0001
130.17
3
<0.0001
51.32
4
<0.0001
41.09
6
<0.0001
7.35
6
<0.0001
10.32
12
<0.0001
B.bassiana
LPSc 9027.66
2
0.0006
1.13
2
0.3258
43.32
3
<0.0001
4.09
4
0.0030
0.73
6
0.6241
0.51
6
0.8021
0.91
12
0.5332
M.anisopliae
LPSc 907377.16
2
<0.0001
4.19
2
0.0160
450.57
3
<0.0001
137.91
4
<0.0001
116.18
6
<0.0001
9.98
6
<0.0001
30.46
12
<0.0001
M.robertsii
LPSc 963308.93
2
<0.0001
11.42
2
<0.0001
302.69
3
<0.0001
171.78
4
<0.0001
93.5
6
<0.0001
28.22
6
<0.0001
40.38
12
<0.0001
3.1.1 Leaf aspersion
The highest number of isolates was recovered from leaves after 7 days inoculation. In general, there was a significant decrease in the percentage of colonized pieces over time. B. bassiana strain LPSc 1098 was the most successful strain, because it showed 100%, 90% and 45% of recovery from leaves, stems, and roots, respectively, after 7 days of inoculation; values that were not obtained for any of the other strains tested (Table 1).
The two isolates belonging to Metarhizium spp. were re-isolated from leaves, stems and roots, but with colonization rates of 60%, 40% and 16% respectively (Table 1).
3.1.2 Root immersion
B. bassiana strains LPSc 1080, LPSc 1156, LPSc 1061, LPSc 1082 and LPSc 902 and Metarhizium strains LPSc 963 and LPSc 907 showed the highest percentages of isolation (55–71%) from roots after 7 days of inoculation. B. bassiana strain LPSc 1062 exhibited the highest frequency of leaf colonization. No isolates of entomopathogenic fungi were registered after 28 days of inoculation (Table 1).
3.1.3 Seed immersion
B. bassiana strains LPSc 1081, LPSc 1082, LPSc 1061 and LPSc 1086 were re-isolated from roots and stems after 7 days of inoculation, with a mean percentage of 40% and 16.6%, respectively. In the case of B. bassiana strains LPSc 1063, LPSc 1066 and LPSc 1083, they were able to colonize the roots and stems but not the leaves after 7, 14 and 21 days of inoculation, exhibiting higher percentages of colonization than the previously mentioned strains.
The other fungal strains were introduced through the seeds and were able to colonize roots, stems, and leaves, even though, after 28 days of inoculation, the only strain re-isolated from roots, stems and leaves was B. bassiana LPSc 1067 (Table 1).
3.2 Experiment-II: effect of fungal inoculation on plant growth and yield
The entomopathogenic fungus B. bassiana promoted the growth of soybean plants, since all growth parameters assessed after inoculation were significantly higher than in the controls: plant height (T = 4.49; df = 58; p < 0.0001), number of branches per plant (T = 4.38; df = 58; p < 0.0001), weight of the pods per branch (T = 3.71; df = 58; p = 0.0005), weight of the pods per plant (T = 4.85; df = 58; p < 0.0001), number of pods per branch (T = 2.87; df = 58; p = 0.0057), number of pods per plant (T = 4.32; df = 58; p < 0.0001), number of seeds per pod (T = 3.48; df = 58; p = 0.0009), number of seeds per branch (T = 4.54; df = 58; p < 0.0001), number of seeds per plant (T = 4.88; df = 58; p < 0.0001), seed weight per branch (T = 1.57; df = 58; p = 0.05), seed weight per plant (T = 4.17; df = 58; p < 0.0001) and yield (T = 2.67 df = 4; p = 0.0456) (Fig. 1). The mean germinative capacity in inoculated plants was significantly higher (T = 8.55, df = 4, p < 0.0010) than in the controls.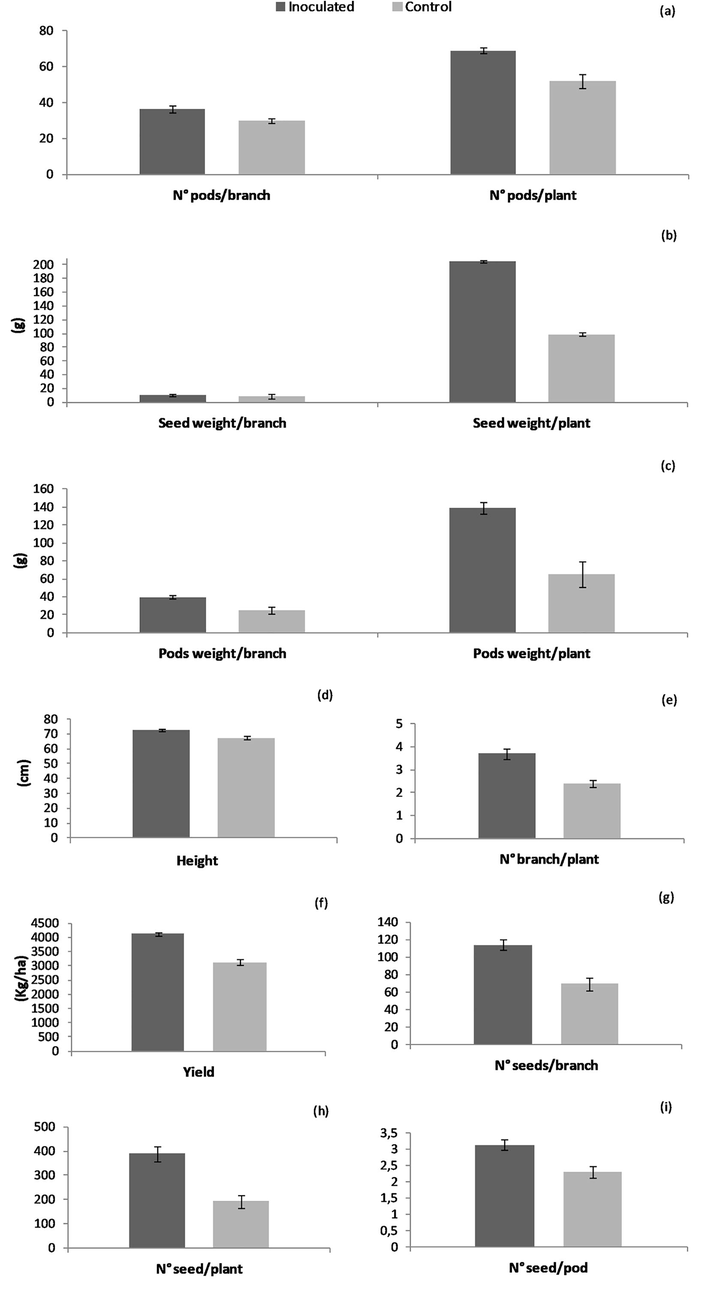
Growth parametres in soybean plants: (a) N° pods/branch and plant, (b) seed weigth/branch and plant (g), (c) pods weigth /branch and plant (g), (d) heigth (cm), (e) N° branch/ plant, (f) yield (kg/ha), (g) N° seed/branch, (h) N° seed/plant and (i) N° seed/pod. Bars indicate ±SEM.
4 Discussion
This study demonstrated that the three inoculation techniques used, i.e. leaf aspersion, root and seed immersion, successfully introduced different strains of B. bassiana into soybean plants. On the contrary, strains of M. anisopliae and M. robertsii were able to establish endophytically exclusively by leaf aspersion and root immersion. The sterilization efficiency was tested by incubating a piece of vegetal tissue onto a solid medium (McKinnion et al., 2017; Reddy et al., 2009). In contrast, most studies to date have tested the efficiency of sterilization by pipetting aliquots of the final water sterilization onto a solid medium (Greenfield et al., 2016; Parsa et al., 2013; Posada and Vega, 2005; Tefera and Vidal, 2009; Vidal and Jaber, 2015), however, this approach may not be an adequate control due to dilution effects and potential failure to remove epiphytes. Plant cuticles are multi-dimensional and hydrophobic so, these surfaces can potentially protect epiphytic microorganisms during submersion and a single viable colony forming unit could yield a false ‘endophyte’ positive. In particular, viable conidia are typically found adhering to the plant surface rather than floating freely in the rinse solution, even when using surfactants (Schulz and Boyle, 2005).
As in the case of poppies (Quesada Moraga et al., 2006), beans (Parsa et al., 2013) and sorghum (Tefera and Vidal, 2009), leaf aspersion was the most efficient technique for the inoculation of different fungal strains into soybean plants. The greatest recovery of B. bassiana and Metarhizium spp. was obtained from leaves after 7 days of inoculation. On the other hand, when the inoculation was performed in the roots or seeds, the entomopathogenic fungi were mostly re-isolated from roots and in less proportion from leaves. This might be the result of a higher colonization frequency in plant organs next to the inoculum than in ones distant to the application place (Greenfield et al., 2016). It is important to mention that the percentage of isolation decreased over time. This result is in agreement with Parsa et al. (2013) in beans, Greenfield et al. (2016) in manioc and Brownbridge et al. (2012) in pine trees. On the contrary, Batta (2013) showed that in rape plants the entomopathogenic fungi Metarhizium sp. and Beauveria sp. were mainly isolated after 4 weeks of inoculation. However, in contrast to our results in which leaf inoculation was a successful technique, some studies showed the greatest recovery of B. bassiana in coffee by direct injection (Posada et al., 2007) and in tomato through the inoculation of roots (Qayyum et al., 2015), indicating that leaves are not appropriate routes of entry for fungal colonization. The low recovery of B. bassiana from leaves could be due to specific cuticular components on the leaf and the lack of stomata on the adaxial side. It is possible that the main components on the leaf cuticle, waxes and cutin, might have a detrimental effect on conidium germination (Posada et al., 2007).
Beauveria bassiana, M. anisopliae and M. robertsii were successfully established as endophytes in soybean plants. As observed by Brownbridge et al. (2012) and Parsa et al. (2013), inoculation of both seeds and roots by entomopathogenic fungi did not reduce seed germination or affect plant growth and we did not observe any damage to roots (data not shown). In agreement with these results, Posada and Vega (2005) and Tefera and Vidal (2009) demonstrated that colonization of the different plant organs differed between Beauveria and Metarhizium. The reason for the greatest colonization found in leaves and roots is yet unclear but might show differences in physiological conditions or in the microbial content between plant organs. Although we used sterile substrates and seeds, we were unable to guarantee that plants were free of native endophytes (Posada and Vega, 2005; Quesada Moraga et al., 2009; Vega, 2008).
In this study, we demonstrated by using the seed and root immersion techniques that as endophytes, the entomopathogenic fungi are able to move throughout the soybean plant tissues, entering through the roots, stems, and leaves. Likewise, the leaf aspersion technique showed the ability of entomopathogenic fungi to enter the plant through these organs, move throughout the different tissues and consequently been isolated from stems, roots, and leaves. The colonization of different plant organs indicated that these fungi are able to move throughout the plant systemically (Akutse et al., 2013; Ownley et al., 2008; Quesada Moraga et al., 2009).
The low inoculum recovery after 28 days of inoculation might be due to the competition with other fungi and bacteria in the system or the host response to fungal colonization. Consequently, there was no balance in the coexistence of both organisms (endophytic relationship), which lead to the growth inhibition of the entomopathogenic fungus (Posada et al., 2007). It is also possible that the efficiency of sterilization methods used minimized the recoverage of fungal propagules (Brownbridge et al. 2012; Quesada Moraga et al., 2009). Considering that we used small sections of the plant organs, the chosen procedure should be optimized for the host plant with respect to the type of tissue and its sensitivity, as stated by Brownbridge et al. (2012). On the other hand, the entomopathogenic fungus B. bassiana positively affected all the growth parameters evaluated. Posada and Vega (2006) obtained similar results in coffee seedlings, without registering harmful impacts on plant health, whereas Griffin et al. (2005), Ownley et al. (2004), and Ownley et al. (2008) observed that the application of B. bassiana as an endophyte in tomato and cotton plants produced a significant increase in the height of these crops. Castillo Lopez and Sword (2015) found an increase in certain growth parameters of cotton plants, such as dry weight and size of the reproductive structures, in response to the inoculation of B. bassiana and Purpureocillium lilacinum (Thom) Samson (Hypocreales: Ophiocordycipitaceae). Likewise, Greenfield et al. (2016) observed an increase in the growth of cassava plants after inoculation with B. bassiana and M. anisopliae. Qayyum et al. (2015) inoculated two different strains of B. bassiana in tomato plants and observed that one of them promoted plant growth while the other caused a delay in the growth and development of the plants and a reduction in the size of the fruits.
Our results showed that the yield increased significantly in inoculated plants, which is in agreement with the results of field studies obtained in onion inoculated with entomopathogenic fungi (Kabaluk and Ericsson, 2007). It has also been demonstrated for root endophytic fungi as Piriformospora indica Sav.Verma, Aj.Varma, Rexer, G.Kost & P.Franken (Sebacinales: Sebacinaceae) to promote growth and yield in soybean plants (Bajaj et al., 2015, 2017a, b).
Unlike Quesada Moraga et al. (2014b), who found that B. bassiana can be transferred vertically in poppy plants, our results could not demonstrate this effect. Even though, we observed that the germinative capacity of seeds in plants inoculated with B. bassiana was considerably higher than in non-inoculated plants.
Regarding the mechanisms related to the promotion of plant growth, previous studies suggested that B. bassiana could reduce the damage caused by insect pests and/or act as an antagonist against certain pathogens (Ownley et al., 2008). Other studies conducted mainly with endophytic and non-entomopathogenic fungi, have suggested that the increase in plant growth can be either due to the production of growth hormones (auxins, gibberellins, and cytokinins) or an increase in the fixation of soil nutrients (Castillo Lopez and Sword, 2015).
Although the aim of the present study was not elucidating the mechanisms that promoted the growth of soybean plants in response to the colonization by the entomopathogenic fungus B. bassiana as an endophyte, it demonstrated a significant increase in the growth and yield of inoculated plants, without adverse effects observed in their development.
5 Conclusions
Our study demonstrated for the first time that the entomopathogenic fungi B. bassiana, M. anisopliae and M. robertsii could associate endophytically with soybean plants. The greatest recovery of the different fungal strains occurred after 7 days inoculation, through the organ that was in direct contact with the fungus during the inoculation. We found that B. bassiana LPSc 1098 inoculated by leaf aspersion is a promising isolate increasing fitness of soybean plants under field conditions.
Acknowledgments
This study was partially supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET PIP 0018), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015-1146), Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CICPBA), Universidad Nacional de La Plata (UNLP, 11/N 773) and Rizobacter Argentina S.A.
MLR and SP conceived and designed research. MC performed morphological determination; CM and AT conducted molecular analysis; MLR, ACS and NA conducted laboratory experiments; MLR and MFV conducted fiel work. MLR, SP and ACS analyzed data. MLV wrote the manuscript. All authors read and approved the manuscript.
Conflict of interest
There is no conflict of interest.
References
- Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol. Exp. Appl.. 2008;129:157-165.
- [Google Scholar]
- Endophytic Beauveria bassiana in banana (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Prot.. 2008;27:1437-1441.
- [Google Scholar]
- Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control.. 2012;61:215-221.
- [Google Scholar]
- Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae) Fungal Ecol.. 2013;6:293-301.
- [Google Scholar]
- The beneficial root endophyte Piriformospora indica reduces egg density of the soybean cyst nematode. Biol. Control. 2015;90:193-199.
- [Google Scholar]
- The role of arbuscular mycorrhizal fungi and the mycorrhizal-like fungus Piriformospora indica in biocontrol of plant parasitic nematodes. In: Varma A., Prasad R., Tuteja N., eds. Mycorrhiza – Eco-Physiology, Secondary Metabolites, Nanomaterials. Cham: Springer; 2017. p. :43-56.
- [Google Scholar]
- Protocol for biocontrol of soybean cyst nematode with root endophytic fungi. In: Varma A., Sharma A.K., eds. Modern Tools and Techniques to Understand Microbes. Switzerland: Springer; 2017. p. :401-412.
- [Google Scholar]
- Efficacy of endophytic and applied Metarhizium anisopliae (Metch.) Sorokin (Ascomycota: Hypocreales) against larvae of Plutella xylostella L. (Yponomeutidae: Lepidoptera) infesting Brassica napus plants. Crop Prot.. 2013;44:128-134.
- [Google Scholar]
- Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control.. 2012;61:194-200.
- [Google Scholar]
- The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea) Biol. Control. 2015;89:53-60.
- [Google Scholar]
- Efecto de la aplicación de fungicidas foliares de distintos grupos químicos en diferentes estadios fenológicos del cultivo de soja sobre la intensidad de “mancha ojo de rana” (Cercospora sojina) y los componentes del rendiemiento. INTA: Publicación Miscelánea; 2010. p. :118-144.
- Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol. Control. 2016;95:40-48.
- [Google Scholar]
- Biocontrol of Rhizoctonia damping-off of cotton with endophytic Beauveria bassiana. Phytopathology 2005 95-S36
- [Google Scholar]
- Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control. 2010;55:34-41.
- [Google Scholar]
- BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucl. Acids Symp. Ser.. 1999;41:95-98.
- [Google Scholar]
- Identification of entomopathogenic fungi. In: Lacey L.A., ed. Manual of Techniques in Invertebrate Pathology. USA: Academic Press; 2012. p. :151-187.
- [Google Scholar]
- InfoStat, 2004. InfoStat versión 2004, Argentina: Grupo InfoStat, Facultad Ciencias Agrarias, Universidad Nacional de Córdoba Primera edición. Editorial Brujas.
- International Seed Testing Association. Zürich: Rules for Seed Testing; 2007.
- Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control. 2016;103:187-195.
- [Google Scholar]
- Seed treatment increases yield of field corn when applied for wireworm control. Agron. J.. 2007;99:1377-1381.
- [Google Scholar]
- The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol.. 2014;98:7089-7096.
- [Google Scholar]
- Efecto de la chinche de los cuernos “Dichelops furcatus” (F.) sobre la calidad de la semilla de soja. Revista Fac Agron Univ Nac La Plata. 2013;112:141-145.
- [Google Scholar]
- Beauveria bassiana as an endophyte: a critical review on associated methodology and biocontrol potential. BioControl. 2017;62:1-17.
- [Google Scholar]
- Beauveria bassiana: endophytic colonization and plant disease control. J. Invertebr. Pathol.. 2008;98:267-270.
- [Google Scholar]
- Beauveria bassiana, a dual purpose biocontrol organism with activity against insect pests and plant pathogens. In: Lartey R.T., Cesar A.J., eds. Emerging Concepts in Plant Health Management. India: Research Signpost; 2004. p. :255-269.
- [Google Scholar]
- Establishing fungal entomopathogens as endophytes: towards endophytic biological control. J. Vis. Exp.. 2013;74:e50360.
- [Google Scholar]
- Inoculation of coffe plants with the fungal entomopathogen Beauveria bassiana (Ascomycota Hypocreales) Mycol. Res.. 2007;111:748-757.
- [Google Scholar]
- Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao) Mycologia. 2005;97:1195-1200.
- [Google Scholar]
- Inoculation and colonization of coffee seedlings (Coffea arabica L.) with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) Mycoscience. 2006;47:284-289.
- [Google Scholar]
- Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. Biol. Control. 2015;90:200-207.
- [Google Scholar]
- Entomopathogenic and nematophagous fungal endophytes. In: Verma V.C., Gange A.C., eds. Advances in Endophytic Research. India: Springer; 2014. p. :85-99.
- [Google Scholar]
- Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia. 2006;161:323-329.
- [Google Scholar]
- The hidden habit of the entomopathogenic fungus Beauveria bassiana: First demonstration of vertical plant transmission. PLoS One. 2014;9:e89278.
- [Google Scholar]
- Systemic Protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales) Environ. Entomol.. 2009;38:723-730.
- [Google Scholar]
- Treatment of millet crop plant (Sorghum bicolor) with the entomopathogen fungus (Beauveria bassiana) to combat infestation by the stem borer, Chilo partellus Swinhoe (Lepidoptera: Pyralidae) J. Asia Pacific. Entomol.. 2009;12:221-226.
- [Google Scholar]
- Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, Hypocreales) Biocontrol Sci. Techn.. 2015;25:475-480.
- [Google Scholar]
- Beauveria bassiana: An entomopathogenic fungus alleviates Fe chlorosis symptoms in plants grown on calcareous substrates. Sci. Hort.. 2015;197:193-202.
- [Google Scholar]
- The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot.. 2012;99:101-107.
- [Google Scholar]
- Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 2009;54:663-669.
- [Google Scholar]
- Fungal entomopathogens: new insights on their ecology. Fungal Ecol.. 2009;2:149-159.
- [Google Scholar]
- Fungal entomopathogens. In: Vega F.E., Kaya H.K., eds. Insect Pathology. San Diego: Academic Press; 2012. p. :171-220.
- [Google Scholar]
- Entomopathogenic fungi as endophytes: plant–endophyte–herbivore interactions and prospects for use in biological control. Current. Sci.. 2015;109:46-54.
- [Google Scholar]
- Species and organ specificity of fungal endophytes in herbaceous grassland plants. J. Ecol.. 2012;100:1085-1092.
- [Google Scholar]
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfend D.H., Sninsky J.J., White T.J., eds. PCR Protocols. A Guide to Methods and Applications. San Diego: Academic Press; 1990. p. :315-322.
- [Google Scholar]
- Do endophytic fungi grow through their hosts systemically? Fungal Ecol.. 2015;13:53-59.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2018.04.008.
Appendix A
Supplementary data
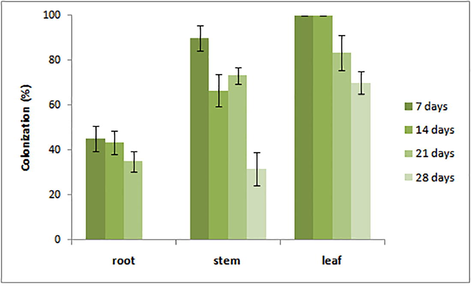
Mean (±SEM) percentage colonization of B. bassiana LPSc 1098 in leaves, stems and roots after 7, 14, 21 and 28 days by leaf aspersión technique.
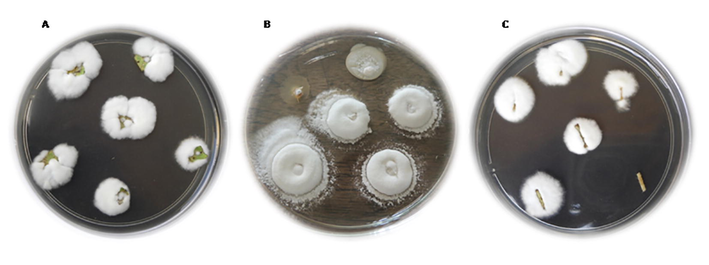
B. bassiana LPSc1098 reisolation from (A) leaf, (B) stem and (C) root.







